Abstract
Hemolysis, a pathological component of many diseases, is associated with thrombosis and vascular dysfunction. Hemolytic products, including cell-free hemoglobin and free heme directly activate platelets. However, the effect of hemolysis on platelet degranulation, a central process in not only thrombosis, but also inflammatory and mitogenic signaling, remains less clear. Our group showed that hemoglobin-induced platelet activation involved the production of mitochondrial reactive oxygen species (mtROS). However, the molecular mechanism by which extracellular hemolysis induces platelet mtROS production, and whether these mtROS regulate platelet degranulation remains unknown. Here, we demonstrate using isolated human platelets that cell free heme is a more potent agonist for platelet activation than hemoglobin, and stimulates the release of a specific set of molecules, including the glycoprotein thrombospondin-1 (TSP-1), from the α-granule of platelets. We uncover the mechanism of heme-mediated platelet mtROS production which is dependent on the activation of platelet toll-like receptor 4 (TLR4) signaling and leads to the downstream phosphorylation and inhibition of complex-V by the serine kinase Akt. Notably, inhibition of platelet TLR4 or Akt, or scavenging of mtROS prevents heme-induced granule release in vitro. Further, heme-dependent granule release is significantly attenuated in vivo in mice lacking TLR4 or those treated with the mtROS scavenger MitoTEMPO. These data elucidate a novel mechanism of TLR4-mediated mitochondrial regulation, establish the mechanistic link between hemolysis and platelet degranulation, and begin to define the heme and mtROS-dependent platelet secretome. These data have implications for hemolysis-induced thrombo-inflammatory signaling and for the consideration of platelet mitochondria as a therapeutic target in hemolytic disorders.
Key points
-
1.
Heme activates platelet toll-like receptor 4 signaling to induce mitochondrial oxidant production.
-
2.
Heme-stimulated platelet granule secretion is regulated by mitochondrial oxidants.
1. Introduction
Intravascular hemolysis occurs in a number of pathologies ranging from genetic hemoglobinopathies [[1], [2], [3]] to more acute conditions such as sepsis [4], pre-eclampsia [5,6], parasitic infection [7,8], or in patients after cardiac surgery [9,10]. It is well established that patients with chronic hemolysis, such as in sickle cell disease, are at significantly greater risk for thrombotic complications [11,12] as well as endothelial dysfunction [13,14] and chronic vasculopathy [[15], [16], [17]], both of which lead to morbidities such as stroke [18,19] and pulmonary hypertension (PH) [[20], [21], [22], [23], [24]]. However, the mechanisms that link hemolysis, thrombosis, and vasculopathy have not been fully elucidated. Platelets, when activated, are central mediators of thrombosis [[25], [26], [27]], sentinels of inflammatory signaling [27,28], and can also propagate vascular responses through the synthesis and release of vasoactive molecules from alpha and dense granules [[29], [30], [31]]. Notably, cell-free hemoglobin (Hb) or free heme released via hemolysis stimulates platelet thrombotic activation [[32], [33], [34], [35], [36]] and promotes inflammatory signaling [37,38]. At a mechanistic level, these effects have been linked to Hb-dependent modulation of platelet mitochondrial function. Specifically, Hb inhibits complex V of the platelet mitochondrial electron transport chain, leading to an increase in mitochondrial reactive oxygen species (mtROS) production, which stimulates platelet activation [32]. Consistent with this mechanism, specific scavengers of mtROS attenuate hemolysis-induced platelet activation [32] and inflammatory signaling [39] ex vivo and thrombosis in murine models [40]. Despite the recognition that Hb-induced mtROS regulates platelet activation and inflammatory signaling, the mechanisms by which hemoglobin or heme released via hemolysis stimulates mtROS production within the platelet is unknown.
Platelet degranulation, while associated with platelet activation, is a distinct and tightly regulated process. Although it is estimated that platelet granules contain over three hundred diverse molecules [41,42], specific patterns of granule contents are released in response to differential platelet agonists [31,[43], [44], [45]]. Thrombospondin-1 (TSP-1), a multifunctional glycoprotein that is stored in and secreted from platelet α-granules, regulates thrombosis [[46], [47], [48]] as well as inflammatory [49,50] and vascular signaling [50,51]. For example, once secreted, TSP-1 interacts with cell adhesion receptors and integrins to potentiate platelet activation and stabilize platelet aggregation [47,52,53]. Through its selective interaction with CD36 or CD47 on macrophages, other leukocytes, and endothelial cells, TSP-1 can stimulate the inflammatory response through the potentiation of NFkB [54] and TGF-β signaling [49,55,56] and the enhancement of leukocyte migration [49,57]. Thrombospondin-1 also inhibits endothelial nitric oxide signaling and enhances matricellular remodeling to propagate vascular remodeling [58,59]. Notably, plasma and platelet TSP-1 levels are significantly increased in conditions with components of hemolysis such as sickle cell disease [60,61] and sepsis [62,63] respectively, and genetic inhibition of TSP-1 signaling attenuates vasculopathy in murine models of sickle cell disease [64] and prevents inflammation and morbidity in a murine model of cecal ligation and puncture induced sepsis [65]. While these studies highlight the role of platelet-derived TSP-1 as a mediator of thrombo-inflammation and vasculopathy, it is unknown whether hemolysis stimulates TSP-1 release, what other granule molecules are released by platelets upon encountering heme, and whether heme-dependent degranulation is regulated by platelet mtROS production.
In this study, we test whether heme and Hb released via hemolysis stimulate platelet granule release and determined the role of mtROS in this process. We demonstrate that cell free heme is a more potent stimulator of platelet mtROS production and TSP-1 release than Hb, and that mtROS production is required for the heme-dependent release of TSP-1 and other granule molecules. Further, we demonstrate that mechanistically, heme-induced mtROS production requires the activation of platelet TLR4 signaling, culminating in the activation of the serine/threonine kinase Akt, which phosphorylates complex V to inhibit its activity, leading to mtROS generation. These data have implications for the regulation of hemolysis induced thrombotic and vascular signaling, as well as for platelet mitochondria as a therapeutic target in hemolytic disease.
2. Materials and methods
All Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and antibodies from BD Biosciences (San Jose, CA) unless otherwise noted.
2.1. Human blood collection and platelet isolation
Venous blood was collected from human participants by standard venipuncture after written informed consent was obtained and in accordance with study #19030018 (approved by the Institutional Review Board of the University of Pittsburgh).
Platelet rich plasma (PRP) was separated from whole blood collected in acid-citrate dextrose (ACD) Solution-A anticoagulant by centrifugation at 500 rpm for 20 min. Platelets were then pelleted in the presence of PGI2 (1 μg/mL) by centrifuging the PRP at 1500×g for 10 min. These platelets were washed with erythrocyte lysis buffer containing PGI2 to remove any residual erythrocytes and resuspended in modified Tyrode's buffer (20 mM HEPES, 128 mM NaCl, 12 mM sodium bicarbonate, 0.4 mM sodium phosphate monobasic, 5 mM dextrose, 1 mM MgCl2, 2.8 mM KCl, pH 7.4). The platelet count was determined by Hemavet® 950.
2.2. Preparation of heme and Hb
Hemin (Cat. No: 51280) was purchased from Sigma-Aldrich (St. Louis, MO), a 50 mM stock solution was made in 1.4 M sodium hydroxide and diluted to 50 μM working stock in modified Tyrode's buffer for all experiments. Human Hb (Cat. No: H7379) was purchased from Sigma-Aldrich (St. Louis, MO) and a stock solution (15 mg/mL) was prepared in PBS (pH-7.2). Methemoglobin (metHb) was prepared by treatment with potassium ferricyanide (1 M), followed by passage through a PD-10 column (Cat. No: 17-0851-01, GE healthcare) as previously described [32]. The purity of heme or metHb was determined using spectrophotometric deconvolution as previously described [32]. Throughout the manuscript, Hb refers to metHb unless otherwise stated.
2.3. Murine studies
TLR4-flox and global TLR4 knockout mice (TLR4−/−) were used in accordance with approval from the University of Pittsburgh Institutional Animal Care and Use Committee. Male mice 10–12 weeks in age (24–27g) were administered cell free heme (110 mg/kg) or saline (vehicle) by tail vein injection. Some groups of mice were pre-treated with MitoTEMPO (300 μM) administered in the drinking water for 72 h. Platelet mtROS (assessed via MitoSOX as described below) and cell free plasma concentrations of TSP-1, interleukin-1 beta (IL-1β), and platelet derived growth factor-B (PDGF-B) were measured 20 min after heme administration.
