Abstract
Superoxide and vascular smooth muscle cells (VSMCs) migration and proliferation play crucial roles in the vascular remodeling. Vascular remodeling contributes to the development and complications of hypertension. Rho family GTPase 3 (RND3 or RhoE), an atypical small Rho-GTPase, is known to be involved in cancer development and metastasis. However, the roles of RND3 in superoxide production and cardiovascular remodeling are unknown. Here, we uncovered the critical roles of RND3 in attenuating superoxide production, VSMCs migration and proliferation, and vascular remodeling in hypertension and its underline mechanisms. VSMCs were isolated and prepared from thoracic aorta of Male Wistar-Kyoto rat (WKY) and spontaneously hypertensive rat (SHR). RND3 mRNA and protein expressions in arteries and VSMCs were down-regulated in SHR. RND3 overexpression in VSMCs reduced NAD(P)H oxidase (NOX) activity, NOX1 and NOX2 expressions, mitochondria superoxide generation, and H2O2 production in SHR. Moreover, the RND3 overexpression inhibited VSMCs migration and proliferation in SHR, which were similar to the effects of NOX1 inhibitor ML171 plus NOX2 inhibitor GSK2795039. Rho-associated kinase 1 (ROCK1) and RhoA expressions and myosin phosphatase targeting protein 1 (MYPT1) phosphorylation in VSMCs were increased in SHR, which were prevented by RND3 overexpression. ROCK1 overexpression promoted NOX1 and NOX2 expressions, superoxide and H2O2 production, VSMCs migration and proliferation in both WKY and SHR, which were attenuated by RND3 overexpression. Adenoviral-mediated RND3 overexpression in SHR attenuated hypertension, vascular remodeling and oxidative stress. These results indicate that RND3 attenuates VSMCs migration and proliferation, hypertension and vascular remodeling in SHR via inhibiting ROCK1-NOX1/2 and mitochondria superoxide signaling.
Keywords: RND3, Superoxide, Vascular remodeling, Hypertension, Migration, Proliferation, Vascular smooth muscle cells
Graphical abstract
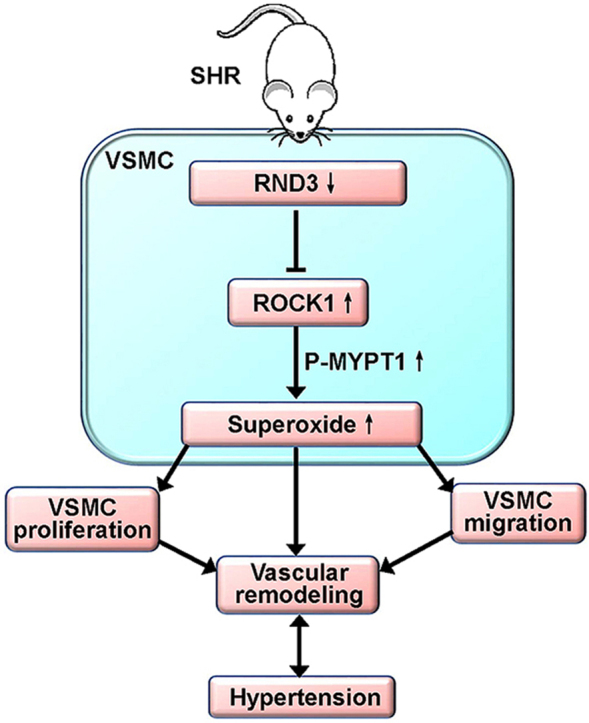
Highlights
-
•
RND3 inhibits superoxide production, VSMCs migration and proliferation in SHR.
-
•
The effects of RND3 are mediated by the ROCK1-NOX1/2 and mitochondria superoxide signaling.
-
•
The RND3 expressions in VSMCs and arteries are down-regulated in SHR.
-
•
RND3 overexpression attenuates oxidative stress, vascular remodeling and hypertension in SHR.
-
•
Upregulation of RND3 may be a therapeutic strategy to reduce vascular remodeling and attenuate hypertension.
Abbreviations
- CCK8
Cell Counting Kit-8
- DHE
dihydroethidium
- EdU
5-ethynyl-2’-deoxyuridine
- MA
mesentery artery
- MYPT1
myosin phosphatase targeting protein 1
- NOX
NAD(P)H oxidase
- OE
overexpression
- PCNA
proliferating cell nuclear antigen
- RhoA
Ras homolog gene family member A
- RND3
Rho family GTPase 3, being also known as RhoE
- ROCK1
Rho-associated kinase 1
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rats
- VSMCs
vascular smooth muscle cells
- WKY
Wistar-Kyoto rats
1. Introduction
Vascular remodeling contributes to the end-organ damage, impacting the development and the complications of hypertension [1]. Rationale for the regression of vascular remodeling may serve as an important therapeutic strategy for hypertension [2]. Vascular smooth muscle cells (VSMCs) are the main component of the arterial cell population. The VSMCs migration and proliferation are the core processes in vascular remodeling, which are influenced by complicated signaling networks [3]. Oxidative stress is one of crucial factors in promoting VSMCs migration and proliferation, and vascular remodeling in hypertension [4].
Misbalance between the oxidation and the antioxidant mechanism leads to oxidative stress that is involved in the vascular remodeling associated with hypertension [5]. Superoxide serves as the messengers to mediate cellular responses via redox reactions in many physiological and pathological processes [6]. However, excessive superoxide generation which exceeds the normal antioxidant mechanism leads to cell damage and inflammation [7]. The oxidative stress promotes VSMCs proliferation, migration and apoptosis, which contributes to vascular remodeling in hypertension [[8], [9], [10]]. Redox-related biomarkers may have a potential prognostic value for cardiovascular diseases [11,12]. The major source of superoxide in the vascular system is the NADPH oxidase (NOX) family composed of seven members [13]. The NOX1 and NOX2 of the NOX isoforms are believed to have the greatest implication in the vascular diseases including hypertension. NOX2 upregulation causes oxidative stress, and NOX2 inhibition reduces reactive oxygen species (ROS) release in peripheral artery diseases [10]. Accumulated evidence indicates that oxidative stress greatly contributes to hypertension and vascular remodeling, and the inhibition of oxidative stress can be considered as a therapeutic strategy [14].
RND3 is also known as RhoE, and belongs to the Rnd subgroup of Rho family of small GTP-binding proteins. It is an atypical GTPase that is able to bind the nucleotide but does not exert the activity seen in other GTPases [15]. RND3 plays roles in the cancer development and metastasis, and shows a diverse expression pattern, upregulation or downregulation, depending on the types of cancer. RND3 behaves as a tumor suppressor or a tumor promoter, probably linked to the status or type of cancer cells [16]. It has been found that RND3 downregulation correlates with cardiac loss of function as in heart failure patients, and mice with RND3 haploinsufficiency are predisposed to hemodynamic stress [17]. However, it is unknown whether RND3 is involved in oxidative stress, VSMCs migration and proliferation, and vascular remodeling in hypertension. This study is designed to investigate the roles of RND3 in redox signaling and vascular remodeling in hypertension.
2. Materials and methods
2.1. Animals
Male Wistar-Kyoto rats (WKY) and spontaneously hypertensive rats (SHR) were purchased from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China). The experiments were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University, and conformed to the Guide for the Care and Use of Laboratory Animal (NIH publication, 8th edition, 2011). The systolic blood pressure (SBP) of SHR used in the present study was higher than 150 mm Hg in this study. Rats were kept in a temperature- and humidity-controlled room with light/dark cycle of 12 h/12 h and free access to standard rodent chow and tap water. The rat was euthanized with intravenous injection of pentobarbital sodium (150 mg/kg).
