Abstract
Immunocompromised patients have an increased risk of persistent COVID‐19 disease. We report here the clinical course of two patients with hematologic malignancies hospitalized due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. In both patients, viral evolution including new spike gene mutations that occurred following treatment with anti‐SARS‐CoV‐2 antibodies preparations, including convalescent plasma and bamlanivimab. These cases demonstrate the possibility of antibody‐resistant SARS‐CoV‐2 infections evolution in immunocompromised patients.
Keywords: bamlanivimab, convalescent plasma, COVID‐19, immunocompromised, SARS‐CoV‐2, Spike gene
Highlights
Patients with hematologic malignancies are at risk of persistent SARS‐CoV‐2 infection.
Using anti‐SRAS‐CoV‐2 antibody preparations in immunocompromised patients may lead to the evolution of antibody‐resistant SRAS‐CoV‐2 infections.
Antibody‐resistant SRAS‐CoV‐2 infections might have severe epidemiological implications.
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic has spread worldwide since late 2019, causing a massive burden of morbidity and mortality. Immunocompromised patients, particularly those with hematologic malignancies 1 have an increased risk of severe disease and higher rates of intensive care admissions and mortality. While most adults with COVID‐19 infection remain infectious up to 10 days after symptoms onset, there are several reports that immunocompromised adults might remain infectious for 3 weeks and even more. 2 , 3 Furthermore, recent studies 4 show that chronic infection may lead to SARS‐CoV‐2 viral mutations, especially in patients treated with convalescent plasma and monoclonal antibody therapy.
In this study, we report the clinical course of two patients with hematologic malignancies who were hospitalized due to SARS‐CoV‐2 infection. We describe the viral evolution during their hospital course in relation to their treatment with various SARS‐CoV‐2 antibody preparations (Figure 1).
Figure 1.
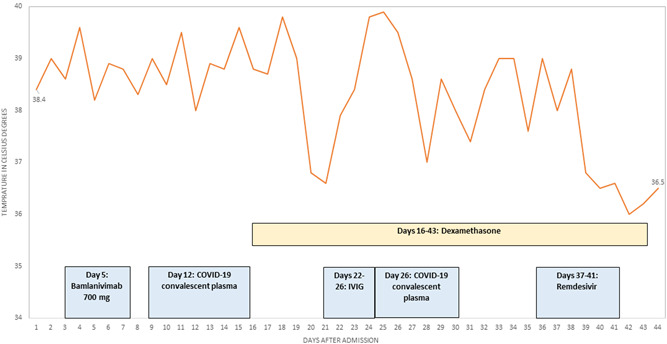
Hospital course of Patient #1. The figure includes the timing of the anti‐SARS‐CoV‐2 treatments
2. METHODS
2.1. Setup
The study was conducted at the Tel Aviv Sourasky Medical Center (TASMC), a 1400‐bed tertiary center. Medical and laboratory data were obtained from the patient's electronic health record.
The study was approved by the Ethics committee of the Tel Aviv Sourasky Medical Center.
2.2. SARS‐CoV‐2 PCR testing
Samples obtained from nasopharyngeal swabs were analyzed at the hospital's virological laboratory. The hospital's virological laboratory performed RT‐PCR testing using several assays: (1) the Seegene Allplex™ 2019‐nCoV assay, targeting the E, N, and RdRP genes; (2) the cobas® SARS‐CoV‐2 assay, targeting the E and the ORF genes; (3) the Xpert® Xpress SARS‐CoV‐2, targeting the E and the N genes; (4) the Simplexa™ COVID‐19 Direct assay, targeting the S and the ORF genes.
2.3. Whole genome sequencing of SARS‐CoV‐2 positive samples
Total nucleic acid was extracted from respiratory specimens using the magLEAD 12gC (Precision System Science Co., Ltd). cDNA synthesis and enrichment were performed on the extracted total nucleic acids using QIAseq SARS‐CoV‐2 Primer Panel (Cat no. 333896; Qiagen). Amplicon libraries for viral genome sequencing using QIAseq FX DNA Library Kit (Cat no. 180475; Qiagen) as instructed by the manufacturer's manual. The library was normalized to 4 nmol and sequenced on the Illumina MiSeq platform, using Illumina MiSeq reagent kit v3 600 cycles (2 × 150 bp), according to the manufacturer's instructions.
