Abstract
Vaccines have been seen as the most important solution for ending the coronavirus disease 2019 (COVID‐19) pandemic. The aim of this study is to evaluate the antibody levels after inactivated virus vaccination. We included 148 healthcare workers (74 with prior COVID‐19 infection and 74 with not). They received two doses of inactivated virus vaccine (CoronaVac). Serum samples were prospectively collected three times (Days 0, 28, 56). We measured SARS‐CoV‐2 IgGsp antibodies quantitatively and neutralizing antibodies. After the first dose, antibody responses did not develop in 64.8% of the participants without prior COVID‐19 infection. All participants had developed antibody responses after the second dose. We observed that IgGsp antibody titers elicited by a single vaccine dose in participants with prior COVID‐19 infection were higher than after two doses of vaccine in participants without prior infection (geometric mean titer: 898 and 607 AU/ml). IgGsp antibodies, participants with prior COVID‐19 infection had higher antibody levels as geometric mean titers at all time points (p < 0.001). We also found a positive correlation between IgGsp antibody titers and neutralizing capacity (r s = 0.697, p < 0.001). Although people without prior COVID‐19 infection should complete their vaccination protocol, the adequacy of a single dose of vaccine is still in question for individuals with prior COVID‐19. New methods are needed to measure the duration of protection of vaccines and their effectiveness against variants as the world is vaccinated. We believe quantitative IgGsp values may reflect the neutralization capacity of some vaccines.
Keywords: coronaVac, immunogenicity, inactivated virus vaccine, neutralizing antibody, SARS‐CoV‐2 antibody
1. INTRODUCTION
Since the first day of clinical studies, coronavirus vaccines have continued to be the most important solution for ending the pandemic. Nine vaccines with emergency‐use approval are being used in different countries all over the world, and over 4 billion doses of vaccine have been administered as of August 11, 2021. 1
Due to the limited global supply of vaccines, the rational use of vaccines and the development of alternative vaccine protocols have gained importance. However, there is still a question about if and when people who have previously had coronavirus disease 2019 (COVID‐19) need to be vaccinated. A small series of studies comparing antibody responses generated by messenger RNA (mRNA) vaccines in people previously infected and not infected with COVID‐19 were recently published. According to the published data, a single dose of mRNA vaccine response may be sufficient in people who have previously been infected with COVID‐19. 2 , 3 , 4
CoronoVac is an inactivated virus vaccine developed by Sinovac Life Sciences in early 2020. After demonstrating the safety and efficacy of the vaccine in Phase 1/2 studies, 5 , 6 Phase 3 studies were initiated in Brazil, Indonesia, Chile, and Turkey. CoronaVac efficacy was found to be 83.5% in the Phase 3 study in Turkey. 7 In an April 2021 report, the World Health Organization (WHO) stated that the CoronaVac vaccine is effective in preventing COVID‐19, and they approved it for emergency use in June 2021. 8 , 9
Individuals and clinicians are trying to understand the effectiveness of vaccines and how long the protection lasts as vaccination rates rise. Assessments of vaccine effectiveness are based on real‐world data that takes time. In vaccination research with influenza, smallpox, and polio, it has been stated that the neutralizing antibody response predicts vaccine protection, and it is known to play an important role in severe acute respiratory syndrome coronavirus 2. 10 However, neutralizing antibody tests are not widely used because of technical and financial issues. Vaccines induce an immune response against viral spike protein. Anti‐spike antibodies are produced by the immune system and may serve as an indicator of protection. 11
The first goal of this study is to follow up the serological responses to the inactivated virus vaccine, namely CoronaVac, among healthcare workers (HCWs) who had or did not have past COVID‐19. The secondary goal is to investigate the impact of the level of quantitative antibody on neutralization capacity.
2. METHODS
2.1. Data sources and searches
We collected plasma samples from HCWs who had received two doses of CoronaVac (one dose of 0.5 ml contains 600 SU of severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2] virus antigen) at a 28 days interval. CoronaVac has been administered in Turkey since January 14, 2021. 12 In our prospective observational study, 444 plasma samples were collected from 148 HCWs, who all signed informed consent forms. Groups with and without prior COVID‐19 infection were created by matching them according to age and gender characteristics.