2.2. Culture of VSMCs
VSMCs were isolated and prepared from the aorta of WKY and SHR as we described previously [18]. Briefly, the thoracic aorta of rat was isolated, and the adventitia including perivascular adipose tissue was removed. The aorta was cut open and the intima was stripped off the media. Then, the media was treated with PBS containing 0.4% collagenase for 30 min. After centrifugation for 10 min, the isolated cells were resuspended in DMEM with 10% fetal bovine serum, penicillin (100 IU/mL) and streptomycin (10 mg/mL) at 37 °C in humidified atmosphere containing 5% CO2. The primary VSMCs were confirmed by negative PECAM-1 (a marker of endothelial cells), negative vimentin (a marker of fibroblasts) and positive α-smooth muscle actin (α-SMA, a marker of VSMCs) [18]. Primary VSMCs from the 2nd to the 5th passages were used in this study. Cell viability was detected using the Cell Counting Kit-8 (CCK8) assay (Dojindo Laboratory, Japan) according to the manufacturer's instructions [19].
2.3. RND3 and ROCK1 overexpression in VSMCs
Both RND3 overexpression plasmid and ROCK1 overexpression plasmid were constructed by RiboBio Co.,LTD. (Guangzhou, Guangdong, China). VSMCs were transfected with pcDNA3.1 plasmid (NC), RND3 overexpression plasmid (50 nmol/L) or ROCK1 overexpression plasmid (50 nmol/L) using lipofectamine 3000 for 24 h.
2.4. Adenoviral-mediated RND3 overexpression in rats
Recombinant adenovirus expressing RND3 or control adenovirus were commercially constructed by Shanghai Genechem Co. Ltd. (Shanghai, China). The WKY and SHR aged at 9 weeks were subjected to intravenous injection of PBS, control adenovirus or adenovirus expressing RND3 (1 × 107 plaque-forming units) via tail vein, respectively. Blood pressure and heart rate (HR) were measured every week, and final experiments were performed 4 weeks after the intravenous injection.
2.5. Evaluation of VSMCs migration
The VSMCs migration was evaluated with both Wound healing assay and Boyden chamber assay as we previously reported [20]. For Wound healing assay, the near-confluent VSMCs in 6-well plates were scraped with a standard 1 mL pipette tip in the central region. Cellular debris was washed with PBS, and fresh medium was added. The wound healing was photographed and calculated.at 0 and 24 h with inverted microscope (Axio Vert. A1, Zeiss, Oberkochen, Germany). For Boyden chamber assay, the VSMCs were seeded onto the upper surface of the chamber with serum-free media, and 10% FBS medium was added to the lower chamber. After 24 h of incubation, the VSMCs were seeded onto the upper surface of the membrane in the 24-well Transwell cell culture chambers with 8-μm sized pores (Merck kGaA, Darmstadt, Germany) and cultured in the medium for 24 h. The VSMCs on the upper surface of the membrane were scraped off with cotton swabs. The migrated VSMCs in the lower surface of the filter were stained with 1% crystal violet. The average number of stained cells from five randomly chosen fields was counted.
2.6. Evaluation of VSMCs proliferation
VSMCs proliferation was evaluated with proliferating cell nuclear antigen (PCNA) protein expression and 5-ethynyl-2’-deoxyuridine (EdU) incorporation assay as we previously reported [21]. The PCNA protein expression was examined with Western blot. The EdU incorporation assay was performed with Cell-Light™ EdU Apollo®567 In Vitro Imaging Kit (Guangzhou RiboBio, Guangzhou, China), which examines the DNA synthesis. The number of EdU stained cells was normalized by the total number of cells.
2.7. Immunohistochemistry
Immunohistochemistry was utilized to detect RND3 expression in aorta, and PCNA expression in aorta and mesenteric artery of WKY and SHR. The primary anti-RND3 antibody (1:100) and primary anti-PCNA antibody (1:100) were obtained from Protein Tech Group Inc (Chicago, IL, USA). Horseradish peroxidase-conjugated goat anti-rabbit antibody was acquired from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Images were taken with a light microscope (BX-51, Olympus, Tokyo, Japan) and quantitative analysis was performed with ImageJ software (v1.80; NIH, Bethesda, Maryland).
2.8. DHE fluorescence staining in VSMCs and arteries
Dihydroethidium (DHE) fluorescence staining was used to evaluate intracellular ROS production in VSMCs and arteries. For VSMCs, the cells about 3 × 105 cells/mL were incubated in six-well plates with PBS containing 10 μM of DHE in a dark and humidified container at 37 °C for 30 min, and then washed three times with PBS. The fluorescence was examined under excitation at 518 nm and emission at 605 nm with a fluorescence microscopy (DP70, Olympus Optical, Tokyo, Japan) [22].
2.9. Determination of NOX activity
NOX activity was examined with lucigenin-derived chemiluminescence method [[23], [24], [25]]. Simply, the photon emission was triggered by both dark-adapted lucigenin (5 μM) and NAD(P)H (100 μM). The values were obtained by averaging ten measurements in 10 min with a luminometer (Model 20/20n, Turner, CA, USA). Background chemiluminescence was measured in the buffer containing lucigenin (5 μM). The NOX activity was expressed as mean light unit (MLU)/min/mg protein.
2.10. Evaluation of superoxide generation
Mitochondrial superoxide generation was measured with MitoSOX Red assay (Life Technologies, Gaithersburg, MD, USA). Simply, VSMCs were seeded in 96-well plates, transfected and incubated for 24 h. Then the cells were incubated with 5 μM of MitoSOX Red for 10 min at 37 °C. Fluorescence was examined at 548 nm absorbance. The relative change in fluorescence is expressed as the fold change [26,27].
2.11. Measurement of H2O2 levels
Cellular H2O2 level in VSMCs was quantified using Amplex Red Assay Kit (Life Technologies, Gaithersburg, MD, USA) according to the manufacturer's instructions. Briefly, the VSMCs were seeded in 96-well plates and cultured with 50 μM of Amplex Red and 0.1 U/mL HRP at a total volume of 100 μL. Hydrogen peroxide production was quantified in absorbance at 560 nm [28,29].
2.12. Masson’ staining
Paraffin-embedded sections of aorta or mesentery artery (MA) were stained with Masson’s trichrome staining under standard protocols. The images were obtained with a light microscope. Vascular remodeling was evaluated by the media thickness, lumen diameter and the ratio of media thickness to lumen diameter [30].
2.13. Measurement of blood pressure
Blood pressure of rat tail artery was measured in waking state with a noninvasive computerized tail-cuff system (NIBP, ADInstruments, Sydney, New South Wales, Australia). The rat was warmed at 28 °C for 10–20 min before measurements to allow the detection of artery pulsations at a steady pulse level. The values were obtained by averaging 10 measurements [18].
2.14. RT-PCR
RND3, NOX1, NOX2 and NOX4 mRNA levels were measured with RT-PCR. Trizol reagent (Life Technologies, Gaithersburg, MD, USA) was used to extract total RNA. Reverse transcriptase reaction was carried out with PrimeScript® RT reagent Kits (Takara Bio Inc, Otsu, Shiga, Japan) and StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Quantitative measurement was performed with SYBR Green RT-PCR (Takara Biotechnology Co., Ltd., Tokyo, Japan) and Stepone Plus system. The values were normalized to GAPDH levels. The primers were obtained from Tiangen Biotech Co., Ltd. (Beijing, China), and the primer sequences were listed in the Online Data Supplement (Table S1).
2.15. Western blot
Total protein content was measured with BCA protein assay kits (Thermo Fisher Scientific, Rockford, IL, USA). The proteins were separated with SDS-PAGE and transferred to the PVDF membrane. RND3 antibody was obtained from Affinity Biosciences (Cincinnati, OH USA). Antibodies of Rho-associated kinase 1 (ROCK1), myosin phosphatase targeting protein (MYPT) and P-MYPT1 were acquired from Cell Signaling Technology (Beverly, MA, USA). NOX1, NOX2, NOX4, PCNA and GAPDH antibodies were purchased from Proteintech Group, Inc. (Rosemont, IL, USA). Ras homolog gene family member A (RhoA) antibody was obtained from Aifang Bio. Co. Ltd. (Changsha, Hunan, China). Secondary antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
2.16. Statistical analysis
Studies were performed in a double-blinded and randomized fashion. The data are expressed as mean ± SE. The difference between two groups was compared with Student’s unpaired t-test. The difference among multiple groups was compared with one-way or two-way ANOVA followed by post hoc Bonferroni test. P < 0.05 was considered significant.
