Background and main findings
The severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) has infected millions worldwide and claimed the lives of many. The severity of SARS‐CoV‐2 infection ranges from asymptomatic to hospitalization and death, with some survivors exhibiting adverse health effects after recovery. Health implications can include decrements in physiological function, autonomic dysfunction, myocarditis and lasting impacts on the respiratory, vascular and nervous systems.
In a recent article published in The Journal of Physiology, Stute et al. (2021) explored autonomic (dys)function and systemic haemodynamics in young, otherwise healthy adults recently infected with SARS‐CoV‐2 (COV+). Muscle sympathetic nerve activity (MSNA), heart rate variability (HRV) and systemic haemodynamics were measured in 12 COV+ participants and 14 healthy control subjects. All COV+ participants experienced mild symptoms following infection (n = 11) or were asymptomatic (n = 1). This group used a comprehensive battery of autonomic cardiovascular assessments, including measurements of resting spontaneous autonomic activity, general sympathoexcitatory conditions (via cold pressor test) and baroreflex‐mediated sympathoexcitatory conditions (via head‐up tilt). The COV+ participants exhibited higher resting MSNA burst frequency (in bursts per minute), burst incidence (in bursts per 100 heartbeats) and total MSNA activity (as total burst area per minute) compared with control subjects. Despite the increased sympathetic vasoconstrictor activity in the COV+ participants, both groups had similar resting heart rate, systolic and diastolic blood pressure. During the cold pressor test, MSNA burst incidence increased equally between the groups, but it remained higher throughout the test in the COV+ group. Consistent with the resting conditions, similar systemic haemodynamic responses were observed throughout the cold pressor test. During the head‐up tilt, HRV was used as an alternative measure of autonomic function, because most MSNA signals were lost during the 60° conditions. Unexpectedly, the COV+ participants had higher overall temporal and frequency domain‐based indices of parasympathetic activity at baseline but exhibited larger drops in parasympathetic activity as the degree of orthostasis was increased. In the subset of participants for whom an MSNA signal was maintained, burst frequency, burst incidence and total activity increased throughout the orthostatic challenge and were consistently higher in the COV+ group (30° tilt, n = 7; 60° tilt, n = 4) compared with the control group (30° and 60° tilt, n = 7). Overall, this study provides a comprehensive and initial investigation into the effects of mild SARS‐CoV‐2 infection on autonomic cardiovascular control in young healthy adults.
Spontaneous sympathetic regulation of arterial blood pressure
Microneurography has been used for more than 50 years to record spontaneous efferent sympathetic nerve activity directed towards the skeletal muscle vasculature. More recently, groups have moved beyond time‐based reports of MSNA burst frequency and burst incidence to include more comprehensive measures of how the sympathetic nervous system influences the vasculature and systemic haemodynamics (i.e. sympathetic transduction). The signal‐averaging technique is a common method to assess sympathetic transduction and has been validated using α‐adrenergic blockades (Fairfax et al. 2013). Using our recently published signal‐averaging analysis program, Stute et al. (2021) reported a moderately high effect size (Cohen's d = 0.74; albeit P = 0.08), observing a larger sympathetic transduction to blood pressure in the COV+ group compared with the control group. This was an important observation considering that the COV+ group also exhibited higher resting burst frequency. This suggests that not only are the COV+ young adults experiencing a greater frequency of sympathetic bursts, but also greater surges in blood pressure for each burst. Generally, it is thought that those with higher burst frequency exhibit decreased sympathetic transduction. These divergent findings might be explained by the acute onset of illnesses such as coronavirus disease 2019 (COVID‐19), in that α‐adrenergic receptors might not have desensitized, in comparison to situations where MSNA increases progressively over time. Alternatively, the larger blood pressure responses following bursts might be attributed to less competing vasodilatory mechanisms. Indeed, Stute et al. (2021) observed smaller falls in blood pressure during periods of sympathetic quiescence in their group of COV+ participants, which might provide some insight into competing vasodilator function. This group also recently demonstrated that COV+ participants exhibit decreased flow‐mediated dilatation. Taken together, SARS‐CoV‐2 infection appears to disrupt the balance between vasodilator and vasoconstrictor function, which might predispose the vascular environment to adverse health consequences. Our understanding of the long‐term health effects of this, specifically in those with more severe illnesses, is unknown.
