Abstract
Tocilizumab has been repurposed against the ‘cytokine storm’ in the setting of coronavirus disease 2019 (COVID‐19). Our aim was to evaluate the efficacy of tocilizumab in the management of hospitalized COVID‐19 patients. We searched MEDLINE, CENTRAL and medRxiv for studies of tocilizumab in hospitalized COVID‐19 patients. Primary objective was the effectiveness of tocilizumab on mortality. Secondary objectives included the need for invasive mechanical ventilation (IMV), composite endpoints of mortality or IMV and intensive care unit (ICU) admission or IMV, length of hospitalization and differences in mortality in subgroups (ICU and non‐ICU patients and patients receiving or not receiving concomitant corticosteroids). We included 52 studies (nine randomized controlled trials [RCTs] and 43 observational) with a total of 27,004 patients. In both RCTs and observational studies, the use of tocilizumab was associated with a reduction in mortality; 11% in RCTs (risk ratio [RR] 0.89, 95% CI 0.82 to 0.96) and 31% in observational studies (RR 0.69, 95% CI 0.58 to 0.83). The need for IMV was reduced by 19% in RCTs (RR 0.81, 95% CI 0.71 to 0.93), while no significant reduction was observed in observational studies. Both RCTs and observational studies showed a benefit from tocilizumab on the composite endpoint of mortality or IMV. Tocilizumab improved mortality both in ICU and non‐ICU patients. Reduction in mortality was evident in observational studies regardless of the use of systemic corticosteroids, while that was not the case in the RCTs. Tocilizumab was associated with lower mortality and other clinically relevant outcomes in hospitalized patients with moderate‐to‐critical COVID‐19.
Keywords: coronavirus disease, COVID‐19, meta‐analysis, mortality, SARS‐CoV‐2, tocilizumab

Tocilizumab administration for the treatment of hospitalized patients with COVID‐19: A systematic review and meta‐analysis
INTRODUCTION
The novel severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), which is the coronavirus disease 2019 (COVID‐19) causing agent, is a highly infectious viral pathogen that is accountable for the ongoing pandemic. 1 As of 30 April 2021, more than 151 million individuals have been infected with SARS‐CoV‐2 worldwide and 3,180,000 deaths globally have been attributed to COVID‐19. 2 COVID‐19 tends to appear with diversity in clinical manifestations ranging from asymptomatic infection to acute respiratory distress syndrome and death. Although the pathogenesis of COVID‐19 is still imprecise, certain patients with severe or critical disease have laboratory evidence of a systemic inflammatory response resembling the cytokine release syndrome (CRS). 3 CRS is characterized by a sharp increase of many proinflammatory cytokines, such as IL‐1β, IL‐6, Granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and Tumor necrosis factor alpha (TNF‐α), and elevated levels of d‐dimers, ferritin and C‐reactive protein (CRP). 4 Proposed immunomodulatory agents with potential use against the cytokine storm include glucocorticoids, 5 colchicine, 6 anakinra, 7 baricitinib 8 and sarilumab. 9 Antiviral drugs, such as remdesivir, 10 and monoclonal antibodies 11 are also used in selected cases.
Tocilizumab is a humanized monoclonal antibody against the IL‐6 receptor. Therapeutic indications include the treatment of severe, active and progressive rheumatoid arthritis 12 ; active systemic juvenile idiopathic arthritis; and chimeric antigen receptor T cell‐induced severe or life‐threatening CRS in adults and paediatric patients of 2 years of age and older. 13 Recently (5 March 2021), the Food and Drug Administration approved tocilizumab for adult patients with systemic sclerosis‐associated interstitial lung disease. IL‐6 plays a crucial role in CRS. 4 In COVID‐19, the primary concept was that intercepting the IL‐6 pathway might reduce the vigorous inflammatory response. 14 Several observational studies and randomized controlled trials (RCTs), with the RECOVERY trial being the largest, 15 have evaluated the administration of tocilizumab for the management of patients with moderate‐to‐severe COVID‐19. 16 , 17 , 18 Nevertheless, tocilizumab should be used cautiously, as it results in increased risk of infection from all microorganisms, viral, bacterial, fungal and parasitic, with serious infections appearing in 2.7% of the treated patients (4.0–4.5/100 patient years of exposure). 19
Data from studies so far have been contradictory regarding the efficacy and effectiveness of tocilizumab in the management of patients with moderate‐to‐critical COVID‐19; this was due to different study designs, populations evaluated and the timing of tocilizumab administration. 20 Based on the available evidence, we performed a systematic review and meta‐analysis of the available data from observational studies and RCTs in order to evaluate the overall effectiveness and efficacy of tocilizumab administration in patients with COVID‐19 on mortality and need for intubation/mechanical ventilation, intensive care unit (ICU) admission and the length of hospitalization, both in usual clinical practice and in the controlled settings of RCTs.
METHODS
Literature search and inclusion criteria
We conducted a systematic literature search, from inception to 31 March 2021, to identify studies that assessed the efficacy of tocilizumab in COVID‐19 in MEDLINE (through PubMed) and the Cochrane Central Register of Controlled Trials (CENTRAL); we also searched medRxiv (https://www.medrxiv.org) for unpublished RCTs. The search strategy algorithm and study selection are shown in detail in Appendix S1 in the Supporting Information. We prospectively submitted the systematic review protocol for registration on PROSPERO (CRD42021247188; Appendix S2 in the Supporting Information). We have followed PRISMA 21 and MOOSE 22 reporting guidelines. A study to be considered as eligible for registration would need to meet the following criteria:
Inclusion of subjects >18 years old hospitalized for COVID‐19.