2.4. Measurement of platelet activation
Platelet activation was measured in washed platelets by staining with anti-CD41a-PE, anti-CD62P (P-selectin)-APC and PAC1 binding antibody-FITC (to bind activated GPIIb/IIIa), and then quantification of these markers and CD41a (as a marker of platelets) by flow cytometry (LSR-Fortessa; Becton Dickinson).
In all experiments, washed platelets (2.0–2.5 × 106/mL) were incubated with heme for 30 min prior to measurement of activation. In some experiments, platelets were pre-treated with TLR4 neutralizing antibody (5 μg/mL), 2 μM of BX795 (TBK1 inhibitor), 100 nM of N-[1-[2-(4-Morpholinyl) ethyl]-1H-benzimidazol-2-yl]-3-nitrobenzamide (IRAK1/4 inhibitor), 5 nM of (5Z)-7-Oxozeaenol (TAK1 inhibitor), and/or 10 μM of MitoTEMPO (mtROS scavenger).
2.5. Measurement of mtROS
Treated washed platelets were pelleted at 1500×g for 5 min, resuspended in HBSS and incubated with 10 μM of MitoSOX™ Red for 5 min, after which fluorescent intensity (510/580 nm) was measured kinetically as previously described [32]. DMSO was used as vehicle control for the pre-treatment of platelets with ARQ092 (10 μM).
2.6. Measurement of Thrombospondin-1 (TSP-1) release from platelets
TSP-1 levels were measured using the Human Thrombospondin-1 DuoSet ELISA kit (R&D systems; DY3074) in the supernatant surrounding treated platelets. DMSO was used as vehicle control for the pre-treatment of platelets with ARQ092 (10 μM).
2.7. Dot blot assay
Conditioned supernatant collected from treated washed platelets was blotted (30 μL) on to a 0.4 μm nitrocellulose membrane using Bio-Dot® Apparatus (Bio-Rad, Cat.No:1706545). The membrane was blocked with SuperBlock™ (TBS) blocking buffer for 30 min at room temperature and followed by incubation with primary antibody overnight at 4 °C (all primary antibodies used for dot blot were purchased form, R&D systems; anti-Cathepsin A, AF1049; anti-Angiostatin, AF226; anti-CD40L, AF617; anti-Kininogen, AF1569; anti-PAI-1, AF1786; anti-Thrombospondin-1, AF3074; AF795; anti-CXCL7, AF393; anti-IL-1β, AF201; anti-PDGF-B, AF220; anti-TGF- β, AF246; anti-FGF basic, AF233) and IRDye® 800CW Donkey anti-Goat IgG secondary antibody for 40min at room temperature. The blots were imaged using a LI-COR imaging system and analyzed using Image Studio software.
2.8. Mitochondrial complex V activity assay
The enzymatic activity of complex V was measured spectrophotometrically by kinetic assay as previously described [32].
2.9. Measurement of pAkt (S473)
Treated platelets were lysed with RIPA lysis buffer including Halt™ protease and phosphatase inhibitor cocktail. The lysates were subjected to western blot by standard procedure using anti-pAkt (S473; Cell Signaling, 4060S) and anti-Akt (pan; Cell Signaling, 4685S) antibodies. The blots were imaged using a LI-COR imaging system and analyzed using Image Studio software.
2.10. Measurement of complex V phosphorylation
The beta subunit of complex V was immunoprecipitated from the lysates of treated platelets using an antibody to the beta subunit of ATP synthase (Millipore, MAB3494) in accordance with the Pierce™ Co-Immunoprecipitation Kit (Cat.No:26149). Immunoprecipitated samples were subjected to western blot by standard procedure using anti- phosphoserine/threonine/tyrosine (Invitrogen, 61–8300) and anti-ATP synthase beta subunit antibodies. The blots were imaged using a LI-COR imaging system and analyzed using Image Studio software.
3. Statistics
Unpaired parametric t-tests were used to compare individual group samples and ANOVA along with Tukey post-hoc test was used to make multiple comparisons. In vitro data was normalized as fold change in most cases, due to the biological variability between platelets from individual human donors. Error bar in the control group was calculated by determining the mean of the biological replicates, setting this value to 1.0 and then plotting the standard error of this mean (SEM) between the biological replicates. Statistical analyses were performed using GraphPad Prism 9 software. P-values <0.05 were considered significant. Data are presented as mean ± standard error of the mean (SEM) unless otherwise specified.
4. Results
4.1. Heme is a more potent platelet agonist than Hb
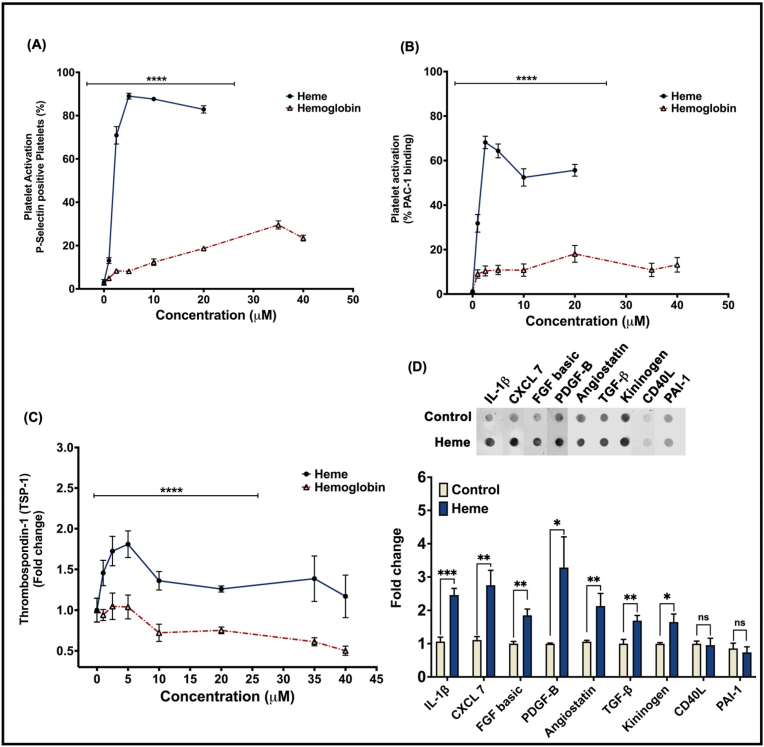
To determine whether free heme stimulates platelet activation as potently as Hb, isolated washed human platelets were incubated with heme (0–20 μM) or metHb (0–40 μM) and platelet activation was measured. Platelets treated with heme or Hb both showed a concentration dependent increase in surface P-selectin levels and PAC-1 antibody binding (as a measure of activated glycoprotein IIb/IIIa -GPIIb/IIIa), indicative of platelet activation (Fig. 1A and B). However, heme treatment stimulated a greater level of surface P-selectin and active GPIIb/IIIa at every concentration measured (70.9 ± 4.03% P-selectin; 68.14 ± 2.76% PAC-1 binding at 2.5 μM) compared to Hb (7.59 ± 0.40% P-selectin; 10.41 ± 2.24% PAC-1 binding at 2.5 μM) (Fig. 1A and B). These results indicate that heme and Hb both activate platelets, but heme is a more potent agonist than Hb for platelet activation. To investigate the role of heme in modulating platelet function beyond activation, we measured the release of TSP-1 from α-granules of heme or Hb treated platelets, as a marker of platelet granule secretion. We observed significantly greater levels of TSP-1 release in heme stimulated platelets compared to those stimulated with Hb (Fig. 1C), similar to the effect on platelet activation.
Fig. 1.
Heme is a more potent platelet agonist than hemoglobin. Platelet activation measured by (A) platelet surface P-selectin or (B) activated GPIIb/IIIa levels in heme (blue line), hemoglobin (red line) treated platelets. (C) Thrombospondin-1 levels quantified in the releasate from heme (blue line) or hemoglobin (red line) treated platelets. (D) Representative dot blot with quantification of IL-1β, CXCL7, FGF basic, PDGF-B, angiostatin, TGFβ, kininogen, CD40L, PAI-1 levels in the releasate from heme (2.5 μM) treated platelets. Data are represented as Mean ± SEM. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns- not significant. n = 3. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Since the platelet granule secretome is agonist specific, we next measured the level of release of a panel of nine common platelet granule molecules in response to heme stimulation. We found that heme induced the release of seven of the granule factors measured (CXCL7, FGF basic, TGFβ, IL-1β, PDGF-B, angiostatin, kininogen), while it did not stimulate the release of CD40L and PAI-1 (Fig. 1D). Notably, we did not observe significant release of these factors by Hb, though we cannot rule out that they were released below the level of detection by our immunoblot method of detection (data not shown). These data demonstrate that heme stimulates the release of a specific pattern of granule molecules.