3. Results
3.1. RND3 expressions
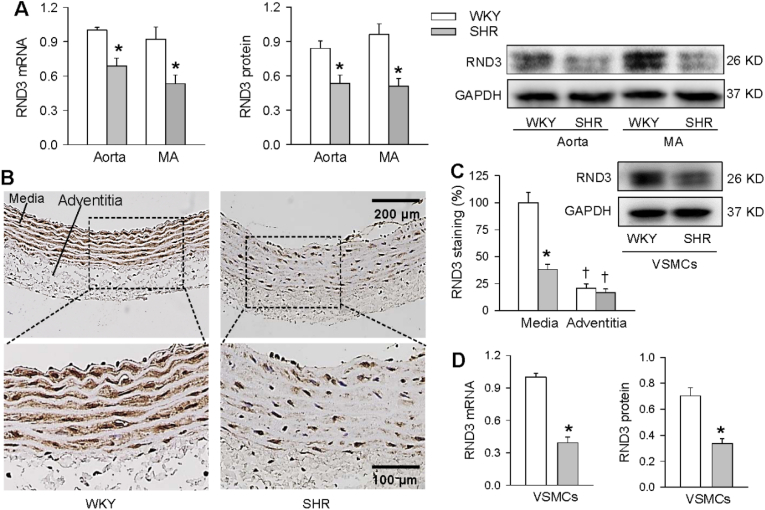
RND3 mRNA and protein expressions in aorta and MA were reduced in SHR (Fig. 1A). Immunohistochemistry for RND3 showed that the RND3 protein expression was much lower in adventitia than that in media of aorta, and the downregulation of RND3 occurred in the media (Fig. 1B and C). It is known that VSMCs are the primary cell component in the media, and VSMCs greatly contribute to the vascular remodeling [3]. We further examined the RND3 expression in the primary VSMCs. As expected, the RND3 mRNA and protein expressions in the VSMCs were lower in SHR than those in WKY (Fig. 1D).
Fig. 1.
RND3 mRNA and protein expressions in WKY and SHR. A, RND3 mRNA and protein expressions in aorta and mesentery artery (MA). B, representative images of immunohistochemistry for RND3 staining (brown color) in aorta. C, bar graph showing the relative density of RND3 staining in media and adventitia of aorta. D, RND3 mRNA and protein expressions in VSMCs of WKY and SHR. Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs media. n = 4 per group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Redox signaling in VSMCs
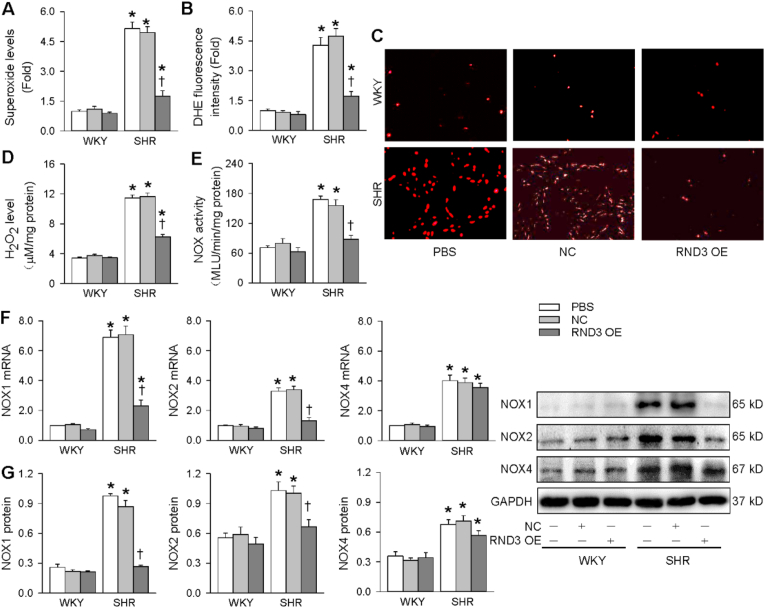
Oxidative stress greatly contributes to VSMCs migration, proliferation and vascular remodeling in hypertension [14]. Thus we examined whether the lower RND3 expression in the VSMCs of SHR may be involved in the redox signaling in hypertension. Both mitochondrial superoxide assay kit and DHE fluorescence assay were used to evaluate superoxide generation. The superoxide generation in VSMCs was increased in SHR compared with WKY, which was attenuated by the RND3 overexpression (Fig. 2A-C). Similarly, H2O2 level in VSMCs was increased in SHR, which was reduced by RND3 overexpression (Fig. 2D). To determine whether NOX is involved in the anti-oxidative stress effects of RND3, we further examined the roles of RND3 in NOX activity and expressions. The NOX activity in the VSMCs was increased in SHR, which was prevented by RND3 overexpression (Fig. 2E). NOX1, NOX2 and NOX4 mRNA and protein expressions in VSMCs were up-regulated in SHR. However, RND3 overexpression only prevented the NOX1 and NOX2 upregulation, but had no significant effect on NOX4 upregulation (Fig. 2F and G). However, RND3 overexpression had no significant effects on superoxide generation, NOX activity and expressions in the VSMCs of WKY. These results indicate that the antioxidant effects of RND3 are based on the NOX1/2 and mitochondria superoxide generation.
Fig. 2.
Effects of RND3 overexpression (OE) on superoxide production and NOX expressions in VSMCs of WKY and SHR. The VSMCs of WKY and SHR were treated with PBS, negative control (NC) or RND3 overexpressed plasmid (50 nM) for 24 h. A, superoxide level. B, relative DHE fluorescence intensity. C, representative images of DHE fluorescence staining. D, H2O2 level. E, NOX activity. F, NOX1, NOX2 and NOX4 mRNA levels. G, NOX1, NOX2 and NOX4 protein expressions. Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs PBS or NC. n = 6 per group in A-E; n = 3 per group in F. n = 4 per group in G.
3.3. VSMCs migration and proliferation
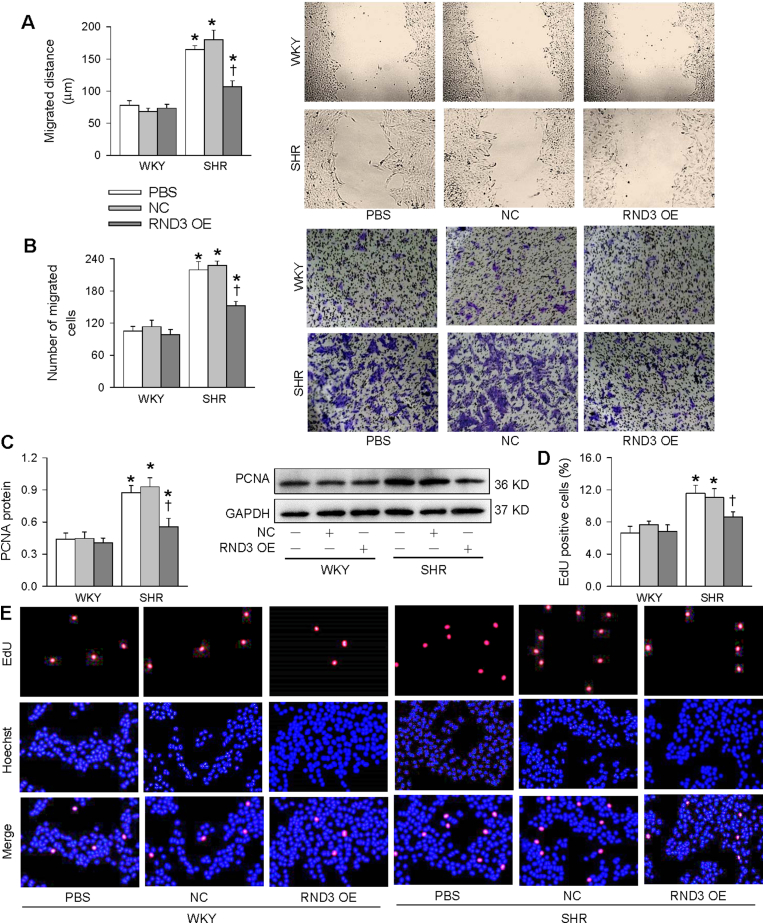
Oxidative stress is known to promote VSMCs migration and proliferation [31]. It is interesting to know whether the antioxidant effects of RND3 may play beneficial roles in attenuating VSMCs migration and proliferation in SHR. Wound healing assay and Boyden chamber assay were used to evaluate VSMCs migration. The VSMCs migration was enhanced in SHR, which was inhibited by RND3 overexpression (Fig. 3A and B). PCNA expression and EdU incorporation assay were used to evaluate VSMCs proliferation. The VSMCs proliferation was enhanced in SHR, which was attenuated by RND3 overexpression (Fig. 3C-E). However, RND3 overexpression had no significant effects on VSMCs migration and proliferation in WKY. These results indicate that RND3 attenuates VSMCs migration and proliferation in SHR.