Inclusion criteria for participants with prior COVID‐19 infection were SARS‐CoV‐2 polymerase chain reaction (PCR) positivity in the preceding months, as well as contact and symptomatic disease in any period before vaccination.
Participants who had not previously been infected with COVID‐19 were eligible if they had not SARS‐CoV‐2 PCR positivity, no history of quarantine, no history of therapy against COVID‐19 and SARS‐CoV‐2 IgGsp levels <50 AU/ml in their serum before vaccination.
Antibody levels were measured just before the first dose (Day 0), just before the second dose (Day 28), and 28 days after the second dose (Day 56).
Serological testing for antibodies to the receptor of the S1 subunit of the viral spike protein (IgGsp) was performed with the Abbott Architect SARS‐CoV‐2 IgG Quant II (Abbott).
Neutralizing antibodies from 31 participants were tested. This included eight samples from Day 0, eight samples from Day 28, and seven samples (eight samples were studied, but one could not be evaluated due to technical reasons) from Day 56 from participants who had previously been infected with COVID‐19. On Day 56, we collected eight samples from participants who had not previously been infected with COVID‐19. Four of the lowest antibody titer samples and four samples that were closest to the geometric mean titer (GMT) of time‐points were chosen from each group.
We used a live virus‐neutralizing antibody test which is the gold standard for measuring protective immunity. For plaque reduction neutralization tests (PRNT) for SARS‐CoV‐2, Vero E6 cells (3 × 105 cells/ml) were seeded in six‐well plates and incubated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% of penicillin, streptomycin, and amphotericin B solution. Patient sera were diluted in DMEM with 5% of FBS and a mixed standard amount of SARS‐CoV‐2 (10−3 PFU/ml) within a ratio of 1:1 (vol/vol). The serum–virus solution was incubated at 37°C for 1 h. After incubation, supernatants were discarded and cells were supplemented with DMEM and 2% methylcellulose solution. After 4 days at 37°C, supernatants were removed and the six‐well plates were fixed and inactivated using 4% paraformaldehyde and stained with crystal violet. Serum dilutions with a PRNT of 50% (PRNT50) are referred to as titers. Unless stated otherwise, cut‐off titers were set at <1:20.
A standardized questionnaire asking about the demographic characteristics of the participants—age, gender, socioeconomic status, marital status, comorbidity, smoking, and alcohol use—and the use of paracetamol and/or nonsteroidal anti‐inflammatory drugs (NSAIDs) was filled out by all participants with the guidance of an infectious diseases specialist. Socioeconomic status was determined using the income and expenditure self‐report. The use of paracetamol and/or NSAIDs was defined for both chronic use and 24 h before and after vaccination.
To evaluate the body mass index (BMI) of participants, their heights and weights were measured on a standard scale using a measuring instrument. The Pittsburgh sleep quality index (PSQI) was applied to assess sleep states, and those with PSQI ≥ 5 were classified as having poor quality sleep. The disease severity of the previously infected participants was categorized into three groups according to WHO classification: mild, moderate, and severe. 13 In any of the participants during follow‐up, there was no symptomatic disease, though this is based on the participants' self‐reports, as SARS‐CoV‐2 PCR tests were not administered.
The design of this study was followed using the Strengthening the Reporting of Observational Studies in Epidemiology checklist.
This study was approved by the Baskent University Institutional Review Board and Ethics Committee (Project no: KA21/51) and was supported by the Baskent University Research Fund.
2.2. Statistical analyses
Antibody levels were presented as GMTs and confidence intervals. Antibody levels were converted to base 10 logarithms and used in statistical analysis for intergroup comparison. By testing the compatibility of numerical data to a normal distribution, parametric data were evaluated using Student's t tests in paired comparison and Mann–Whitney U tests for those who did not have parametric properties. Analysis of the nominal data χ 2 test or Fisher's exact test was used. When changes of numerical variables were obtained with more than two measurements in a single group, the Friedman test was used if the variables were not normally distributed, and analysis of variance was used for repeated measures. Spearman's correlation coefficient was used to measure the strength of association between antibody levels and neutralized antibody results. Statistical analysis was done with IBM® SPSS© 25 software. Situations below 5% of the type 1 error level were interpreted as statistically significant.