4.2. Heme- induced mtROS production stimulates platelet granule release
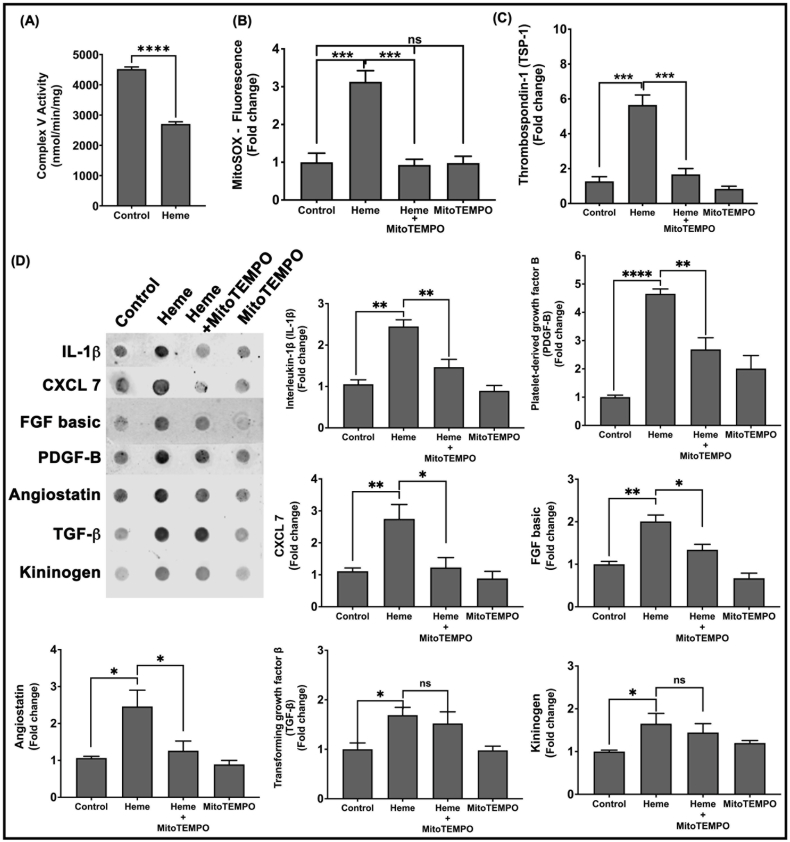
We previously showed that Hb inhibits platelet mitochondrial complex V activity, which increases mitochondrial inner membrane potential to promote mtROS production, an effect that was associated with platelet activation [32]. To test whether heme similarly modulates platelet mitochondrial function, we treated platelets with heme and measured complex V activity and mtROS production. Heme treatment significantly decreased platelet mitochondrial complex V activity (Fig. 2A), and concomitantly increased mtROS production (Fig. 2B).
Fig. 2.
Heme inhibits platelet complex V activity and induces mtROS production that stimulates granule release. Platelets were treated with heme (2.5 μM) in the presence or absence of MitoTEMPO (10 μM) or with mitoTEMPO alone and (A) Platelet mitochondrial complex V activity and (B) MitoSOX fluorescence were measured. (C) Thrombospondin-1 levels in the platelet releasate were measured by ELISA. (D) Representative dot blot along with the quantification of levels of IL-1β, CXCL7, FGF basic, PDGF-B, angiostatin, TGFβ and kininogen in the platelet releasate. Data are Mean ± SEM. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns- not significant. n = 4.
To determine whether mtROS production was required for platelet granule release, we measured the levels of release of the eight granule factors identified to be stimulated by heme (TSP1, CXCL7, FGF basic, TGFβ, IL-1β, PDGF-B, angiostatin, kininogen) from heme treated platelets in the presence and absence of MitoTEMPO (10 μM), a mtROS scavenger. Treatment of platelets with MitoTEMPO significantly decreased heme-induced mtROS levels (Fig. 2B) and also significantly attenuated the heme-induced release of TSP1, CXCL7, FGF basic, IL-1β, PDGF-B, and angiostatin, but had no effect on TGFβ, kininogen release (Fig. 2C and D).
4.3. Heme inhibits mitochondrial complex V and induces mtROS in a TLR4 dependent manner
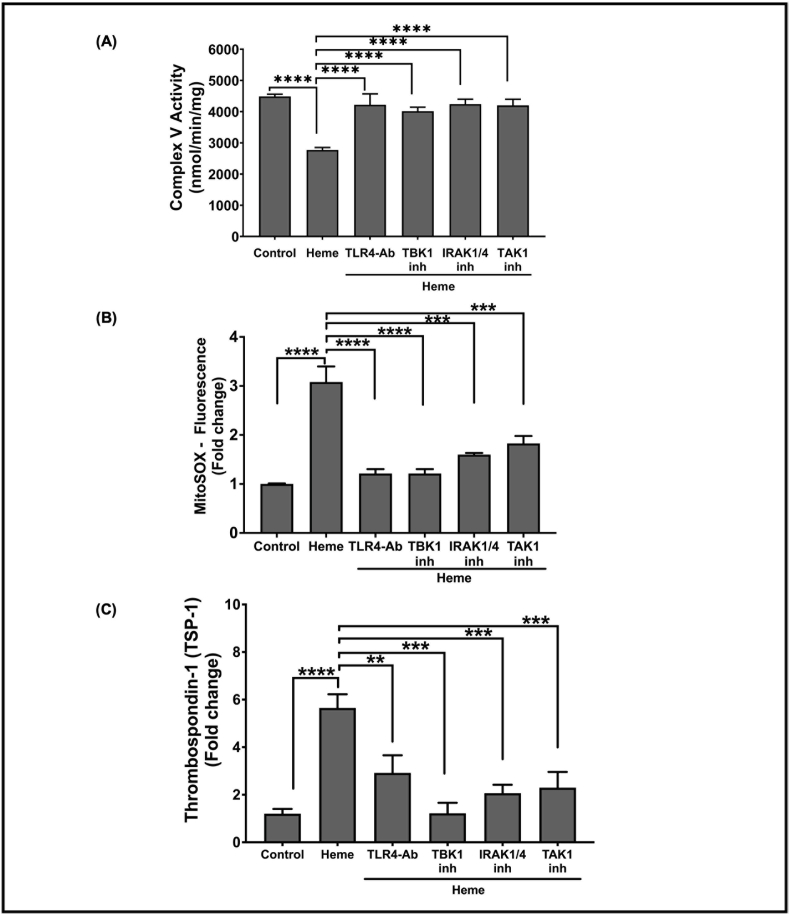
To determine the mechanism by which extracellular heme mediates intra-platelet signaling, we tested whether a platelet surface receptor was required for heme-mediated platelet granule secretion, focusing on TSP-1 as a marker of heme induced granule release. Since TLR4 is known to mediate heme-dependent responses in other cell types [67,68], we blocked platelet TLR4 with TLR4 neutralizing antibody (5 μg/mL) and measured complex V activity and mtROS production in platelets after treatment with heme (2.5 μM). Blocking platelet TLR4 attenuated heme-dependent complex V inhibition (Fig. 3A) and significantly decreased heme-induced mtROS production (Fig. 3B). Consistent with heme induced TSP-1 secretion being dependent on mtROS production, the presence of TLR4 neutralizing antibody also significantly decreased heme-induced TSP-1 secretion (Fig. 3C).
Fig. 3.
Heme inhibits platelet complex V activity to induce mtROS production and TSP-1 release via TLR4. (A) Platelet mitochondrial complex V activity, (B) MitoSOX fluorescence, and (C) thrombospondin-1 levels in the releasate were measured in heme-treated platelets in the presence or absence of TLR4 neutralizing antibody (5 μg/mL), pharmacological inhibitors of TBK1 (2 μM of BX795), IRAK1/4 (100 nM of N-[1-[2-(4-Morpholinyl)ethyl]-1H-benzimidazol-2-yl]-3-nitrobenzamide), TAK1 (5 nM of (5Z)-7-Oxozeaenol). Data are represented as Mean ± SEM. ****p < 0.0001, ***p < 0.001, **p < 0.01. n = 4.