2.4. Bioinformatic analysis
FASTAQ files were imported into CLC Genomics Workbench version 21.02.2 (Qiagen), raw reads trimming and mapping to the Wuhan SARS‐CoV‐1 reference genome (MN908947.3) 5 producing a consensus sequence. We filtered mutations that met a coverage of >30X and the frequency >70%. The global phylogenetic placement was determined using the Nextclade database (https://clades.nextstrain.org/) and Phylogenetic Assignment of Named Global Outbreak Lineages (PANGOLIN).
3. RESULTS
3.1. Cases description
Patient #1: A 68‐year‐old male with chronic lymphocytic leukemia known since 2012, was diagnosed with symptomatic SARS‐CoV‐2 infection at the beginning of January 2021. The patient was treated with two cycles of fludarabine, cyclophosphamide, and rituximab in 2017, then with prednisone due to autoimmune hemolytic anemia since 2019 and with venetoclax plus rituximab since July 2020.
Ten days following the diagnosis, the patient was admitted to TASMC due to bilateral pneumonia and was treated with ceftriaxone, levofloxacin, and bamlanivimab. Although his respiratory symptoms improved, the patient had persistent fever and positive COVID‐19 tests on Day 12 of his admission. Hence, treatment with dexamethasone was initiated followed by COVID‐19 convalescent plasma.
On Day 21 of his admission, the patient was given a course of intravenous immunoglobulin treatment due to persistent fever. No other source for his symptoms besides COVID‐19 was identified despite extensive evaluation and hence the second course of COVID‐19 convalescent plasma was administered. On Day 37 of his admission, he started a course of remdesivir for 5 days, which was followed by resolution of the fever and an improvement of his general condition. The patient was discharged on Day 43 of his admission while continuing to have positive COVID‐19 PCR tests. The patient continued his medical follow‐up outside our institution.
Patient #2: A 33‐year‐old male with previously untreated Hodgkin lymphoma (HL) was admitted to TASMC at the beginning of February 2021 due to worsening dyspnea. The patient was diagnosed with HL 4 months before his admission that was complicated by pleural and pericardial effusion and a right cardiac ventricle thrombus. He was treated with anticoagulants but refused chemotherapy. Five days before his admission, the patient was tested positive by COVID‐19 PCR and was admitted later due to worsening dyspnea. On admission, the patient and was found to have recurrent bilateral pleural effusions, a pleural embolism, and an inferior vena cava obstruction. Hence, it was unclear whether his symptoms were due to SARS‐CoV‐2 infection or due to his underlying illness. The patient was initially treated with high‐flow oxygen, pleurocentesis, and dexamethasone. On the 15th day of his admission and due to persistence of positive COVID‐19 PCR, the patient received bamlanivimab. The patient's condition continued to deteriorate and 1 month after his admission he agreed to chemotherapy with dacarbazine, doxorubicin, and vincristine and showed improvement. In the following 2 months, the patient was hospitalized twice due to fever. COVID‐19 PCR was persistently positive throughout that period.
3.2. Molecular analysis of COVID‐19 genomes
The spike gene mutations, the total number of mutations (compared with the wild‐type strain MN908947), as well as the PCR C t values, are presented in Tables 1 and 2.
Table 1.
Molecular evolution of the SARS‐CoV‐2 strain in Patient #1
| Test date | C t value | Spike mutations | Number of mutation | |
|---|---|---|---|---|
| 1 | Jan 17, 2021 | ORF = 22, E = 22 | Leu5Phe | 23 |
| His69_Val70del | ||||
| Tyr145del | ||||
| Asn501Tyr | ||||
| Ala570Asp | ||||
| Asp614Gly | ||||
| Pro681His | ||||
| Thr716Ile | ||||
| Ser982Ala | ||||
| Asp1118His | ||||
| 2 | Feb 8, 2021 | E = 30, N = 30 | Leu5Phe | 28 |
| His69_Val70del | ||||
| Tyr145del | ||||
| Glu484Gln | ||||
| Asn501Tyr | ||||
| Ala570Asp | ||||
| Asp614Gly | ||||
| Pro681His | ||||
| Thr716Ile | ||||
| Ser982Ala | ||||
| Asp1118His | ||||
| 3 | Feb 19, 2021 | E = 23, N = 25, RdrP = 24 | Leu5Phe | 28 |
| His69_Val70del | ||||
| Tyr145del | ||||
| Glu484Gln | ||||
| Asn501Tyr | ||||
| Ala570Asp | ||||
| Asp614Gly | ||||
| Pro681His | ||||
| Thr716Ile | ||||
| Ser982Ala | ||||
| Asp1118His | ||||
| 4 | Feb 28, 2021 | E = 19, N = 21, RdrP = 21 | Leu5Phe | 30 |
| His69_Val70del | ||||
| Tyr145del | ||||
| Glu484Gln | ||||
| Asn501Tyr | ||||
| Ala570Asp | ||||
| Asp614Gly | ||||
| Pro681His | ||||
| Thr716Ile | ||||
| Ser982Ala | ||||
| Asp1118His |
Note: The total number of mutations and the detailed spike gene mutations are in comparison with the Wuhan SARS‐CoV‐1 reference genome (MN908947.3).