3. RESULTS
3.1. Demographic characteristics
Of the 148 HCW participants included in the study, 104 (70.3%) were women, and the median age was 39 years (min 22–max 64). Forty‐five of the participants (30.4%) had comorbidity; the most prevalent being thyroid dysfunction (13/148, 8.8%). This was followed by hypertension (11/148, 7.4%) and rheumatic disease (10/148, 6.8%). The demographic characteristics of the participants are given in Table S1.
3.2. Evaluation of IgGsp antibody titers
IgGsp antibodies were evaluated as GMTs in all groups. After the first dose (Day 28) IgGsp antibodies were not detected in 64.8% (n = 48/74) of participants without prior COVID‐19 infection. After the second dose (Day 56), all individuals had IgGsp antibodies of more than 50 AU/ml (the positive value of the test). Although there was no significant difference between age groups, antibody responses were highest in the ages 18–34 group, while the mean antibody decreased as the age increased. Antibody titers were significantly higher in females than in males (Day 56 GMT = 1150 AU/ml in females, GMT = 908 AU/ml in males [p = 0.038]). The antibody titers were significantly lower in smokers than in nonsmokers (GMT = 825, 1202 AU/ml, respectively [p = 0.007]). No difference in IgGsp titers was detected in groups categorized by BMI, marital status, socioeconomic status, presence of comorbidities, alcohol use, caregiving to COVID‐19 patients, and paracetamol and/or NSAIDs use (Table 1).
Table 1.
Factors that may affect the vaccine response
| Factors | Day 56 GMT (AU/ml) | 95% CI | p Value |
|---|---|---|---|
| Age | |||
| 18–34 | 1165.47 | 956.7–1419.3 | 0.538 |
| 35–49 | 1017.42 | 840–1231.9 | |
| ≥50 | 984.2 | 581.5–1668 | |
| Gender | |||
| Female | 1150.80 | 986.7–1342.1 | 0.038 * |
| Male | 908.24 | 706.6–1167.6 | |
| BMI | |||
| <30 | 1062.18 | 923.6–1221.5 | 0.716 |
| ≥30 | 1133.97 | 761.9–1687.7 | |
| Marital status | |||
| Married | 1014.14 | 859.6–1196.4 | 0.326 |
| Single | 1164.39 | 936.9–1447.4 | |
| Socioeconomic status | |||
| Income < expenditure | 831.7 | 492.2–1171.2 | 0.1 |
| Income = expenditure | 1222.3 | 758.4–1686.1 | |
| Income > expenditure | 1098.8 | 823.1–1374.6 | |
| Comorbidities | |||
| Yes | 1175.71 | 945.3–1462.1 | 0.344 |
| No | 1030.39 | 874.5–1213.9 | |
| Smoking | |||
| Yes | 825.28 | 663.28–1027.07 | 0.007 * |
| No | 1202.54 | 1025.6–1410.2 | |
| Alcohol consumption | |||
| Yes | 912.85 | 711.2–1171.6 | 0.201 |
| No | 1113.78 | 957.4–1295.6 | |
| Sleep | |||
| Good quality | 994.95 | 822–1203.9 | 0.318 |
| Bad quality | 1171.93 | 980.3–1400.5 | |
| COVID‐19 patients care | |||
| Caregiver | 1015.08 | 831–1239.9 | 0.139 |
| Noncaregiver | 1126.94 | 923.6–1374.6 | |
| Paracetamol and/or NSAID usage | |||
| Yes | 973.52 | 670.9–1276 | 0.511 |
| No | 1093.9 | 741.5–1575 | |
Abbreviations: BMI, body mass index; CI, confidence intervals; COVID‐19, coronavirus disease 2019; GMT, geometric mean titer; NSAID, nonsteroidal anti‐inflammatory drugs.
p < 0.05.
Participants with prior COVID‐19 infection had higher antibody titers at all three measurements. (p < 0.001). The antibody responses of the participants with and without prior COVID‐19 infection and all measurement times are listed in Table S2.