Fig. 3.
Roles of RND3 overexpression (OE) in VSMCs migration and proliferation of WKY and SHR. The VSMCs of WKY and SHR were treated with PBS, negative control (NC) or RND3 overexpressed plasmid (50 nM) for 24 h. A, VSMCs migration evaluated with wound healing assay. B, VSMCs migration evaluated with Boyden chamber assay. C, PCNA protein expression. D, percentage of EdU-positive cells. E, representative images of EdU-positive cells. The red fluorescence represents proliferating cells, and the blue fluorescence represents total cells. Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs PBS or NC. n = 4 per group in C; n = 6 per group in others. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Roles of NOX1 inhibitor, NOX2 inhibitor and PEG-SOD
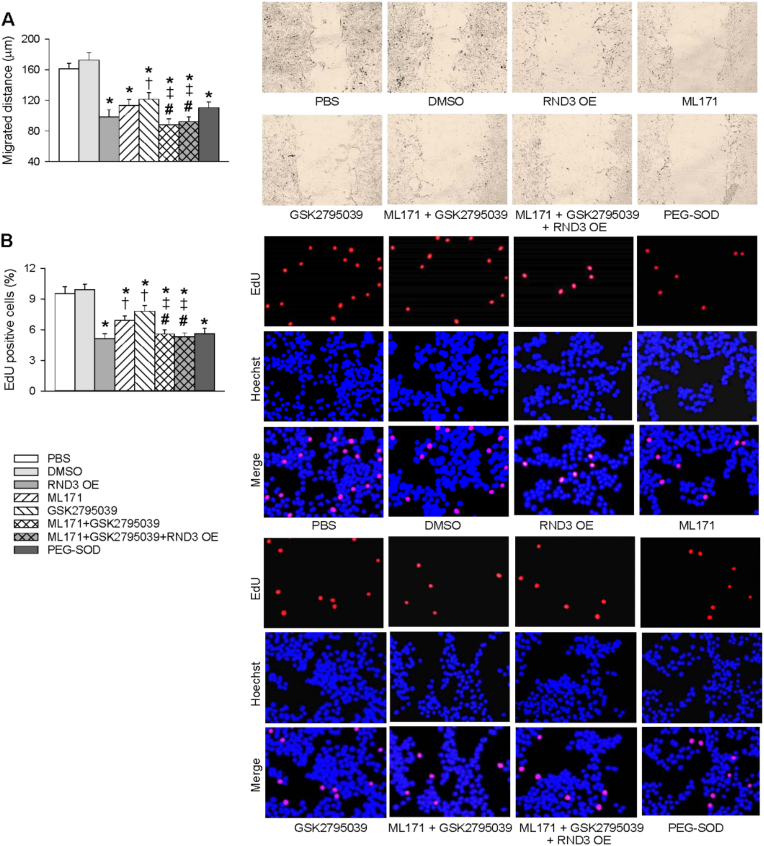
In order to determine whether RND3-induced inhibition in superoxide generation might contribute to VSMCs migration and proliferation, the effects of selective NOX1 inhibitor ML171 [32], NOX2 inhibitor GSK2795039 [33] and antioxidant enzyme PEG-SOD on VSMCs migration and proliferation of SHR were examined. The roles of either ML171 or GSK2795039 in inhibiting VSMCs migration and proliferation of SHR were less than those of RND3 overexpression, but the roles of combined treatment with ML171 and GSK2795039 were significantly greater than those of ML171 alone or GSK2795039 alone. Furthermore, RND3 overexpression failed to enhance the roles of combined treatment with ML171 and GSK2795039 in inhibiting VSMCs migration and proliferation of SHR (Fig. 4A and B). However, the limitation with respect to the specificity of NOX1 inhibitor and NOX2 inhibitor should be taken into account in analyzing the roles of ML171 or GSK2795039.
Fig. 4.
Comparing the effects of ML171 (a NOX1 inhibitor), GSK2795039 (a NOX2 inhibitor) and PEG-SOD with the effects of RND3 overexpression (RND3 OE) on VSMCs migration and proliferation in VSMCs of SHR. The VSMCs migration and proliferation were respectively evaluated with wound healing assay (A) and EdU incorporation assay (B). The VSMCs of SHR were treated with PBS, 1% DMSO (vehicle), RND3 overexpressed plasmid (50 nM), ML171 (10 μM), GSK2795039 (25 μM) or PEG-SOD (25 U/mL) for 24 h. Values are mean ± SE.. *P < 0.05 vs PBS or DMSO; †P < 0.05 vs RND3; ‡P < 0.05 vs ML171. #P < 0.05 vs GSK2795039. n = 6 per group.
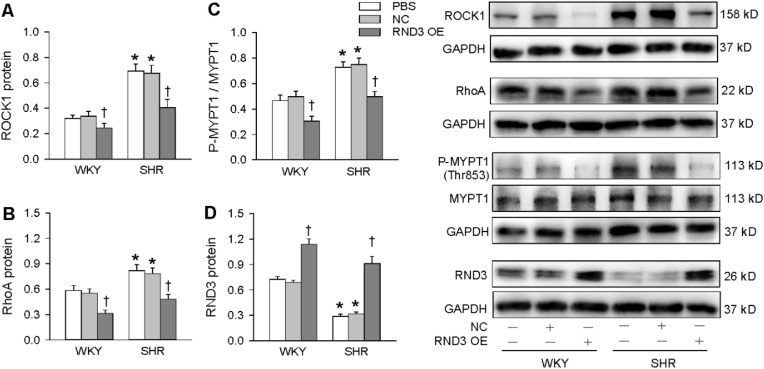
3.5. ROCK1 expression and MYPT1 phosphorylation
RND3 upregulation inhibits human trophoblast migration and proliferation via inhibiting ROCK1 signaling pathway [34]. RhoA is a small GTPase protein in the Rho family, and is inhibited by RND3 [35], while inhibition of RhoA reduces ROCK1 expression [36]. Thus, we wondered if ROCK1 signaling could be involved in the effects of RND3. Both ROCK1 and RhoA protein expressions in VSMCs were upregulated in SHR, which were prevented by RND3 overexpression (Fig. 5A and B). It is known that the activation of ROCK1 results in its downstream MYPT1 phosphorylation in rat aorta [37]. ROCK1 activity was evaluated by the phosphorylation level of a major ROCK1 substrate, MYPT-1, at Thr853 with Western blot. The phosphorylated MYPT1 in VSMCs was increased in SHR, which was normalized by RND3 overexpression (Fig. 5C). Furthermore, RND3 overexpression also reduced ROCK1 expression and MYPT1 phosphorylation in the VSMCs of WKY. These results indicate that RND3 attenuates the ROCK1 signaling in the VSMCs. On the other hand, the validity of RND3 overexpression was confirmed by the increased RND3 protein expression in the VSMCs of both WKY and SHR (Fig. 5D).
Fig. 5.
ROCK1 expression and phosphorylation of MYPT1 in the VSMCs of WKY and SHR treated with RND3 overexpression. The VSMCs were treated with PBS, negative control (NC) or RND3 overexpressed plasmid (50 nM) for 24 h. A, ROCK1 protein expression. B, RhoA protein expression. C. ROCK1 activity evaluated with the phosphorylation of MYPT1. D, RND3 protein expression. Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs PBS or NC. n = 4 per group.