Impacts of habitual activity, resting blood pressure and sex
Many factors can contribute to spontaneous sympathetic transduction, and there remains uncertainty regarding what it means to have a larger sympathetic transduction (Fig. 1). Our group has demonstrated that healthy individuals who spend less time sedentary (i.e. waking time spent sitting/lying), a healthy lifestyle behaviour, exhibit lower sympathetic transduction (O'Brien et al. 2021). Meanwhile, Kobetic & colleagues (2021) recently reported lower sympathetic transduction in a sample of individuals with untreated hypertension compared with normotensive control subjects. Although Stute et al. (2021) studied a sample of individuals with mild symptoms, it is likely that individuals who have recently been sick with COVID‐19 had to isolate, in addition to resting in order to recover from their illness. If so, an increase in sedentary time might have contributed to the observed higher sympathetic transduction. However, it should be noted that many factors can influence sympathetic transduction (Fig. 1). Interestingly, both groups studied by Stute et al. (2021) appeared to have stage 1 hypertension or prehypertension (COV+, 132/78 mmHg; and control, 129/75 mmHg). It is unclear whether different results would be observed in normotensive individuals or a more hypertensive sample following acute SARS‐CoV‐2 infection.
Figure 1. Factors that might contribute to spontaneous sympathetic neurohaemodynamic or neurovascular transduction of bursts of muscle sympathetic nerve activity.
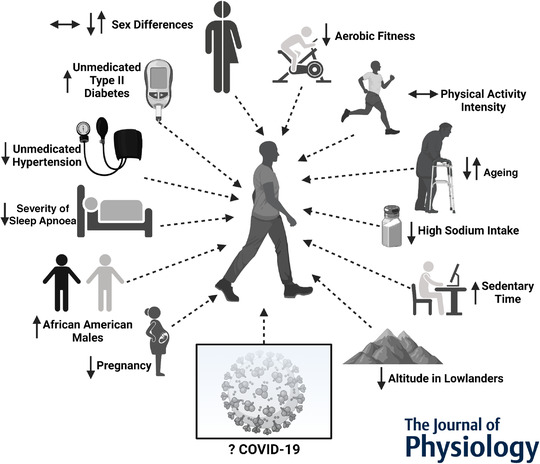
The direction of each arrow indicates the effect of the factor on sympathetic transduction. Multiple arrows are provided for conflicting reports. COVID‐19, coronavirus disease 2019.
There remains conflicting evidence regarding whether sex influences sympathetic neurovascular or neurohaemodynamic transduction in young adults (Fig. 1). The COV+ group in this study was 33% females (4 of 12), whereas the control group was 65% females (9 of 14). Young females can have attenuated α‐adrenergically mediated vasoconstriction in response to noradrenaline, possibly attributable to counteracting β‐adrenergically mediated vasodilatation (Hissen & Taylor, 2020). Thus, sex might have played a role in the differences in the sympathetic regulation of blood pressure observed in this study, in that the larger transduction responses might be attributable to the male‐dominant COV+ sample. Nevertheless, recruitment and testing of COV+ patients is inherently challenging. It is difficult to tease out the individual contributions of sex‐, activity‐ and illness‐related impacts with a cross‐sectional study design, and future longitudinal work following COVID‐19 survivors might provide more insight into these factors.