Given that blocking platelet surface TLR4 resulted in decreased heme-induced platelet mtROS production by improving complex V activity and attenuated TSP-1 secretion from heme treated platelets, we sought to determine how TLR4 downstream signaling inhibits complex V activity. We used pharmacological inhibitors to inhibit key downstream kinases in the TLR4 pathway. We blocked signaling associated with the TLR4 adaptor protein MyD88 with inhibitors of kinases downstream of MyD88 - IRAK1/4 and TAK1. Additionally, we used inhibitors of TBK1 kinase to test the role of MyD88-independent signaling. Inhibition of TBK1, IRAK1/4 or TAK1 individually significantly attenuated heme-dependent complex V inhibition (Fig. 3A). Similarly, mtROS production in heme-treated platelets was significantly decreased in the presence of the inhibitors of TBK1, IRAK1/4 or TAK1. (Fig. 3B). Consistent with the requirement for complex V inhibition and mtROS production to induce TSP-1 release by heme, inhibitors of downstream TLR4 signaling also significantly attenuated TSP-1 release from the heme-treated platelets (Fig. 3C). Collectively, these data suggest that heme-dependent TLR4 activation inhibits mitochondrial complex V and induces mtROS production through both MyD88 dependent and independent pathways.
4.4. Heme mediated TLR4 signaling promotes Akt phosphorylation to inhibit complex V activity
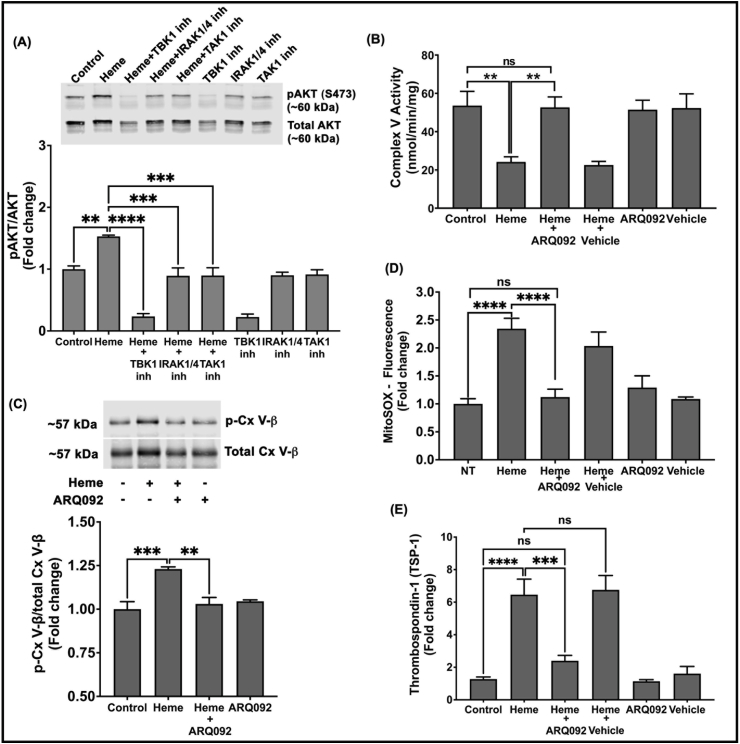
Akt is a serine/threonine-specific protein kinase that can be activated downstream of TLR4 and is known to bind to and phosphorylate a number of mitochondrial proteins, including the α and β subunits of complex V, to regulate their function [69,70]. To determine whether heme-induced TLR4 activation requires Akt activation to inhibit complex V activity, we examined the phosphorylation status of Akt in heme treated platelets by blocking TLR4 downstream signaling with TBK1, IRAK1/4 or TAK1 inhibitors. Heme treated platelets showed a significant increase in the level of Akt phosphorylation at serine 473 (pAkt -S473), which was decreased in heme-treated platelets pre-treated with blockers of TLR4 signaling (Fig. 4A). These data demonstrate that heme-mediated activation of TLR4 signaling stimulates downstream Akt phosphorylation.
Fig. 4.
Heme-induced TLR4 signaling activates AKT to inhibit complex V activity.
(A) Representative western blot and quantification of pAKT/total AKT in platelets treated with heme (2.5 μM) in the presence or absence of BX795 (TBK1 inh; 2 μM), N-[1-[2-(4-Morpholinyl) ethyl]-1H-benzimidazol-2-yl]-3-nitrobenzamide (IRAK1/4 inh; 100 nM) or (5Z)-7-Oxozeaenol (TAK1 inh; 5 nM). (B) Platelet mitochondrial complex V activity (C) the ratio of phosphorylated to total complex V beta subunit, (D) MitoSOX fluorescence and (E) thrombospondin-1 release in heme treated platelets pre-treated with or without ARQ092 (10 μM). Data are represented as Mean ± SEM. ****p < 0.0001, ***p < 0.001, **p < 0.01, ns – not significant. n = 4.
To determine whether heme-induced activation of Akt regulates complex V activity, we treated platelets with heme in the presence and absence of ARQ092, a small molecule that prevents phosphorylation of Akt at S473 [71], and measured complex V activity. While heme treatment inhibited complex V activity, this effect was significantly attenuated when Akt phosphorylation was blocked (Fig. 4B). Immunoprecipitation of the beta subunit of complex V from these platelets showed that heme induced significant phosphorylation of complex V, and this phosphorylation was attenuated when Akt activation was blocked with ARQ092 (Fig. 4C). Collectively, these data demonstrate that heme-induced TLR4 activation stimulates the downstream activation of Akt, which phosphorylates complex V and inhibits its activity. Further, pre-treatment of platelets with ARQ092 significantly attenuated heme-induced platelet mtROS production and TSP-1 release (Fig. 4D and E).
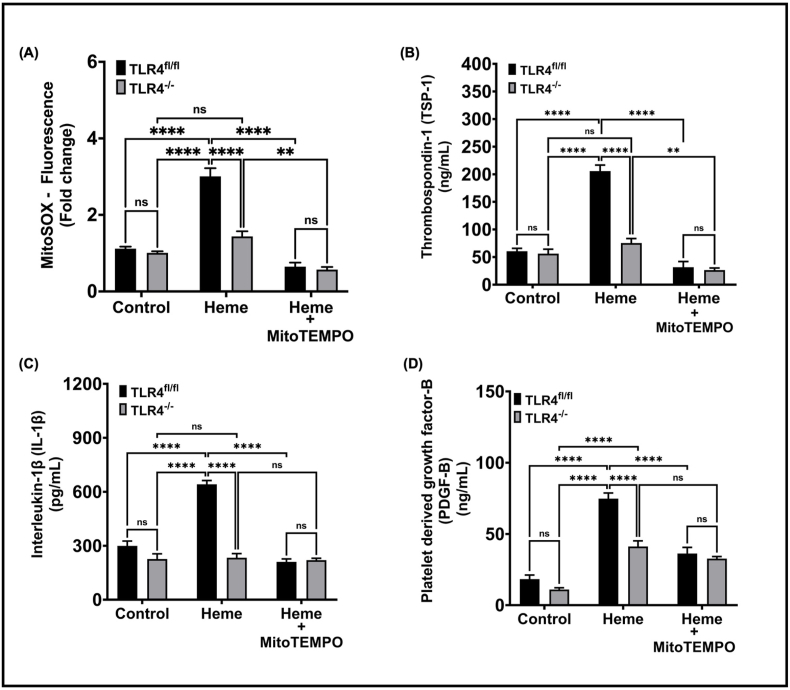
To determine whether the heme-induced pathway elucidated ex vivo was relevant in the physiological milieu in vivo, TLR4−/− mice and corresponding control mice (TLR4 fl/fl) were administered cell free heme (110 mg/kg) to mimic hemolysis and platelet mtROS was measured. While heme induced platelet mtROS production in control mice, this effect was significantly attenuated in TLR4−/− mice (Fig. 5A). Measurement of plasma levels of TSP-1, PDGF-B, and IL-1β showed that heme-induced release of these factors was also significantly attenuated in TLR4−/− mice. Consistent with the dependence of mtROS on TLR4 signaling, treatment with MitoTEMPO attenuated heme-induced granule secretion in control mice but had no effect in TLR4−/− mice (Fig. 5B).
Fig. 5.
Heme-induced platelet mtROS production and granule release is attenuated in TLR4−/− mice. (A) MitoSOX fluorescence in platelets, plasma levels of (B) TSP-1, (C) IL-1β and (D) Platelet derived growth factor-B were measured in TLR4fl/fl (control) and TLR4−/− mice injected with i.v. heme (110 mg/kg) with or without pre-treatment with Mitotempo (300 μM). Data are represented as Mean ± SEM. ****p < 0.0001, **p < 0.001, ns- not significant. n = 5 mice per group in control and heme treated groups; n = 3 to 4 mice in MitoTEMPO treated groups.