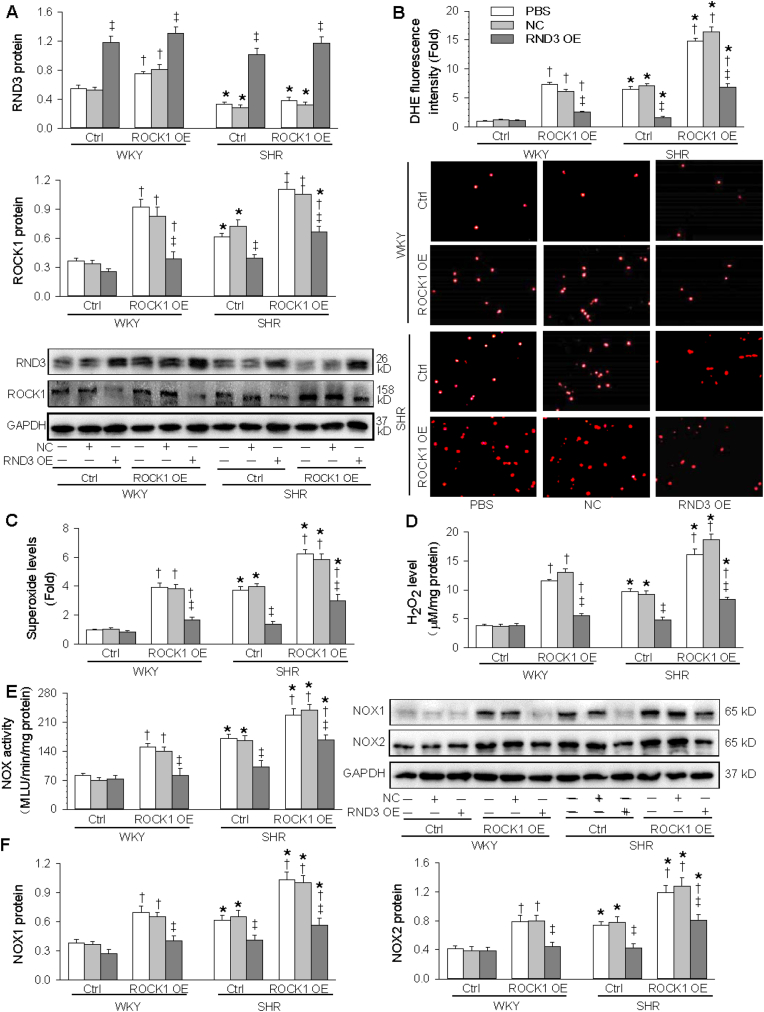
3.6. Interaction of ROCK1 and RND3 overexpression in inhibiting oxidative stress
To examine whether the ROCK1 signaling mediates the roles of RND3 in inhibiting oxidative stress, RND3 overexpression combined with ROCK1 overexpression was carried out in the VSMCs. The cell viability was not damaged by overexpression of both RND3 and ROCK1 (Fig. S1). The validity of RND3 and ROCK1 overexpression was confirmed by the upregulation of RND3 and ROCK1 expressions in the VSMCs, respectively. ROCK1 overexpression had no significant effects on the RND3 overexpression-induced RND3 upregulation, but ROCK1 overexpression-induced ROCK1 upregulation was attenuated by RND3 overexpression (Fig. 6A). These results further confirmed that ROCK1 is a target of RND3. ROCK1 overexpression increased superoxide and H2O2 generation in the VSMCs of both WKY and SHR, which were inhibited by the RND3 overexpression (Fig. 6B-D). Similarly, ROCK1 overexpression increased NOX activity, NOX1 and NOX2 expressions in the VSMCs of both WKY and SHR, which were attenuated by RND3 overexpression (Fig. 6E and F). These results indicate that ROCK1 signaling mediates the roles of RND3 in inhibiting NOX1 and NOX2-related superoxide generation in the VSMCs of SHR.
Fig. 6.
Effects of ROCK1 OE on the roles of RND3 OE in inhibiting VSMCs oxidative stress. The VSMCs were treated with PBS, negative control (NC) or RND3 OE plasmid (50 nmol/L) and ROCK1 OE plasmid (50 nmol/L) for 24 h. A, RND3 and ROCK1 protein expressions. B, DHE fluorescence staining for detecting ROS in VSMCs C, Superoxide level. D, H2O2 level. E, NOX activity. F, NOX1 and NOX2 protein expressions. Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs Ctrl; ‡P < 0.05 vs PBS or NC. n = 3 per group in A and G. n = 6 per group in others.
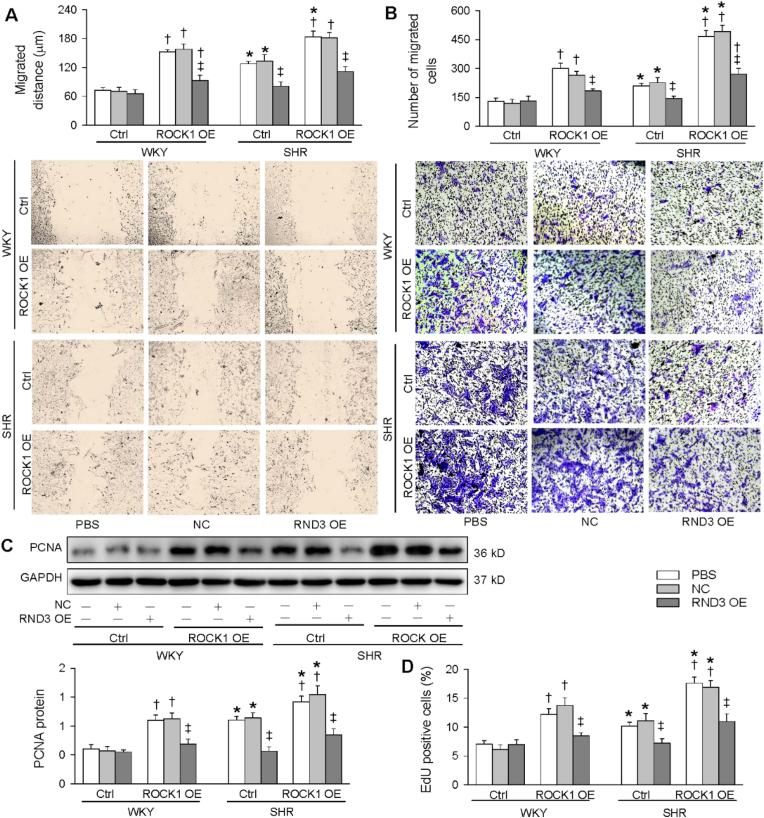
3.7. Interaction of ROCK1 and RND3 overexpression in inhibiting VSMCs migration and proliferation
RND3 overexpression combined with ROCK1 overexpression was performed in the VSMCs to determine whether the ROCK1 signaling mediates the roles of RND3 in inhibiting VSMCs migration and proliferation. ROCK1 overexpression promoted VSMCs migration in both WKY and SHR, which was attenuated by RND3 overexpression (Fig. 7A and B). ROCK1 overexpression stimulated the VSMCs proliferation in both WKY and SHR, which was inhibited by RND3 overexpression (Fig. 7C, D and S2). These results indicate that ROCK1 signaling mediates the roles of RND3 in inhibiting VSMCs migration and proliferation in SHR.
Fig. 7.
Effects of ROCK1 OE on the roles of RND3 OE in inhibiting VSMCs migration and proliferation. The VSMCs were treated with PBS, negative control (NC) or RND3 OE plasmid (50 nmol/L) and ROCK1 OE plasmid (50 nmol/L) for 24 h. A & B, VSMCs migration evaluated with wound healing assay (A) and Boyden chamber assay (B). C & D, VSMCs proliferation were evaluated with PCNA protein expression (C) and EdU-positive cells (D). Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs Ctrl; ‡P < 0.05 vs PBS or NC. n = 6 per group.