Heart rate variability in COVID‐19 survivors
Based on the higher MSNA, COVID‐19 is certainly getting on our nerves. However, based on the HRV results, it might not be getting on our nerves as one would have expected. Heart rate variability describes beat‐to‐beat fluctuations in heart rate and is determined by cardiac autonomic modulation (i.e. both sympathetic and parasympathetic nerve activity). At rest, Stute et al. (2021) observed that their COV+ group had higher cardiac vagal activity using either time‐domain (root mean square of successive cardiac interval differences) or frequency‐domain analyses (high‐frequency band). As identified by the authors, this is a surprising result. It is peculiar that COVID‐19 survivors would exhibit both increased sympathetic vasoconstrictor activity (measured directly as MSNA) and elevated parasympathetic cardiac activity (estimated indirectly from changes in heart rate variability), indicating greater autonomic activity in general. In contrast, the responses during head‐up tilt were as expected, with both control and COV+ groups experiencing decreased parasympathetic activity with the magnitude of the orthostatic challenge. The discrepancy between resting HRV and HRV during the head‐up tilt in the COV+ group is an interesting finding. It is unclear whether these findings will have any future implications for the neurocardiovascular health of these young adults.
Conclusion
The careful research conducted by Stute et al. (2021) represents an important contribution to our understanding of the impact of COVID‐19 on autonomic function, which might have implications for the development of neurocardiovascular conditions resulting from SARS‐CoV‐2 infection. Importantly, a robust experimental protocol was conducted using invasive, direct measures of sympathetic activity across spontaneous and multiple sympathoexcitatory conditions. This work sets the stage for further research aimed at understanding the impact of the COVID‐19 on the sympathetic regulation of arterial pressure across different clinical populations using interventional designs in the immediate and long‐term aetiology of COVID‐19‐induced health consequences. A better understanding of the neurohaemodynamic mechanisms of COVID‐19 might lead to targeted therapies that improve the treatment of COVID‐19 survivors with ‘long COVID’ symptoms.
Additional information
Competing interests
None declared.
Author contributions
Conception or design of work: J.L.P. and M.W.O. Analysis and interpretation of the work: all authors. Drafting the work or revising it critically for important intellectual content: all authors. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
Jennifer L. Petterson was supported by a Canadian Institute of Health Research Fredrick Banting and Charles Best Master's Award. Myles W. O'Brien was supported by a Heart and Stroke Foundation of Nova Scotia BrightRed Scholarship, a Nova Scotia Graduate Scholarship, a Research Nova Scotia–Scotia Scholars Award, a Killam PreDoctoral Scholarship and a Canadian Institute of Health Research Fredrick Banting and Charles Best Doctoral Award.
Supporting information
Peer Review History
Acknowledgements
We acknowledge Dr Derek Kimmerly for his input and discussion of the article contents.
Edited by: Harold Schultz & Vaughan Macefield
Linked articles: This Journal Club article highlights an article by Stute et al. To read this article, visit https://doi.org/10.1113/JP281888.
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282362#support‐information‐section).
References
- Fairfax ST, Holwerda SW, Credeur DP, Zuidema MY, Medley JH, Dyke PC, Wray DW, Davis MJ & Fadel PJ (2013). The role of α‐adrenergic receptors in mediating beat‐by‐beat sympathetic vascular transduction in the forearm of resting man. J Physiol 591, 3637–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissen SL & Taylor CE (2020). Sex differences in vascular transduction of sympathetic nerve activity. Clin Auton Res 30, 381–392. [DOI] [PubMed] [Google Scholar]
- Kobetic MD, Burchell AE, Ratcliffe LEK, Neumann S, Adams ZH, Nolan R, Nightingale AK, Paton JFR & Hart EC (2021). Sympathetic transduction in untreated hypertension. J Hum Hypertens 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MW, Ramsay D, Johnston W & Kimmerly DS (2021). The association between habitual posture and intensity‑related physical activity with sympathetic neurohemodynamic transduction in young males. Clin Auton Res 31, 339–341. [DOI] [PubMed] [Google Scholar]
- Stute NL, Stickford JL, Province VM, Augenreich MA, Ratchford SM & Stickford ASL (2021). COVID‐19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS‐CoV‐2. J Physiol 599, 4269–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Peer Review History