5. Discussion
In this study we demonstrate that while heme and hemoglobin both stimulate platelet activation and TSP-1 release, heme is a more potent platelet agonist than Hb. Mechanistically, we show that heme-mediated TSP-1 release relies on the production of platelet mtROS generation. Further, we elucidate the mechanism by which heme stimulates mtROS production, and find that mtROS production is dependent on heme-mediated activation of TLR4 signaling, culminating in the Akt-dependent phosphorylation of complex V and inhibition of its activity (see Fig. 6). This pathway has implications for not only understanding the pathogenesis of hemolysis-induced thrombotic and inflammatory diseases, but also for the development of potential therapeutics for these conditions.
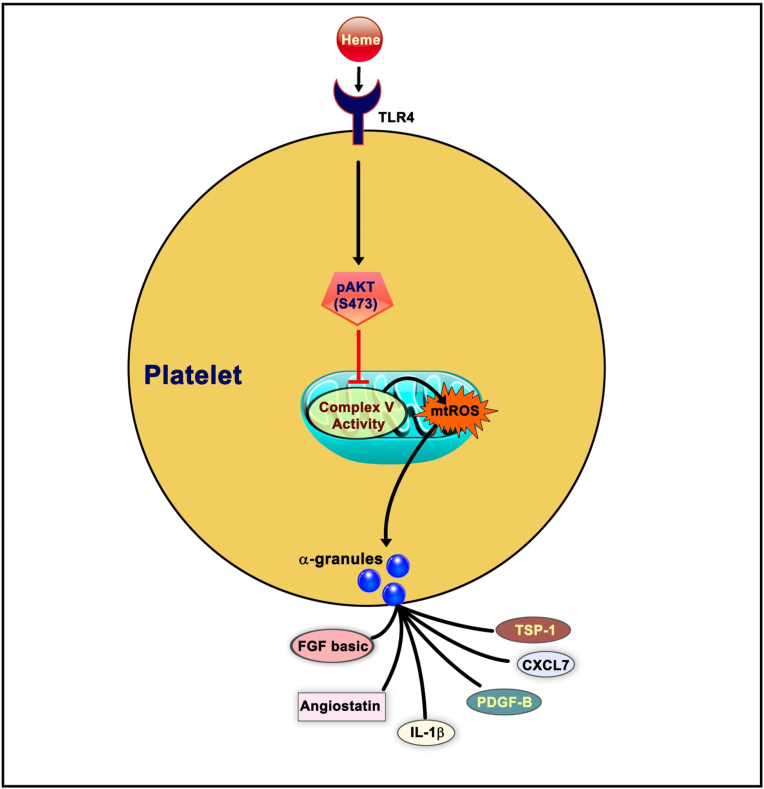
Fig. 6.
Schematic showing the mechanism of heme-induced platelet granule release. Heme activates multiple pathways of TLR4 signaling, which leads to the downstream phosphorylation of Akt at Ser473. Phosphorylated Akt(S473) inhibits mitochondrial complex V activity, resulting in the generation of mtROS which ultimately drive the release of TSP-1, IL1-β, PDGF-B, CXCL7, FGF basic and angiostatin from platelet α-granules.
Prior studies have independently reported that heme [35,36] and Hb [[32], [33], [34]] stimulate platelet activation, however their potency in the context of platelet agonism has previously not been compared. We show here that heme is a more potent mediator of both platelet activation and TSP-1 release than Hb. It is unclear what factors underlie this increased potency of heme. Heme and Hb are biochemically distinct species and heme can selectively bind to multiple receptors and transcription factors [35,68,72,73]. Thus, it is likely that heme is either a stronger activator of TLR4 or stimulates multiple signaling pathways that concomitantly contribute to platelet activation and/or TSP-1 secretion. While prior studies demonstrate heme-induced activation of TLR4 [68,72], it remains unclear whether heme activates TLR4 through traditional ligand binding, and whether Hb acts similarly. Given that heme iron is a potential source of ROS [74], it is possible that iron mediated ROS production outside the platelet plays a role in TLR4 activation. However, consistent with the potential of heme activating concomitant pathways of platelet agonism, Bourne and colleagues showed that heme-mediated activation of C-type-lectin-like-receptor-2 (CLEC-2) contributes to platelet activation [35]. Notably, CLEC-2 activation was observed with higher concentrations of heme (6.25 μM) [35] than used in our study (2.5 μM). Given the biphasic effect of heme observed by both groups, in which maximal platelet agonism is observed at ∼5–6 μM heme, it is possible that while both pathways contribute to heme-mediated platelet agonism, the contribution of these pathways potentially shifts with increasing concentrations of heme. Further study is required to delineate the contribution of each pathway, their potential cross-talk, and whether stimulation of multiple pathways makes heme a more efficient platelet agonist than Hb.
Though heme-mediated platelet activation is well documented [35,36], heme-induced platelet granule release has not been extensively studied. It is estimated that platelet granules store over three hundred molecules, including mitogens, chemokines, and thrombotic regulators, which are released in discreet patterns dependent on the specific stimulus [31,[43], [44], [45]]. The distribution of these molecules is heterogenous and spatially segregated within platelets into distinct zones. This enables platelets to respond to specific agonists to release granule contents in an agonist-specific pattern [75]. The current study begins to define the heme-dependent platelet secretome. While the complete secretomes of all traditional platelet agonists have not been comprehensively defined, Jonnalagadda and colleagues previously compared the platelet secretion profiles stimulated by thrombin, convulxin, PAR1 and PAR4 [45]. Comparison of the heme-induced secretome to that of these agonists suggests considerable overlap, characterized by the release of PDGF-B, IL-1β, and angiostatin by all five agonists. In addition, heme was similar to thrombin and PAR1 in its stimulation of the release of TGFβ, and similar to thrombin and convulxin in its release of FGF basic [45]. Notably, heme did not stimulate CD40L release, which was found in the secretome of the other molecules, while the secretion of TSP-1, kininogen, and CXCL-7 was specific to heme and not the other agonists.
Identification of the heme specific platelet secretome may provide a critical link between hemolysis and pathogenic vascular signaling. For example, platelet derived TSP-1 can promote both thrombosis and vascular pathogenesis through its interaction with CD36 on circulating cells and CD47 in endothelial cells [47,[76], [77], [78]]. In severe sepsis patients, plasma TSP-1 levels have been found to be significantly elevated [63], and recent murine studies of sepsis models show that independent of pathogen load, free heme promotes thrombosis [79]. Thus, it is interesting to speculate that heme-induced platelet TSP-1 release potentially drives pathogenic thrombosis in sepsis. Similarly, in sickle cell disease, platelet TSP-1 has been implicated in the pathogenesis of pulmonary hypertension [80], which is a major cause of morbidity in these patients and is also associated with hemolysis [60]. Notably, plasma TSP-1 levels are significantly elevated in patients with sickle cell disease in steady state [60,81]. In patients who are in vaso-occlusive crisis, which is associated with even higher rates of hemolysis than in steady state, TSP-1 levels associate with lower rates of hemolysis [60]. This is likely consistent with the biphasic curve (Fig. 1) for heme-dependent TSP-1 release that we demonstrate in this study.
While prior studies have demonstrated that heme induces ROS production in the platelet, this study is the first to demonstrate the mitochondrion as a significant source of heme-induced ROS and to define the mechanism by which heme induces mtROS. Our data demonstrate that heme inhibits mitochondrial complex V to induce mtROS production, and that this inhibition of complex V requires heme-mediated activation of platelet TLR4 signaling which ultimately results in Akt activation and association with complex V. Our data are consistent with accumulating reports demonstrating that phosphorylated Akt can translocate and accumulate in the mitochondria, and specifically that Akt can phosphorylate mitochondrial complexes, including the β-subunit of complex-V [69,70]. Our data are not entirely consistent with other reports of Akt-dependent complex V phosphorylation which show that this association leads to activation of the complex V rather than the inhibition shown in this study. However, inhibitory and activating phosphorylation sites have both been identified on complex V β-subunit. Thus, it is possible that separate stimuli propagate Akt-dependent phosphorylation of different sites. Further study identifying the heme-dependent phosphorylation site is required for comparison with other stimuli.
The data presented in this study demonstrate that mtROS regulate heme-dependent granule release. While a growing number of reports have established the association between mtROS production and platelet activation in multiple pathologies [[82], [83], [84]], the specific role of mtROS in regulating granule secretion in response to specific agonists, is less clear. Notably, a recent study demonstrates that agonists such as thrombin receptor activator peptide-6 (TRAP-6) activates platelets in a mtROS independent manner, and while scavenging mtROS does not affect TRAP-6 dependent platelet activation, it significantly attenuates platelet aggregation [83]. These data are consistent with mtROS regulation of platelet granule secretion independent of regulation of platelet activation. Further study is required to dissect mtROS regulation of platelet activation versus granule secretion as well as elucidate the mechanisms by which mtROS cause granule release. It is known that platelet degranulation is dependent on the fusion of the granule with the platelet membrane, a phenomenon that is catalyzed by a variety of SNARE proteins localized on both the platelet membrane as well as on the granule itself [85]. These SNARE proteins are activated by Protein Kinase C (PKC). Notably, oxidation of PKC can result in its activation [86,87]. Thus, while this study did not identify a target of mtROS in the platelet, it is interesting to speculate that the mtROS generated potentially oxidize PKC or directly modify reactive cysteines on the SNARE proteins to activate SNARE proteins and initiate degranulation.