Antibody titers in the previously infected group increased significantly (p < 0.001): Days 0 (GMT = 294.93 AU/ml), 28 (GMT = 607.02 AU/ml), and 56 (GMT = 1280.27 AU/ml), as shown in Figure 1A,B.
Figure 1.
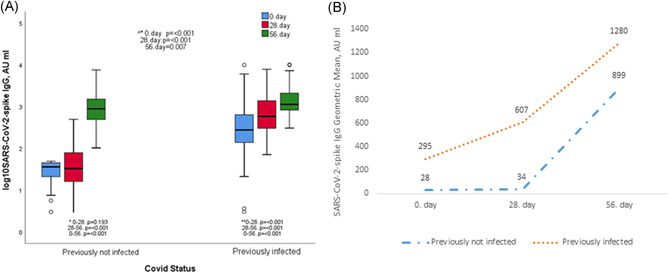
(A) Anti‐SARS‐CoV‐2 IgGsp responses on Days 0, 28, 56 in groups with and without COVID‐19 history. (B) Showing the geometric mean titers with line graphs of COVID‐19 previously infected and not‐infected groups at all measurement times. ^*Comparison between groups. *Not previously infected participants intergroup comparison. **Previously infected participants intergroups comparison. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Participants with prior COVID‐19 infection were divided into three groups based on the duration after infection; less than 3 months (<3 months, n = 29), between 3–6 months (3–6 months, n = 34), and more than 6 months (>6 months, n = 11). Antibody titers on Day 0 were the lowest in the group more than 6 months (GMT: 135.11 AU/ml). Antibody responses were higher in the 3–6 month group than the other time groups in the measurements performed on Days 28 and 56 (Figure 2A,B).
Figure 2.
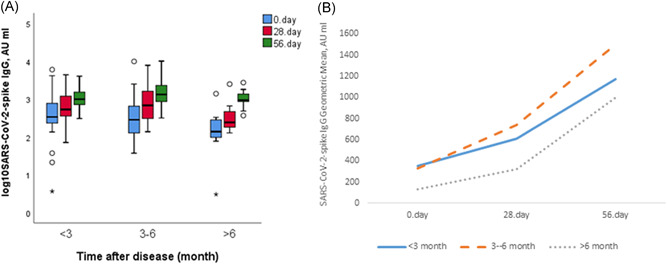
(A) Anti‐SARS‐CoV‐2 IgGsp responses by time after disease in the group with prior COVID‐19. (B) Showing the geometric mean titers with line graphs of the groups according to by the time after disease with prior COVID‐19 at all measurement times. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Participants with prior moderate/severe COVID‐19 infection had higher antibody responses than the group with mild disease on Day 56 (Figure 3A,B).
Figure 3.
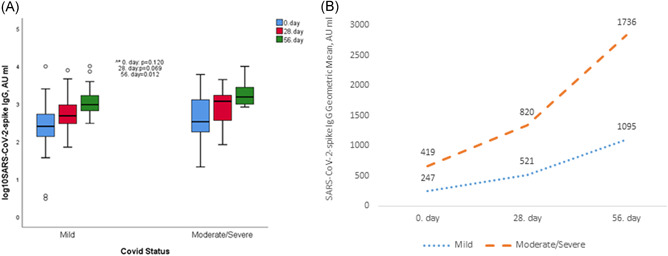
(A) Anti‐SARS‐CoV‐2 IgGsp responses according to disease severity in the group with prior COVID‐19. (B) Showing the geometric mean titers with line graphs of the groups according to the severity of the disease with prior COVID‐19 at all measurement times. ^*Comparisons between groups. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3. Evaluation neutralizing antibodies
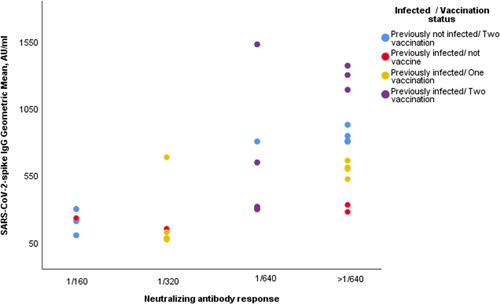
The serological response to the vaccine, natural immune response, and correlation with IgGsp antibody titers was evaluated. Sera from 31 participants were evaluated by neutralization assays and a response equal to or greater than 1/160 dilution was detected (IgGsp antibody titers ranged from 70 to 1526 AU/ml). Neutralizing antibody responses without vaccine were detected 1/320 dilution for those who had past COVID‐19 infection and the lowest antibody titers. However, the lowest antibody titers in second‐dose vaccinated participants who had not been previously infected with COVID‐19 had 1/160 dilution of neutralizing capacity (Figure 4). We found a positive correlation between IgGsp antibody titers and neutralizing capacity—r s = 0.697, p < 0.001—(Figure 5).