3.8. Effects of RND3 overexpression on blood pressure and vascular remodeling in WKY and SHR
Intravenous injection of adenovirus expressing RND3 was carried out to determine the therapeutical effects of RND3 overexpression on the blood pressure and vascular remodeling. The validity of RND3 overexpression was identified by the increased RND3 expression in the aorta and mesenteric artery (MA) of both WKY and SHR (Fig. S3). RND3 overexpression persistently reduced blood pressure and heart rate in SHR, lasting at least 4 weeks and reaching its maximal effects at about 2 weeks after the transfection (Fig. 8A). The media thickness and the ratio of media thickness to lumen diameter in aorta and MA were increased in SHR, which were attenuated by the RND3 overexpression. The lumen diameter of aorta was increased while the lumen diameter of MA was reduced in SHR. RND3 overexpression had no significant effects on the increased lumen diameter of aorta, but prevented the reduction in the lumen diameter of MA in SHR (Fig. 8B). Immunohistochemistry for PCNA showed that PCNA expression was increased in both aorta and MA, which was prevented by RND3 overexpression (Fig. 8D and E). These results indicate that RND3 overexpression effectively attenuates hypertension and vascular remodeling in SHR.
Fig. 8.
Therapeutic effects of adenoviral (Ad)-mediated RND3 overexpression on hypertension and vascular remodeling. The rat in WKY or SHR group was subjected to intravenous administration of PBS, Ad-scrambled RND3 (NC) or Ad-RND3 (RND3 OE, 1 × 107 plaque forming units). The blood pressure was measured every week in awake state. The vascular remodeling was examined 4 weeks after the intravenous injection. A, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and heart rate (HR) in WKY and SHR. B, bar graph showing the Masson's staining analysis for media thickness, lumen diameter and the ratio of medium thickness to lumen diameter in aorta and mesentery artery (MA). C, representative images of Masson's staining of aorta and MA. D, bar graph showing the relative values of the PCNA immunohistochemistry analyses. E, representative images of PCNA immunohistochemistry (brown color) in aorta and mesentery artery (MA). Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs. PBS or NC. n = 6 per group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
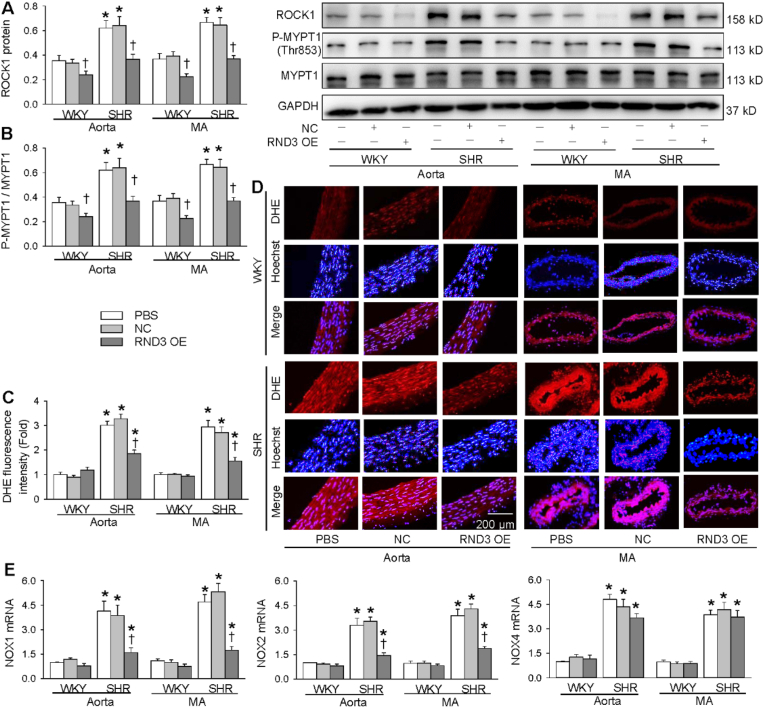
3.9. Effects of RND3 overexpression on arterial ROCK1 signaling in WKY and SHR
We further examined the effects of RND3 overexpression on arterial ROCK1 signaling with in vivo study to confirm our findings in VSMCs. ROCK1 protein expression and MYPT1 phosphorylation were increased in the aorta and MA of SHR. RND3 overexpression reduced the ROCK1 protein expression and MYPT1 phosphorylation in the arteries of both WKY and SHR (Fig. 9A and B). On the other hand, RND3 overexpression prevented the increased superoxide generation in the aorta and MA of SHR (Fig. 9C and D). Moreover, RND3 overexpression attenuated the NOX1 and NOX2 mRNA upregulation in the aorta and MA of SHR (Fig. 9E). These in vivo findings further support that RND3 attenuates vascular remodeling in SHR via inhibiting ROCK1-NOX1/2 and mitochondria superoxide signaling.
Fig. 9.
Roles of adenoviral (Ad)-mediated RND3 overexpression in WKY and SHR in PCNA and ROCK1 protein expressions, phosphorylation of MYPT1 and oxidative stress. The rat was subjected to intravenous administration of PBS, Ad-scrambled RND3 (NC) or Ad-RND3 (RND3 OE, 1 × 107 plaque forming units). The measurements were performed 4 weeks after the intravenous injection. A, ROCK1 protein expression. B, MYPT1 phosphorylation. C, bar graph showing the relative values of the DHE fluorescence staining analyses. D, representative images of DHE fluorescence staining, a marker of ROS production in aorta and MA. E, NOX1, NOX2 and NOX4 mRNA levels. Values are mean ± SE. *P < 0.05 vs WKY; †P < 0.05 vs. PBS or NC. n = 6 per group.
4. Discussion
Vascular remodeling greatly contributes to the development and the complications of hypertension [1], while oxidative stress plays important roles in promoting VSMCs proliferation and migration, and vascular remodeling [9]. RND3 is involved in the cancer development and metastasis [15]. The primary novel findings are that RND3 attenuates oxidative stress, and VSMCs migration and proliferation of SHR, which are mediated by inhibiting ROCK1 signaling. RND3 overexpression in SHR inhibits oxidative stress, vascular remodeling and hypertension in SHR. RND3 may serve as an antioxidant, and the upregulation of RND3 may be a new strategy to attenuate vascular remodeling in hypertension and several other vascular diseases.
RND3 is an atypical Rho-GTPase without the activity seen in other GTPases. The RND3 expression is primarily regulated by the balance between transcription/translation and degradation, or posttranslational modifications. RND3 is involved in the remodeling of actin cytoskeleton, cell proliferation, differentiation, motility, adhesion and survival [16]. Previous studies for RND3 mainly focused on its roles in cancer development and metastasis. In the present study, RND3 expressions were reduced in VSMCs and arteries of SHR. Both in vitro and in vivo studies showed that RND3 overexpression attenuated oxidative stress in VSMCs and arteries of SHR. The inhibitory effects of RND3 on oxidative stress at least partially attributed to its roles in inhibiting NOX1 and NOX2 expression and activity. RND3 may be an important endogenous antioxidant, and contributes to the balance of oxidation and anti-oxidation processes. It is known that oxidative stress promotes VSMCs proliferation and migration [9]. Therefore, the roles of RND3 in attenuating VSMCs proliferation and migration in SHR are partially secondary its anti-oxidation effect. Oxidative stress is closely related to inflammation [38], and both oxidative stress and inflammation are major triggers for vascular diseases [39]. NLRP3 inflammasome activation promotes VSMCs phenotypic transformation and proliferation in SHR [30]. Inhibition of oxidative stress attenuates NLRP3-mediated inflammation [40]. We speculate that the antioxidant effects of RND3 may inhibit inflammation, which also play roles in attenuating VSMCs migration and proliferation, and vascular remodeling in SHR.