Our in vivo data are consistent with the molecular pathway elucidated in which heme-mediated platelet granule release is dependent on platelet TLR4 and mtROS. However, a significant limitation of these data is the use of the global TLR4 knockout mouse given that other vascular cell types including, macrophages, monocytes, and endothelial cells express TLR4 [88,89]. Further, while platelets are the major source of circulating TSP-1 [80,90], these other cell types can also secrete both TSP-1 and IL-1β [91,92]. Similarly, our results showing that MitoTEMPO attenuates TSP-1 and IL-1β levels in the plasma combined with our prior study showing the mtROS scavenger attenuates platelet activation and thrombosis in murine models of hemolysis [40] are consistent with a role in vivo for platelet mtROS in granule release. However, the study is limited by the lack of specificity of MitoTEMPO for the platelet. Despite these limitations and the need for further testing in platelet specific models, our data suggest that mtROS scavenging (or TLR4 inhibition to prevent mtROS production) is a potentially viable option in preventing heme-induced platelet dysfunction.
In conclusion, this study demonstrates the mechanism by which extracellular heme signals through platelet TLR4 to induce platelet mtROS production. Further, we demonstrate that heme-induced mtROS stimulates platelet granule secretion. The data begin to define the heme-induced platelet secretome and its regulation by mtROS. Overall, these studies advance the understanding of the mechanisms that link hemolysis to platelet dysfunction. While further study of the secretome is required to determine whether heme-induced secreted products are responsible for hemolysis-associated inflammation and vasculopathy, the data herein demonstrate a central role for the mitochondrion in heme-dependent platelet dysfunction and suggest this organelle as a potential therapeutic target in hemolytic conditions.
Authorship contributions
GA, DND, MR, YW, LK, BZ and SS designed the study and performed the research. LK and BZ performed murine experiments and collected the data.GA, DND, MR and SS analyzed the data. GA and SS wrote the manuscript.
Declaration of competing interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by NIH grants HL133003-01A1 (to SS), R01 GM113816-04 (to BZ and SS), 1 R01 HL130268-03 (to SS and BZ) and The Hemophilia Center of Western Pennsylvania (to SS).
References
- 1.Piel F.B., Steinberg M.H., Rees D.C. Sickle cell disease. N. Engl. J. Med. 2017;376(16):1561–1573. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- 2.Hill A., DeZern A.E., Kinoshita T., Brodsky R.A. Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers. 2017;3:17028. doi: 10.1038/nrdp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fibach E., Rachmilewitz E.A. Pathophysiology and treatment of patients with beta-thalassemia - an update. F1000Res. 2017;6:2156. doi: 10.12688/f1000research.12688.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Effenberger-Neidnicht K., Hartmann M. Mechanisms of hemolysis during sepsis. Inflammation. 2018;41(5):1569–1581. doi: 10.1007/s10753-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 5.Burwick R.M., Rincon M., Beeraka S.S., Gupta M., Feinberg B.B. Evaluation of hemolysis as a severe feature of preeclampsia. Hypertension. 2018;72(2):460–465. doi: 10.1161/HYPERTENSIONAHA.118.11211. [DOI] [PubMed] [Google Scholar]
- 6.Ciantar E., Walker J.J. Pre-eclampsia, severe pre-eclampsia and hemolysis, elevated liver enzymes and low platelets syndrome: what is new? Womens Health (Lond) 2011;7(5):555–569. doi: 10.2217/whe.11.57. [DOI] [PubMed] [Google Scholar]
- 7.Orf K., Cunnington A.J. Infection-related hemolysis and susceptibility to Gram-negative bacterial co-infection. Front. Microbiol. 2015;6:666. doi: 10.3389/fmicb.2015.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narurkar R., Mamorska-Dyga A., Nelson J.C., Liu D. Autoimmune hemolytic anemia associated with babesiosis. Biomark Res. 2017;5:14. doi: 10.1186/s40364-017-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilmann C., Geisen U., Benk C., Berchtold-Herz M., Trummer G., Schlensak C., Zieger B., Beyersdorf F. Haemolysis in patients with ventricular assist devices: major differences between systems. Eur. J. Cardio. Thorac. Surg. 2009;36(3):580–584. doi: 10.1016/j.ejcts.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Vercaemst L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: a review in search of a treatment algorithm. J. Extra Corpor. Technol. 2008;40(4):257–267. [PMC free article] [PubMed] [Google Scholar]
- 11.Ataga K.I. Hypercoagulability and thrombotic complications in hemolytic anemias. Haematologica. 2009;94(11):1481–1484. doi: 10.3324/haematol.2009.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappellini M.D. Hematology Am Soc Hematol Educ Program; 2007. Coagulation in the Pathophysiology of Hemolytic Anemias; pp. 74–78. [DOI] [PubMed] [Google Scholar]
- 13.Rother R.P., Bell L., Hillmen P., Gladwin M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. J. Am. Med. Assoc. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 14.Minneci P.C., Deans K.J., Zhi H., Yuen P.S., Star R.A., Banks S.M., Schechter A.N., Natanson C., Gladwin M.T., Solomon S.B. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris C.R. Hematology Am Soc Hematol Educ Program; 2008. Mechanisms of Vasculopathy in Sickle Cell Disease and Thalassemia; pp. 177–185. [DOI] [PubMed] [Google Scholar]
- 16.Kato G.J., Hebbel R.P., Steinberg M.H., Gladwin M.T. Vasculopathy in sickle cell disease: biology, pathophysiology, genetics, translational medicine, and new research directions. Am. J. Hematol. 2009;84(9):618–625. doi: 10.1002/ajh.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato G.J., Steinberg M.H., Gladwin M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Invest. 2017;127(3):750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed O., Jakobleff W.A., Forest S.J., Chinnadurai T., Mellas N., Rangasamy S., Xia Y., Madan S., Acharya P., Algodi M., Patel S.R., Shin J., Vukelic S., Sims D.B., Reyes Gil M., Billett H.H., Kizer J.R., Goldstein D.J., Jorde U.P. Hemolysis and nonhemorrhagic stroke during venoarterial extracorporeal membrane oxygenation. Ann. Thorac. Surg. 2019;108(3):756–763. doi: 10.1016/j.athoracsur.2019.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubert M., Elion J., Tolo A., Diallo D.A., Diop S., Diagne I., Sanogo I., Belinga S., Guifo O., Wamba G., Ngo Sack F., Boidy K., Kamara I., Traore Y., Diakite C.O., Gbonon V., Faye B.F., Seck M., Deme Ly I., Chelo D., N'Guetta R., Diop I.B., Gaye B., Jouven X., Ranque B. Degree of anemia, indirect markers of hemolysis, and vascular complications of sickle cell disease in Africa. Blood. 2017;130(20):2215–2223. doi: 10.1182/blood-2016-12-755777. [DOI] [PubMed] [Google Scholar]
- 20.Wahl S., Vichinsky E. Pulmonary hypertension in hemolytic anemias. F1000 Med. Rep. 2010;2 doi: 10.3410/M2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado R.F., Gladwin M.T. Pulmonary hypertension in hemolytic disorders: pulmonary vascular disease: the global perspective. Chest. 2010;137(6 Suppl):30S–38S. doi: 10.1378/chest.09-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rafikova O., Williams E.R., McBride M.L., Zemskova M., Srivastava A., Nair V., Desai A.A., Langlais P.R., Zemskov E., Simon M., Mandarino L.J., Rafikov R. Hemolysis-induced lung vascular leakage contributes to the development of pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 2018;59(3):334–345. doi: 10.1165/rcmb.2017-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haw A., Palevsky H.I. Pulmonary hypertension in chronic hemolytic anemias: pathophysiology and treatment. Respir. Med. 2018;137:191–200. doi: 10.1016/j.rmed.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Morris C.R., Kato G.J., Poljakovic M., Wang X., Blackwelder W.C., Sachdev V., Hazen S.L., Vichinsky E.P., Morris S.M., Jr., Gladwin M.T. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. J. Am. Med. Assoc. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: an update. Eur. Heart J. 2017;38(11):785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurbel P.A., Jeong Y.H., Navarese E.P., Tantry U.S. Platelet-mediated thrombosis: from bench to bedside. Circ. Res. 2016;118(9):1380–1391. doi: 10.1161/CIRCRESAHA.115.307016. [DOI] [PubMed] [Google Scholar]