Fıgure 4.
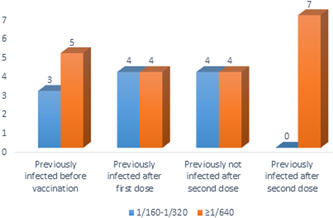
Neutralizing capacity pre‐ and postvaccination with prior COVID‐19 and postvaccination without prior COVID‐19 (n = 31). COVID‐19, coronavirus disease 2019
Figure 5.
Correlation between IgGsp antibodies and neutralizing capacity. IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
4. DISCUSSION
Due to the shortage of vaccine supply, alternative vaccination strategies are needed to combat the pandemic. Modified vaccination strategies in terms of the dose and duration are commonly used. The efficacy of a single dose vaccine being sufficient for individuals with past COVID‐19 infection is under evaluation. Recently, studies on mRNA vaccines comparing antibody responses after vaccination in individuals with and without the previous disease have been published. It has been concluded that the antibody response after a single dose mRNA vaccine in patients with a previous infection is similar to the response after two doses of vaccine for those who did not have a prior infection. 2 , 3 , 4 In our study, we evaluated the serological response to inactivated virus vaccine among the HCWs with and without prior COVID‐19 infection.
There is no established threshold for seroprotection, but 50 AU/ml is considered as positive in our SARS‐CoV‐2 IgGsp test according to the SARS‐CoV‐2 Quant Assay user manual. 14 In this study, we detected that all participants had antibody responses after two doses of the CoronaVac vaccine.
We observed that while antibody levels after the first vaccine were very low in participants without previous COVID‐19, a high titer was observed in antibody levels after the second dose. Among those who had previous COVID‐19 infection, antibody titers after the first dose of vaccine were found to be close to the titers obtained after the second dose in the previously not infected group. Higher antibody titers were detected among previously infected HCWs, compared with not previously infected, during the whole study period.
In our study, we also noticed that older people, men, and smokers have a lower antibody response. Due to the small number of participants in our study, it is impossible to remark on this issue.
CoronaVac has been shown to be effective in preventing symptomatic or severe illness. 7 It was also found to be effective against several SARS‐CoV‐2 variants, albeit some variants drastically reduced neutralizing antibody efficacy. 15 , 16 Neutralizing antibodies have been shown to be highly predictive of SARS‐CoV‐2 infection protection. 17 According to our findings, a single dose of CoronaVac did not produce a reliable antibody response in those who had not previously been infected (n = 48/74, 64.8%), whereas two doses of the vaccine produced antibodies 28 days after the second dose. Individuals without prior COVID‐19 infection had lower antibody responses during the whole study period than those in the previously COVID‐19‐infected group. The SARS‐CoV‐2 Quant Assay user manual estimates a 1050 AU/ml SARS‐CoV‐2 IgG titer, which corresponds to a 95% probability of being at or above the 1:80 neutralization dilutions. However, we detected higher neutralizing capacity at lower quantitative antibody values. This could be due to nonstandardized neutralizing antibody measurements and working with not the same strain. The strain used in our study is the most widely circulating one in our country.
It still remains unclear how long immunity lasts after COVID‐19 infection, the protective level of antibody titer protect against reinfection, and when people should be vaccinated. These are all important questions in terms of the future trajectory of the SARS‐CoV‐2 virus.