Table 2.
Molecular evolution of the SARS‐CoV‐2 strain in Patient #2
| Test date | C t value | Spike mutations | Number of mutation | |
|---|---|---|---|---|
| 1 | Feb 1, 2021 | E = 12, N = 16, RdrP = 14 | Leu452Arg | 14 |
| Asp614Gly | ||||
| Leu1063Phe | ||||
| 2 | Feb 10, 2021 | E = 20, N = 20, RdrP = 19 | Leu452Arg | 14 |
| Asp614Gly | ||||
| Leu1063Phe | ||||
| 3 | Feb 17, 2021 | E = 29, N = 26, RdrP = 28 | Leu452Arg | 14 |
| Asp614Gly | ||||
| Leu1063Phe | ||||
| 4 | Mar 16, 2021 | E = 27, N = 28, RdrP = 26 | Phe140del | 18 |
| Leu452Arg | ||||
| Gly485Arg | ||||
| Asp614Gly | ||||
| Leu1063Phe | ||||
| 5 | Mar 25, 2021 | E = 25, N = 25 | Pro139_Tyr144del | 19 |
| Trp258Cys | ||||
| Leu452Arg | ||||
| Gly485Arg | ||||
| Asp614Gly | ||||
| Leu1063Phe | ||||
| 6 | Apr 22, 2021 | ORF = 30, E = 31 | Pro139_Tyr144del | 21 |
| Trp258Cys | ||||
| Leu452Arg | ||||
| Gly485Arg | ||||
| Asp614Gly | ||||
| Leu1063Phe |
Note: The total number of mutations and the detailed spike gene mutations are in comparison with the Wuhan SARS‐CoV‐1 reference genome (MN908947.3).
Patient #1 was infected by a virus of the alpha (B.1.1.7) SARS‐CoV‐2 lineage. Altogether, seven mutations evolved during his 44 days of hospitalization. Following treatment with convalescent plasma, additional spike mutation was noted (Glu484Gln) followed by a decline in the C t values.
Patient #2 was infected by a virus of the B.1.362 SARS‐CoV‐2 lineage. Altogether, seven mutations evolved during his 3 months of hospitalization. Following treatment with bamlanivimab, an additional three spike mutations evolved. The C t values remained stable below 30.
4. DISCUSSION
In this study, we described the clinical course of two patients with hematologic malignancies with persistent SARS‐CoV‐2 infection. In both of them, viral evolution, including spike gene mutations occurred despite treatment with various anti‐SARS‐CoV‐2 antibodies preparations. Patient #1 was treated with convalescent plasma and bamlanivimab. Genomic sequencing of the sample taken on Day 22 of his admission, identified evolution with an E484Q (Glu484Gln) mutation that is located within the receptor‐binding domain of the SRAS‐CoV‐2 spike protein. Several studies demonstrated the phenomenon of mutations emerging after treatment with bamlanivimab 6 , 7 , 8 , 9 , 10 or convalescent plasma therapy, especially in immunocompromised patients. 11 , 12 One study had demonstrated that SRAS‐CoV‐2 infection with this mutation has reduced susceptibility to monoclonal antibodies preparations such as bamlanivimab. 13 Hence, it is possible that the evolution of this mutation had contributed to the persistency of his infection. In his case, the patient improved only after remdesivir treatment as previously described. 14
Patient #2 was infected by a virus of the B.1.362 lineage with the L452R mutation. This variant harbored the L452R mutation, which is associated with the Delta and Epsilon variants and was shown to cause a reduction in neutralization in pseudoviruses. 15 It infected 270 individuals in Israel between December 2020 and March 2021, until diminishing due to the gain in the dominance of the Alpha variant in February 2021. 16
The patient received bamlanivimab 13 days after his admission, which was subsequently followed by the evolution of three new spike gene mutations (Table 2). These mutations have not been previously described in the context of specific biological significance, but several studies 17 , 18 recently demonstrated that similar immune escape mutations occur during cocktail monoclonal antibody therapy, making this a serious concern. However, it signifies the ability of the SRAS‐CoV‐2 virus to evolve in the case of persistent infection, despite treatment with bamlanivimab. Together, these cases demonstrate the clinical dilemma regarding the use of bamlanivimab and other anti‐SARS‐CoV‐2 antibody preparations in immunocompromised patients. Although these patients are prone to severe or persistent SARS‐CoV‐2 infection, the use of these preparations may lead to the evolution of antibody‐resistant SARS‐CoV‐2 infections that in addition to the patient himself, might have serious epidemiological implications. Hence, further studies are required to define the patient's population that can benefit the most from this treatment.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Yotam Bronstein, Yair Herishanu, and Haytham Katash provided clinical care for the patients. Katia Levytskyi, Ora Halutz, and Amos Adler performed and interpreted laboratory studies. Yotam Bronstein and Katia Levytskyi wrote the manuscript. Amos Adler revised the manuscript. All authors reviewed and commented on the revised manuscript before submission.