- 27.Koupenova M., Clancy L., Corkrey H.A., Freedman J.E. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ. Res. 2018;122(2):337–351. doi: 10.1161/CIRCRESAHA.117.310795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rolfes V., Ribeiro L.S., Hawwari I., Bottcher L., Rosero N., Maasewerd S., Santos M.L.S., Prochnicki T., Silva C.M.S., Wanderley C.W.S., Rothe M., Schmidt S.V., Stunden H.J., Bertheloot D., Rivas M.N., Fontes C.J., Carvalho L.H., Cunha F.Q., Latz E., Arditi M., Franklin B.S. Platelets fuel the inflammasome activation of innate immune cells. Cell Rep. 2020;31(6):107615. doi: 10.1016/j.celrep.2020.107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker R.C., Sexton T., Smyth S.S. Translational implications of platelets as vascular first responders. Circ. Res. 2018;122(3):506–522. doi: 10.1161/CIRCRESAHA.117.310939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi G., Morrell C.N. Platelets as initiators and mediators of inflammation at the vessel wall. Thromb. Res. 2011;127(5):387–390. doi: 10.1016/j.thromres.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golebiewska E.M., Poole A.W. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardenes N., Corey C., Geary L., Jain S., Zharikov S., Barge S., Novelli E.M., Shiva S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123(18):2864–2872. doi: 10.1182/blood-2013-09-529420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Annarapu G.K., Singhal R., Gupta A., Chawla S., Batra H., Seth T., Guchhait P. HbS binding to GP1balpha activates platelets in sickle cell disease. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singhal R., Annarapu G.K., Pandey A., Chawla S., Ojha A., Gupta A., Cruz M.A., Seth T., Guchhait P. Hemoglobin interaction with GP1balpha induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis. Haematologica. 2015;100(12):1526–1533. doi: 10.3324/haematol.2015.132183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourne J.H., Colicchia M., Di Y., Martin E., Slater A., Roumenina L.T., Dimitrov J.D., Watson S.P., Rayes J. Heme induces human and mouse platelet activation through C-type-lectin-like receptor-2. Haematologica. 2020 doi: 10.3324/haematol.2020.246488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NaveenKumar S.K., SharathBabu B.N., Hemshekhar M., Kemparaju K., Girish K.S., Mugesh G. The role of reactive oxygen species and ferroptosis in heme-mediated activation of human platelets. ACS Chem. Biol. 2018;13(8):1996–2002. doi: 10.1021/acschembio.8b00458. [DOI] [PubMed] [Google Scholar]
- 37.Conran N., De Paula E.V. Thromboinflammatory mechanisms in sickle cell disease - challenging the hemostatic balance. Haematologica. 2020;105(10):2380–2390. doi: 10.3324/haematol.2019.239343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belcher J.D., Nath K.A., Vercellotti G.M. Vasculotoxic and proinflammatory effects of plasma heme: cell signaling and cytoprotective responses. ISRN Oxidative Med. 2013. 2013 doi: 10.1155/2013/831596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vats R., Brzoska T., Bennewitz M.F., Jimenez M.A., Pradhan-Sundd T., Tutuncuoglu E., Jonassaint J., Gutierrez E., Watkins S.C., Shiva S., Scott M.J., Morelli A.E., Neal M.D., Kato G.J., Gladwin M.T., Sundd P. Platelet extracellular vesicles drive inflammasome-IL-1beta-dependent lung injury in sickle cell disease. Am. J. Respir. Crit. Care Med. 2020;201(1):33–46. doi: 10.1164/rccm.201807-1370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Annarapu G., Nolfi-Donegan D., Reynolds M., Wang Y., Shiva S. Mitochondrial reactive oxygen species scavenging attenuates thrombus formation in a murine model of sickle cell disease. J. Thromb. Haemost. 2021 doi: 10.1111/jth.15298. [DOI] [PubMed] [Google Scholar]
- 41.Maynard D.M., Heijnen H.F., Horne M.K., White J.G., Gahl W.A. Proteomic analysis of platelet alpha-granules using mass spectrometry. J. Thromb. Haemost. 2007;5(9):1945–1955. doi: 10.1111/j.1538-7836.2007.02690.x. [DOI] [PubMed] [Google Scholar]
- 42.Whiteheart S.W. Platelet granules: surprise packages. Blood. 2011;118(5):1190–1191. doi: 10.1182/blood-2011-06-359836. [DOI] [PubMed] [Google Scholar]
- 43.Chatterjee M., Huang Z., Zhang W., Jiang L., Hultenby K., Zhu L., Hu H., Nilsson G.P., Li N. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood. 2011;117(14):3907–3911. doi: 10.1182/blood-2010-12-327007. [DOI] [PubMed] [Google Scholar]
- 44.Golebiewska E.M., Poole A.W. Secrets of platelet exocytosis - what do we really know about platelet secretion mechanisms? Br. J. Haematol. 2013 doi: 10.1111/bjh.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonnalagadda D., Izu L.T., Whiteheart S.W. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120(26):5209–5216. doi: 10.1182/blood-2012-07-445080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prakash P., Kulkarni P.P., Chauhan A.K. Thrombospondin 1 requires von Willebrand factor to modulate arterial thrombosis in mice. Blood. 2015;125(2):399–406. doi: 10.1182/blood-2014-06-581942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuijpers M.J., de Witt S., Nergiz-Unal R., van Kruchten R., Korporaal S.J., Verhamme P., Febbraio M., Tjwa M., Voshol P.J., Hoylaerts M.F., Cosemans J.M., Heemskerk J.W. Supporting roles of platelet thrombospondin-1 and CD36 in thrombus formation on collagen. Arterioscler. Thromb. Vasc. Biol. 2014;34(6):1187–1192. doi: 10.1161/ATVBAHA.113.302917. [DOI] [PubMed] [Google Scholar]
- 48.Bonnefoy A., Daenens K., Feys H.B., De Vos R., Vandervoort P., Vermylen J., Lawler J., Hoylaerts M.F. Thrombospondin-1 controls vascular platelet recruitment and thrombus adherence in mice by protecting (sub)endothelial VWF from cleavage by ADAMTS13. Blood. 2006;107(3):955–964. doi: 10.1182/blood-2004-12-4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Dee Z., Pidcock K., Gutierrez L.S. Thrombospondin-1: multiple paths to inflammation. Mediat. Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stein E.V., Miller T.W., Ivins-O'Keefe K., Kaur S., Roberts D.D. Secreted thrombospondin-1 regulates macrophage interleukin-1beta production and activation through CD47. Sci. Rep. 2016;6:19684. doi: 10.1038/srep19684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isenberg J.S., Martin-Manso G., Maxhimer J.B., Roberts D.D. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat. Rev. Cancer. 2009;9(3):182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leung L.L. Role of thrombospondin in platelet aggregation. J. Clin. Invest. 1984;74(5):1764–1772. doi: 10.1172/JCI111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonnefoy A., Hantgan R., Legrand C., Frojmovic M.M. A model of platelet aggregation involving multiple interactions of thrombospondin-1, fibrinogen, and GPIIbIIIa receptor. J. Biol. Chem. 2001;276(8):5605–5612. doi: 10.1074/jbc.M010091200. [DOI] [PubMed] [Google Scholar]
- 54.Xing T., Wang Y., Ding W.J., Li Y.L., Hu X.D., Wang C., Ding A., Shen J.L. Thrombospondin-1 production regulates the inflammatory cytokine secretion in THP-1 cells through NF-kappaB signaling pathway. Inflammation. 2017;40(5):1606–1621. doi: 10.1007/s10753-017-0601-x. [DOI] [PubMed] [Google Scholar]
- 55.Ahamed J., Janczak C.A., Wittkowski K.M., Coller B.S. In vitro and in vivo evidence that thrombospondin-1 (TSP-1) contributes to stirring- and shear-dependent activation of platelet-derived TGF-beta1. PLoS One. 2009;4(8) doi: 10.1371/journal.pone.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen Y., Leask A., Abraham D.J., Kennedy L., Shi-Wen X., Denton C.P., Black C.M., Verjee L.S., Eastwood M. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4(1):9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Z., Morgan S., Ren J., Wang Q., Annis D.S., Mosher D.F., Zhang J., Sorenson C.M., Sheibani N., Liu B. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ. Res. 2015;117(2):129–141. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Isenberg J.S., Frazier W.A., Roberts D.D. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell. Mol. Life Sci. 2008;65(5):728–742. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isenberg J.S., Wink D.A., Roberts D.D. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc. Res. 2006;71(4):785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 60.Novelli E.M., Kato G.J., Ragni M.V., Zhang Y., Hildesheim M.E., Nouraie M., Barge S., Meyer M.P., Hassett A.C., Gordeuk V.R., Gladwin M.T., Isenberg J.S. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am. J. Hematol. 2012;87(3):326–330. doi: 10.1002/ajh.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adegoke S.A., Smith O.S., Adeniyi A.T., Adekile A.D. Thrombospondin-1 and vitamin D in children with sickle cell anemia. J. Pediatr. Hematol. Oncol. 2019;41(8):e525–e529. doi: 10.1097/MPH.0000000000001368. [DOI] [PubMed] [Google Scholar]