According to current evidence, the natural immune response can last up to 9 months after COVID‐19 infection. 18 We also demonstrated that the antibody response persisted for up to 6 months after infection, declining over time. According to the SIREN trial, past COVID‐19 infection reduced the probability of reinfection by 84% and reduced the risk of symptomatic infection by 93%. 19 In our study, the highest antibody titers of participants with prior COVID‐19 infection—in terms of the time passed after the infection—were seen in those who were vaccinated between 3 and 6 months postinfection. This is due to the presence of both basal high IgG‐spike antibody titers and high neutralizing antibodies following the first vaccine dose. A new vaccination schedule can be considered, including extending the dosing interval in those who had prior COVID‐19 infection.
It has been observed in the literature that there is a positive correlation between disease severity and antibody responses. 20 , 21 , 22 , 23 In our study, the basal antibody levels of the participants in the group with moderate/severe disease were higher than those in the group with mild disease. The antibody titers acquired as a result of the participants' immunological response to the vaccine were higher in the moderate/severe disease group than in the mild disease group.
The main limitation of our study was the small number of participants. Larger cohorts are needed to both evaluate antibody responses and assess differences between demographic and clinical subgroups after vaccination. More studies are needed to determine whether a certain interval would be optimal for timing vaccination in people previously infected with COVID‐19. Although no symptomatic disease developed during the follow‐up of our study, the probability of encountering SARS‐CoV‐2 is still high, as our group consisted of HCWs and we were unable to prove an absence of infection using PCR tests. We were unable to measure both neutralizing antibody and IgG‐spike antibody titers for all participants at different times due to limited resources. Our study shows neutralizing antibodies against the most widely circulating strain of SARS‐CoV‐2 in our country, but we do not have data regarding variants.
5. CONCLUSION
It is well known that not only spike antibodies but also T‐ and B‐cell mediated immune factors play a role in the immune response against SARS‐CoV‐2. The most important solution in the fight against COVID‐19 still seems to be vaccines that reach the greatest number of individuals in the shortest amount of time.
In the present study after two doses of vaccination, all vaccinated individuals developed an IgGsp antibody response. These responses were found to be significantly lower than mRNA vaccine responses in previous studies of the same quantitative test. 4 On the other hand, neutralizing antibodies were detected in all cases (≥1/160 dilution) of participants receiving a single dose of vaccine with prior COVID‐19 infection and participants receiving two doses of vaccine without prior infection.
In our research, we observed a positive correlation between IgGsp antibody levels and neutralization capacity obtained after CoronaVac vaccination. We believe quantitative antibody tests are important because it is well known that neutralizing antibody levels decrease over time and neutralizing antibody measurement is not routinely available. Vaccination around the world necessitates the development of standard and rapid new methods for measuring vaccine duration of protection and effectiveness against variants. From now on, comparing booster doses developed with various vaccines, particularly in our country, with antibody titers and, if possible, neutralization results follow‐up will be useful in evaluating the pandemic's course, vaccine effectiveness follow‐up, and booster timing. This is a pioneering study, and more comprehensive research on the subject is required.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Tuğba Y. Yalçın, Nuran Sarı, and Çiğdem Erol contributed to data interpretation, writing. Deniz İ. Topcu contributed severe acute respiratory syndrome coronavirus 2 IgGsp assessment. Saliha Aydın contributed to statistic analysis. Özlem Doğan, Zeynep E. Kuloğlu, and Füsun Can contributed to neutralizing antibody tests. Hande Arslan and Özlem Azap contributed to proof‐reading and supervision.
Supporting information
Supporting information.
Yalçın TY, Topçu Dİ, Doğan Ö, et al. Immunogenicity after two doses of inactivated virus vaccine in healthcare workers with and without previous COVID‐19 infection: prospective observational study. J Med Virol. 2021;94:279‐286. 10.1002/jmv.27316
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author (Dr. Tuğba Y. Yalçın). The data are not publicly available due to patient privacy.