ROCK is a downstream effector of the small GTP-binding protein, including 2 isoforms, ROCK1 and ROCK2. ROCK pathway is involved in various fundamental cellular functions such as proliferation, apoptosis and contraction as well as the pathogenesis of hypertension. ROCK1 signaling is one of downstream pathways of RND3 [41]. The phosphorylation level of MYPT1 (a major ROCK1 substrate) can be used to reflect ROCK1 activity [34]. ROCK1 expression and MYPT1 phosphorylation in the VSMCs were increased in SHR, which were prevented by RND3 overexpression. ROCK1 overexpression did not affect the RND3 overexpression-induced RND3 upregulation, but RND3 overexpression attenuated ROCK1 overexpression-induced ROCK1 upregulation. These findings indicate that ROCK1 is the downstream signal molecule of the RND3. ROCK1 overexpression not only stimulated NOX1 and NOX2 expression and oxidative stress, but also the VSMCs migration and proliferation in SHR, which were attenuated by RND3 overexpression. These results provide a solid evidence that the roles of RND3 in inhibiting oxidase stress, VSMCs migration and proliferation were mediated by inhibiting ROCK1 signaling. On the other hand, RND3 overexpression reduced ROCK1 and P-MYPT1 levels in WKY, but had no effects on NOX1 and NOX2 levels, cell migration and cell proliferation in WKY. The results suggest a possibility that some other signaling mechanisms may be involved in regulating NOX1 and NOX2 levels, cell migration and cell proliferation in physiological condition.
Vascular remodeling in hypertension contributes to the increased peripheral resistance, impacting both development and complications of hypertension [42]. Large arteries in hypertension undergo outward hypertrophic remodeling, contributing to the stiffness of large arteries and the increased pulse pressure. Small artery remodeling is associated with the increased media thickness and media/lumen ratio. The reduced lumen diameter increases peripheral resistance and blood pressure. The increased media/lumen ratio may be the first manifestation of end-organ damage in hypertension [43]. Patients with the high media/lumen ratio have an increased incidence of cardiovascular events in hypertension [44]. In this study, we took aorta and MA as the representatives of large artery and small artery respectively. The increased media thickness and media/lumen ratio in aorta and MA of SHR were attenuated by RND3 overexpression. The enlarged diameter in aorta of SHR was not affected by RND3 overexpression, while reduced diameter in MA of SHR was prevented by RND3 overexpression. Moreover, RND3 overexpression caused a slowly and persistently anti-hypertension effect. The role of RND3 overexpression in attenuating vascular remodeling may play important roles in preventing the development and complications of hypertension. The anti-hypertension effect may be related to the roles of RND3 overexpression in attenuating vascular remodeling and oxidative stress. Furthermore, RND3 overexpression in SHR reduced PCNA and ROCK1 expressions, MYPT1 phosphorylation and ROS production in aorta and MA of SHR. The results of both the in vivo study and the in vitro study indicate that RND3 attenuates oxidative stress and vascular remodeling via inhibiting ROCK1 signaling. The roles of RND3 in inhibiting VSMCs migration, proliferation and oxidative stress play an important role in attenuating vascular remodeling in hypertension.
In conclusion, RND3 inhibits NOX activity, NOX1 and NOX2 expressions, mitochondria superoxide generation, and attenuates cell migration and proliferation in VSMCs of SHR, which are mediated by ROCK1 signaling. RND3 overexpression in SHR attenuates hypertension, oxidative stress and vascular remodeling. RND3 may serve as a crucial endogenous antioxidant, and contributes to the balance between oxidation and antioxidation in VSMCs. The antioxidation role of RND3 in SHR at least partially contributes to its beneficial roles of RND3 in attenuating VSMCs migration and proliferation, vascular remodeling and hypertension. The upregulation of RND3 is a potential therapeutic strategy in attenuating oxidative stress, vascular remodeling and hypertension.
Declaration of competing interest
None declared.
Acknowledgments
This study was supported by National Natural Science Foundation of China (32071106, 81970356 & 31871148).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102204.
Contributor Information
Guo-Qing Zhu, Email: gqzhucn@njmu.edu.cn.
Ye-Bo Zhou, Email: zhouyebo666@njmu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lee R.M., Dickhout J.G., Sandow S.L. Vascular structural and functional changes: their association with causality in hypertension: models, remodeling and relevance. Hypertens. Res. 2017;40:311–323. doi: 10.1038/hr.2016.145. [DOI] [PubMed] [Google Scholar]
- 2.Brown I.A.M., Diederich L., Good M.E., DeLalio L.J., Murphy S.A., Cortese-Krott M.M., Hall J.L., Le T.H., Isakson B.E. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler. Thromb. Vasc. Biol. 2018;38:1969–1985. doi: 10.1161/ATVBAHA.118.311229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M., Fukagawa N.K. Age-related changes in redox signaling and VSMC function. Antioxidants Redox Signal. 2010;12:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka L.Y., Laurindo F.R.M. Vascular remodeling: a redox-modulated mechanism of vessel caliber regulation. Free Radic. Biol. Med. 2017;109:11–21. doi: 10.1016/j.freeradbiomed.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Redondo A.B., Aguado A., Briones A.M., Salaices M. NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 2016;114:110–120. doi: 10.1016/j.phrs.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Obradovic M., Essack M., Zafirovic S., Sudar-Milovanovic E., Bajic V.P., Van N.C., Trpkovic A., Stanimirovic J., Bajic V.B., Isenovic E.R. Redox control of vascular biology. Biofactors. 2020;46:246–262. doi: 10.1002/biof.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sena C.M., Leandro A., Azul L., Seica R., Perry G. Vascular oxidative stress: impact and therapeutic approaches. Front. Physiol. 2018;9:1668. doi: 10.3389/fphys.2018.01668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Q.B., Wan M.Y., Wang P.Y., Zhang C.X., Xu D.Y., Liao X., Sun H.J. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox. Biol. 2018;14:656–668. doi: 10.1016/j.redox.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durgin B.G., Straub A.C. Redox control of vascular smooth muscle cell function and plasticity. Lab. Invest. 2018;98:1254–1262. doi: 10.1038/s41374-018-0032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismaeel A., Brumberg R.S., Kirk J.S., Papoutsi E., Farmer P.J., Bohannon W.T., Smith R.S., Eidson J.L., Sawicki I., Koutakis P. Oxidative stress and arterial dysfunction in peripheral artery disease. Antioxidants. 2018;7:145. doi: 10.3390/antiox7100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daiber A., Hahad O., Andreadou I., Steven S., Daub S., Munzel T. Redox-related biomarkers in human cardiovascular disease - classical footprints and beyond. Redox. Biol. 2021;42:101875. doi: 10.1016/j.redox.2021.101875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordoni L., Fedeli D., Piangerelli M., Pelikant-Malecka I., Radulska A., Samulak J.J., Sawicka A.K., Lewicki L., Kalinowski L., Olek R.A., Gabbianelli R. Gender-related differences in trimethylamine and oxidative blood biomarkers in cardiovascular disease patients. Biomedicines. 2020;8:238. doi: 10.3390/biomedicines8080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poznyak A.V., Grechko A.V., Orekhova V.A., Khotina V., Ivanova E.A., Orekhov A.N. NADPH oxidases and their role in atherosclerosis. Biomedicines. 2020;8:206. doi: 10.3390/biomedicines8070206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brito R., Castillo G., Gonzalez J., Valls N., Rodrigo R. Oxidative stress in hypertension: mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes. 2015;123:325–335. doi: 10.1055/s-0035-1548765. [DOI] [PubMed] [Google Scholar]
- 15.Jie W., Andrade K.C., Lin X., Yang X., Yue X., Chang J. Pathophysiological functions of Rnd3/RhoE. Comp. Physiol. 2015;6:169–186. doi: 10.1002/cphy.c150018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paysan L., Piquet L., Saltel F., Moreau V. Rnd3 in cancer: a review of the evidence for tumor promoter or suppressor. Mol. Cancer Res. 2016;14:1033–1044. doi: 10.1158/1541-7786.MCR-16-0164. [DOI] [PubMed] [Google Scholar]