- 62.Gawaz M., Dickfeld T., Bogner C., Fateh-Moghadam S., Neumann F.J. Platelet function in septic multiple organ dysfunction syndrome. Intensive Care Med. 1997;23(4):379–385. doi: 10.1007/s001340050344. [DOI] [PubMed] [Google Scholar]
- 63.van der Wekken R.J., Kemperman H., Roest M., de Lange D.W. Baseline thrombospondin-1 concentrations are not associated with mortality in septic patients: a single-center cohort study on the intensive care unit. Intensive Care Med. Exp. 2017;5(1):7. doi: 10.1186/s40635-017-0120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novelli E.M., Little-Ihrig L., Knupp H.E., Rogers N.M., Yao M., Baust J.J., Meijles D., St Croix C.M., Ross M.A., Pagano P.J., DeVallance E.R., Miles G., Potoka K.P., Isenberg J.S., Gladwin M.T. Vascular TSP1-CD47 signaling promotes sickle cell-associated arterial vasculopathy and pulmonary hypertension in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316(6):L1150–L1164. doi: 10.1152/ajplung.00302.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McMaken S., Exline M.C., Mehta P., Piper M., Wang Y., Fischer S.N., Newland C.A., Schrader C.A., Balser S.R., Sarkar A., Baran C.P., Marsh C.B., Cook C.H., Phillips G.S., Ali N.A. Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS One. 2011;6(5) doi: 10.1371/journal.pone.0019654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Belcher J.D., Chen C., Nguyen J., Milbauer L., Abdulla F., Alayash A.I., Smith A., Nath K.A., Hebbel R.P., Vercellotti G.M. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Figueiredo R.T., Fernandez P.L., Mourao-Sa D.S., Porto B.N., Dutra F.F., Alves L.S., Oliveira M.F., Oliveira P.L., Graca-Souza A.V., Bozza M.T. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007;282(28):20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 69.Bijur G.N., Jope R.S. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003;87(6):1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li C., Li Y., He L., Agarwal A.R., Zeng N., Cadenas E., Stiles B.L. PI3K/AKT signaling regulates bioenergetics in immortalized hepatocytes. Free Radic. Biol. Med. 2013;60:29–40. doi: 10.1016/j.freeradbiomed.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Y., Savage R.E., Eathiraj S., Meade J., Wick M.J., Hall T., Abbadessa G., Schwartz B. Targeting AKT1-E17K and the PI3K/AKT pathway with an allosteric AKT inhibitor, ARQ 092. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janciauskiene S., Vijayan V., Immenschuh S. TLR4 signaling by heme and the role of heme-binding blood proteins. Front. Immunol. 2020;11:1964. doi: 10.3389/fimmu.2020.01964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mense S.M., Zhang L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 2006;16(8):681–692. doi: 10.1038/sj.cr.7310086. [DOI] [PubMed] [Google Scholar]
- 74.Chiabrando D., Vinchi F., Fiorito V., Mercurio S., Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front. Pharmacol. 2014;5:61. doi: 10.3389/fphar.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heijnen H., van der Sluijs P. Platelet secretory behaviour: as diverse as the granules ... or not? J. Thromb. Haemost. 2015;13(12):2141–2151. doi: 10.1111/jth.13147. [DOI] [PubMed] [Google Scholar]
- 76.Nergiz-Unal R., Lamers M.M., Van Kruchten R., Luiken J.J., Cosemans J.M., Glatz J.F., Kuijpers M.J., Heemskerk J.W. Signaling role of CD36 in platelet activation and thrombus formation on immobilized thrombospondin or oxidized low-density lipoprotein. J. Thromb. Haemost. 2011;9(9):1835–1846. doi: 10.1111/j.1538-7836.2011.04416.x. [DOI] [PubMed] [Google Scholar]
- 77.Rogers N.M., Sharifi-Sanjani M., Csanyi G., Pagano P.J., Isenberg J.S. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kale A., Rogers N.M., Ghimire K. Thrombospondin-1 CD47 signalling: from mechanisms to medicine. Int. J. Mol. Sci. 2021;22(8) doi: 10.3390/ijms22084062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Larsen R., Gozzelino R., Jeney V., Tokaji L., Bozza F.A., Japiassu A.M., Bonaparte D., Cavalcante M.M., Chora A., Ferreira A., Marguti I., Cardoso S., Sepulveda N., Smith A., Soares M.P. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010;2(51):51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 80.Kaiser R., Frantz C., Bals R., Wilkens H. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir. Res. 2016;17(1):96. doi: 10.1186/s12931-016-0412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Browne P.V., Mosher D.F., Steinberg M.H., Hebbel R.P. Disturbance of plasma and platelet thrombospondin levels in sickle cell disease. Am. J. Hematol. 1996;51(4):296–301. doi: 10.1002/(SICI)1096-8652(199604)51:4<296::AID-AJH8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 82.Masselli E., Pozzi G., Vaccarezza M., Mirandola P., Galli D., Vitale M., Carubbi C., Gobbi G. ROS in platelet biology: functional aspects and methodological insights. Int. J. Mol. Sci. 2020;21(14) doi: 10.3390/ijms21144866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mendez D., Arauna D., Fuentes F., Araya-Maturana R., Palomo I., Alarcon M., Sebastian D., Zorzano A., Fuentes E. Mitoquinone (MitoQ) inhibits platelet activation steps by reducing ROS levels. Int. J. Mol. Sci. 2020;21(17) doi: 10.3390/ijms21176192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Melchinger H., Jain K., Tyagi T., Hwa J. Role of platelet mitochondria: life in a nucleus-free zone. Front Cardiovasc Med. 2019;6:153. doi: 10.3389/fcvm.2019.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joshi S., Whiteheart S.W. The nuts and bolts of the platelet release reaction. Platelets. 2017;28(2):129–137. doi: 10.1080/09537104.2016.1240768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cosentino-Gomes D., Rocco-Machado N., Meyer-Fernandes J.R. Cell signaling through protein kinase C oxidation and activation. Int. J. Mol. Sci. 2012;13(9):10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J., Wang X., Vikash V., Ye Q., Wu D., Liu Y., Dong W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell Longev 2016. 2016:4350965. doi: 10.1155/2016/4350965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ward J.R., Francis S.E., Marsden L., Suddason T., Lord G.M., Dower S.K., Crossman D.C., Sabroe I. A central role for monocytes in Toll-like receptor-mediated activation of the vasculature. Immunology. 2009;128(1):58–68. doi: 10.1111/j.1365-2567.2009.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu Z., Li Y., Jin J., Zhang X., Lopes-Virella M.F., Huang Y. Toll-like receptor 4 activation in microvascular endothelial cells triggers a robust inflammatory response and cross talk with mononuclear cells via interleukin-6. Arterioscler. Thromb. Vasc. Biol. 2012;32(7):1696–1706. doi: 10.1161/ATVBAHA.112.251181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Starlinger P., Moll H.P., Assinger A., Nemeth C., Hoetzenecker K., Gruenberger B., Gruenberger T., Kuehrer I., Schoppmann S.F., Gnant M., Brostjan C. Thrombospondin-1: a unique marker to identify in vitro platelet activation when monitoring in vivo processes. J. Thromb. Haemost. 2010;8(8):1809–1819. doi: 10.1111/j.1538-7836.2010.03908.x. [DOI] [PubMed] [Google Scholar]
- 91.Jaffe E.A., Ruggiero J.T., Falcone D.J. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65(1):79–84. [PubMed] [Google Scholar]
- 92.Kobayashi S., Yamamoto T. The molecular biologic study of the expression of thrombospondin in vascular smooth muscle cells and mesangial cells. J. Diabet. Complicat. 1991;5(2-3):121–123. doi: 10.1016/0891-6632(91)90040-v. [DOI] [PubMed] [Google Scholar]