REFERENCES
- 1. WHO Health Emergency Dashboard . WHO (COVID‐19). Homepage; 2021. Accessed May 30, 2021. https://covid19.who.int/
- 2. Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS‐CoV‐2 infection on humoral and T‐cell responses to single‐dose BNT162b2 vaccine. Lancet. 2021;397(10280):1178‐1181. 10.1016/S0140-6736(21)00502-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saadat S, Rikhtegaran Tehrani Z, Logue J, et al. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS‐CoV‐2. JAMA. 2021;325(14):1467‐1469. 10.1001/jama.2021.3341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebinger JE, Fert‐Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS‐CoV‐2. Nat Med. 2021;27(6):981‐984. 10.1038/s41591-021-01325-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18‐59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. 10.1016/S1473-3099(20)30843-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(6):803‐812. 10.1016/S1473-3099(20)30987-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole‐virion SARS‐CoV‐2 vaccine (CoronaVac): interim results of a double‐blind, randomised, placebo‐controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213‐222. 10.1016/S0140-6736(21)01429-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evidence Assessment: Sinovac/CoronaVac COVID‐19 Vaccine . For recommendatıon by the Strategıc Advısory Group of Experts (Sage) on Immunızatıon. Prepared by the SAGE Working Group on COVID‐19 vaccines; 2021. Accessed May 30, 2021. https://cdn.who.int/media/docs/default-source/immunization/sage/2021/april/5_sage29apr2021_critical-evidence_sinovac.pdf
- 9. World Health Organization . WHO validates Sinovac COVID‐19 vaccine for emergency use and issues interim policy recommendations; 2021. Accessed June 6, 2021. https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations
- 10. Suhandynata RT, Hoffman MA, Huang D, et al. Commercial serology assays predict neutralization activity against SARS‐CoV‐2. Clin Chem. 2021;67(2):404‐414. 10.1093/clinchem/hvaa262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eyre DW, Lumley SF, Wei J, et al. Quantitative SARS‐CoV‐2 anti‐spike responses to Pfizer‐BioNTech and Oxford‐AstraZeneca vaccines by previous infection status [published online ahead of print, 2021 Jun 7]. Clin Microbiol Infect. 2021;S1198‐743X(21)00289‐5. doi:10.1016/j.cmi.2021.05.041 [DOI] [PMC free article] [PubMed]
- 12.Public attention; 2021. Accessed May 13, 2021. https://www.titck.gov.tr/haber/kamuoyunun-dikkatine-13012021185623
- 13.COVID‐19 clinical management: living guidance; 2021. Accessed May 30, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
- 14.SARS‐CoV‐2 IgG II Quant Assay User Manual. Abbott Laboratories; 2020.
- 15. Chen Y, Shen H, Huang R, Tong X, Wu C. Serum neutralising activity against SARS‐CoV‐2 variants elicited by CoronaVac. Lancet Infect Dis. 2021;21(8):1071‐1072. 10.1016/S1473-3099(21)00287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang GL, Wang ZY, Duan LJ, et al. Susceptibility of circulating SARS‐CoV‐2 variants to neutralization. N Engl J Med. 2021;384(24):2354‐2356. 10.1056/NEJMc2103022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐CoV‐2 infection. Nat Med. 2021;27(7):1205‐1211. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 18.Achiron A, Gurevich M, Falb R, Dreyer‐Alster S, Sonis P, Mandel M. SARS‐CoV‐2 antibody dynamics and B‐cell memory response over time in COVID‐19 convalescent subjects. Clin Microbiol Infect. 2021;27(9):1349.e1‐1349.e6. doi: 10.1016/j.cmi.2021.05.008 [DOI] [PMC free article] [PubMed]
- 19. Hall VJ, Foulkes S, Charlett A, et al. SARS‐CoV‐2 infection rates of antibody‐positive compared with antibody‐negative health‐care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet. 2021;397(10283):1459‐1469. 10.1016/S0140-6736(21)00675-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS‐CoV‐2‐specific neutralizing antibody responses in COVID‐19. Signal Transduct Target Ther. 2020;5(1):180. 10.1038/s41392-020-00301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang AT, Garcia‐Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. 10.1038/s41467-020-18450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Zhang L, Sang L, et al. Kinetics of viral load and antibody response in relation to COVID‐19 severity. J Clin Invest. 2020;130(10):5235‐5244. 10.1172/JCI138759 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author (Dr. Tuğba Y. Yalçın). The data are not publicly available due to patient privacy.