Bronstein Y, Adler A, Katash H, Halutz O, Herishanu Y, Levytskyi K. Evolution of spike mutations following antibody treatment in two immunocompromised patients with persistent COVID‐19 infection. J Med Virol. 2022;94:1241‐1245. 10.1002/jmv.27445
Yotam Bronstein and Amos Adler contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID‐19: a systematic review and meta‐analysis of 3377 patients. Blood. 2020;136:2881‐2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avanzato VA, Matson MJ, Seifert SN, et al. Case study: prolonged infectious SARS‐CoV‐2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901‐1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aydillo T, Gonzalez‐Reiche AS, Aslam S, et al. Shedding of viable SARS‐CoV‐2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586‐2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kemp SA, Collier DA, Datir RP, et al. SARS‐CoV‐2 evolution during treatment of chronic infection. Nature. 2021;592:277‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sabin AP, Richmond CS, Kenny PA. Acquisition and onward transmission of a SARS‐CoV‐2 E484K variant among household contacts of a bamlanivimab‐treated patient. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lohr B, Niemann D, Verheyen J. Bamlanivimab treatment leads to rapid selection of immune escape variant carrying E484K mutation in a B.1.1.7 infected and immunosuppressed patient. Clin Infect Dis. Published online May 1, 2021. [DOI] [PubMed] [Google Scholar]
- 8. Choudhary MC, Chew KW, Deo R, et al. Emergence of SARS‐CoV‐2 resistance with monoclonal antibody therapy. medRxiv. 2021. [Google Scholar]
- 9. Peiffer‐Smadja N, Bridier‐Nahmias A, Ferré VM, et al. Emergence of E484K mutation following bamlanivimab monotherapy among high‐risk patients infected with the alpha variant of SARS‐CoV‐2. Viruses. 2021;13(8):1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Truffot A, Andréani J, Le Maréchal M, et al. SARS‐CoV‐2 variants in immunocompromised patient given antibody monotherapy. Emerg Infect Dis. 2021;27:2725‐2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verghese M, Jiang B, Iwai N, et al. A SARS‐CoV‐2 variant with L452R and E484Q neutralization resistance mutations. J Clin Microbiol. 2021;59:e0074121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisblum Y, Schmidt F, Zhang F, et al. Escape from neutralizing antibodies by SARS‐CoV‐2 spike protein variants. eLife. 2020;9:e61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID‐19. Nat Rev Immunol. 2021;21:382‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helleberg M, Niemann CU, Moestrup KS, et al. Persistent COVID‐19 in an immunocompromised patient temporarily responsive to two courses of remdesivir therapy. J Infect Dis. 2020;222:1103‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Motozono C, Toyoda M, Zahradnik J, et al. SARS‐CoV‐2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29:1124‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mor O, Mandelboim M, Fleishon S, et al. The rise and fall of an emerging SARS‐CoV‐2 variant with the spike protein mutation L452R. Vaccines. 2021;9(8):937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Focosi D, Novazzi F, Genoni A, et al. Emergence of SARS‐COV‐2 spike protein escape mutation Q493R after treatment for COVID‐19. Emerg Infect Dis. 2021;27:2728‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guigon A, Faure E, Lemaire C, et al. Emergence of Q493R mutation in SARS‐CoV‐2 spike protein during bamlanivimab/etesevimab treatment and resistance to viral clearance. J Infect. 2021;S0163‐4453:(21):00435‐00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