- 17.Yue X., Yang X., Lin X., Yang T., Yi X., Dai Y., Guo J., Li T., Shi J., Wei L., Fan G.C., Chen C., Chang J. Rnd3 haploinsufficient mice are predisposed to hemodynamic stress and develop apoptotic cardiomyopathy with heart failure. Cell Death Dis. 2014;5:e1284. doi: 10.1038/cddis.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren X.S., Tong Y., Qiu Y., Ye C., Wu N., Xiong X.Q., Wang J.J., Han Y., Zhou Y.B., Zhang F., Sun H.J., Gao X.Y., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. MiR155-5p in adventitial fibroblasts-derived extracellular vesicles inhibits vascular smooth muscle cell proliferation via suppressing angiotensin-converting enzyme expression. J. Extracell. Vesicles. 2020;9:1698795. doi: 10.1080/20013078.2019.1698795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T.Y., Shi C.X., Gao R., Sun H.J., Xiong X.Q., Ding L., Chen Q., Li Y.H., Wang J.J., Kang Y.M., Zhu G.Q. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. (Lond.) 2015;129:839–850. doi: 10.1042/CS20150009. [DOI] [PubMed] [Google Scholar]
- 20.Tong Y., Ye C., Ren X.S., Qiu Y., Zang Y.H., Xiong X.Q., Wang J.J., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. Exosome-mediated transfer of ACE (angiotensin-converting enzyme) from adventitial fibroblasts of spontaneously hypertensive rats promotes vascular smooth muscle cell migration. Hypertension. 2018;72:881–888. doi: 10.1161/HYPERTENSIONAHA.118.11375. [DOI] [PubMed] [Google Scholar]
- 21.Ye C., Tong Y., Wu N., Wan G.W., Zheng F., Chen J.Y., Lei J.Z., Zhou H., Chen A.D., Wang J.J., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. Inhibition of miR-135a-5p attenuates vascular smooth muscle cell proliferation and vascular remodeling in hypertensive rats. Acta Pharmacol. Sin. 2021;42:1798–1807. doi: 10.1038/s41401-020-00608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu N., Ye C., Zheng F., Wan G.W., Wu L.L., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. MiR155-5p inhibits cell migration and oxidative stress in vascular smooth muscle cells of spontaneously hypertensive rats. Antioxidants. 2020;9:204. doi: 10.3390/antiox9030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheu M.L., Shen C.C., Chen Y.S., Chiang C.K. Ochratoxin A induces ER stress and apoptosis in mesangial cells via a NADPH oxidase-derived reactive oxygen species-mediated calpain activation pathway. Oncotarget. 2017;8:19376–19388. doi: 10.18632/oncotarget.14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S., Zhao Y., Jin C., Yu L., Ding F., Fu G., Zhu J. PKC/NADPH oxidase are involved in the protective effect of pioglitazone in high homocysteine-induced paracrine dyfunction in endothelial progenitor cells. Am. J. Transl. Res. 2017;9:1037–1048. [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Y., Zheng F., Ye C., Chen A.D., Wang J.J., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. Angiotensin type 1 receptors and superoxide anion production in hypothalamic paraventricular nucleus contribute to capsaicin-induced excitatory renal reflex and sympathetic activation. Neurosci. Bull. 2020;36:463–474. doi: 10.1007/s12264-019-00460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu C., Wu Z., Huang Z., Hao X., Wang S., Deng J., Yin Y., Tan C. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021;45:102051. doi: 10.1016/j.redox.2021.102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvis A.M., McCormick M.L., Spitz D.R., Kiningham K.K. Redox balance influences differentiation status of neuroblastoma in the presence of all-trans retinoic acid. Redox Biol. 2016;7:88–96. doi: 10.1016/j.redox.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Z., Zhuo Q., Hu Q., Xu X., Mengqi L., Zhang Z., Xu W., Liu W., Fan G., Qin Y., Yu X., Ji S. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells, Redox. Biol. 2021;38:101807. doi: 10.1016/j.redox.2020.101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idelman G., Smith D.L.H., Zucker S.D. Bilirubin inhibits the up-regulation of inducible nitric oxide synthase by scavenging reactive oxygen species generated by the toll-like receptor 4-dependent activation of NADPH oxidase. Redox. Biol. 2015;5:398–408. doi: 10.1016/j.redox.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun H.J., Ren X.S., Xiong X.Q., Chen Y.Z., Zhao M.X., Wang J.J., Zhou Y.B., Han Y., Chen Q., Li Y.H., Kang Y.M., Zhu G.Q. NLRP3 inflammasome activation contributes to VSMC phenotypic transformation and proliferation in hypertension. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Ji L., Jiang R., Zheng L., Liu D. Oxidized high-density lipoprotein induces the proliferation and migration of vascular smooth muscle cells by promoting the production of ROS. J. Atherosclerosis Thromb. 2014;21:204–216. doi: 10.5551/jat.19448. [DOI] [PubMed] [Google Scholar]
- 32.Gianni D., Taulet N., Zhang H., DerMardirossian C., Kister J., Martinez L., Roush W.R., Brown S.J., Bokoch G.M., Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem. Biol. 2010;5:981–993. doi: 10.1021/cb100219n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirano K., Chen W.S., Chueng A.L.W., Dunne A.A., Seredenina T., Filippova A., Ramachandran S., Bridges A., Chaudry L., Pettman G., Allan C., Duncan S., Lee K.C., Lim J., Ma M.T., Ong A.B., Ye N.Y., Nasir S., Mulyanidewi S., Aw C.C., Oon P.P., Liao S., Li D., Johns D.G., Miller N.D., Davies C.H., Browne E.R., Matsuoka Y., Chen D.W., Jaquet V., Rutter A.R. Discovery of GSK2795039, a novel small molecule NADPH oxidase 2 inhibitor. Antioxidants Redox Signal. 2015;23:358–374. doi: 10.1089/ars.2014.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X.L., Li X., Tian F.J., Zeng W.H., Zhang J., Mo H.Q., Qin S., Sun L.Q., Zhang Y.C., Zhang Y., Lin Y. Upregulation of RND3 affects trophoblast proliferation, apoptosis, and migration at the maternal-fetal interface. Front. Cell. Dev. Biol. 2020;8:153. doi: 10.3389/fcell.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breslin J.W., Daines D.A., Doggett T.M., Kurtz K.H., Souza-Smith F.M., Zhang X.E., Wu M.H., Yuan S.Y. Rnd3 as a novel target to ameliorate microvascular leakage. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X.X., Yue G.G.-L., Dong J.R., Lam C.W.-K., Wong C.K., Qiu M.H., Lau C.B.-S. Actein inhibits tumor growth and metastasis in HER2-positive breast tumor bearing mice via suppressing AKT/mTOR and Ras/Raf/MAPK signaling pathways. Front. Oncol. 2020;10:854. doi: 10.3389/fonc.2020.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q., Wo D., Huang Y., Yu N., Zeng J., Chen H., Wang H., Bao L., Lin S., Chu J., Peng J. Alkaloids from Nelumbinis Plumula (AFNP) ameliorate aortic remodeling via RhoA/ROCK pathway. Biomed. Pharmacother. 2019;112:108651. doi: 10.1016/j.biopha.2019.108651. [DOI] [PubMed] [Google Scholar]
- 38.Poznyak A.V., Melnichenko A.A., Wetzker R., Gerasimova E.V., Orekhov A.N. NLPR3 inflammasomes and their significance for atherosclerosis. Biomedicines. 2020;8:205. doi: 10.3390/biomedicines8070205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M.T., Vujacic-Mirski K., Helmstadter J., Kroller-Schon S., Munzel T., Daiber A. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019;2019:7092151. doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sho T., Xu J. Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnol. Appl. Biochem. 2019;66:4–13. doi: 10.1002/bab.1700. [DOI] [PubMed] [Google Scholar]
- 41.Shimokawa H., Satoh K. 2015 ATVB Plenary Lecture: translational research on rho-kinase in cardiovascular medicine. Arterioscler. Thromb. Vasc. Biol. 2015;35:1756–1769. doi: 10.1161/ATVBAHA.115.305353. [DOI] [PubMed] [Google Scholar]
- 42.Intengan H.D., Schiffrin E.L. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38:581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- 43.Schiffrin E.L. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–374. doi: 10.1161/HYPERTENSIONAHA.111.187021. [DOI] [PubMed] [Google Scholar]
- 44.Perticone F., Ceravolo R., Pujia A., Ventura G., Iacopino S., Scozzafava A., Ferraro A., Chello M., Mastroroberto P., Verdecchia P., Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.