Randomized clinical trials, retrospective observational cohort studies, retrospective case–control studies and prospective case–control studies.
Intravenous or subcutaneous administration of tocilizumab for COVID‐19 treatment.
The group of patients receiving tocilizumab was compared with a control arm (standard of care treatment or other approved drugs).
Objectives
Primary objective
The primary objective was to determine whether treatment with tocilizumab reduces mortality in patients hospitalized with COVID‐19.
Secondary objectives
Secondary objectives included the evaluation of differences between the tocilizumab and control groups in:
The need for intubation or invasive mechanical ventilation (IMV).
A composite endpoint of mortality or IMV.
A composite endpoint of ICU admission or IMV.
The length of hospitalization.
Mortality in non‐ICU and ICU‐treated patients.
Mortality in patients according to the concomitant use of systemic corticosteroids.
Data extraction and risk of bias assessment
Two authors (CK and AG) reviewed concurrently all the eligible studies to perform data extraction. The reviewers worked independently during study data extraction; disagreements, if any, were resolved by discussion to obtain consensus, with unresolved conflicts decided by a third reviewer (KK). Obtained data were validated by a third independent author (GN). Studies published in languages other than English were excluded.
From each eligible study, we recorded information about the first author, publication year, journal, study design, follow‐up time, population characteristics, total and tocilizumab‐treated sample size, treatment indication, tocilizumab and comparator dose, ICU setting and use of corticosteroids. Moreover, we extracted information on mortality, intubation (or IMV) and days of hospitalization along with their effect estimates. Risk ratios (RRs) along with their CIs were calculated for mortality and intubation, assessed as binary outcomes, and median differences were calculated for days of hospitalization. Risk of bias of eligible trials was assessed by applying the Cochrane Collaboration's tool. 23
Data analysis
We calculated RR and summary median differences, along with the corresponding 95% CI, by pooling the study‐specific estimates using random‐effects models. 24 Days of hospitalization, in most of the studies, were provided as medians and interquartile ranges (IQRs). Hence, in order to synthesize these estimates, we used a formula that converts medians and IQRs to mean and SDs. 25 , 26 The presence and the degree of heterogeneity were assessed with I 2 (ranging from 0% to 100%). 27 When more than three studies were included in the meta‐analysis, prediction intervals (PI) were calculated to describe the uncertainty we expect in the summary effect if a new study is included in the meta‐analysis. 28 Subgroup analysis was performed regarding the ICU setting and the use of corticosteroids. We further assessed the possible small study effects (an indication of publication bias) by visual inspection of funnel plots and Egger's test. 29 The presence of heterogeneity was estimated with the Cochran's Q statistic and it was quantified with I 2. 30 Finally, we accounted for the inter‐study variability using a meta‐regression approach. The covariates that were considered in the meta‐regression model were age of participants, gender, the type of centres that the study engaged (single/multicentre) and the continent where the study was performed. All analyses were performed using Stata (version 14; StataCorp, College Station, TX, USA).
RESULTS
Study identification and selection
The search of the electronic databases (MEDLINE and CENTRAL) on 31 March 2021 identified a total of 873 studies, with further six RCTs identified through preprint servers; these six RCTs identified in medRxiv were subsequently identified in their final form in PubMed. Following removal of duplicates, screening and full‐text review, 52 articles published worldwide were shortlisted for inclusion. Nine of them were RCTs and 43 were observational cohort studies involving a control arm (Table 1). Figure 1 shows the flow chart of the study selection process. The data from the RECOVERY trial were updated on 1 May when the study was published in its final form. 15
TABLE 1.
Characteristics of included studies and sample size for treatment (n) and total patients included (N)
| Author, year | n, N | Study country | Centre | Study design |
|---|---|---|---|---|
| Albertini et al., 2021 31 | 22, 44 | France | Single centre | Prospective study |
| Biran et al., 2020 32 | 210, 630 | USA | Multicentre | Retrospective study |
| Campochiaro et al., 2020 33 | 32, 65 | Italy | Single centre | Retrospective study |
| Canziani et al., 2020 34 | 64, 168 | Italy | Multicentre | Retrospective study |
| Capra et al., 2020 35 | 62, 85 | Italy | Single centre | Retrospective study |
| Chachar et al., 2021 36 | 33, 93 | Pakistan | Single centre | Retrospective study |
| Chilimuri et al., 2020 37 | 87, 1225 | USA | Single centre | Retrospective study |
| Colaneri et al., 2020 38 | 21, 112 | Italy | Single centre | Retrospective study |
| De Rossi et al., 2020 39 | 90, 158 | Italy | Single centre | Retrospective study |
| Eimer et al., 2021 40 | 29, 87 | Sweden | Single centre | Retrospective study |
| Fisher et al., 2021 41 | 45, 115 | USA | Single centre | Retrospective study |
| Galván‐Román et al., 2021 42 | 58, 146 | Spain | Single centre | Retrospective study |
| Gokhale et al., 2021 43 | 70, 161 | India | Single centre | Retrospective study |
| Gordon et al., 2021 (REMAP‐CAP) 16 | 353, 865 | International a | Multicentre | RCT |
| Guaraldi et al., 2020 44 | 179, 544 | Italy | Multicentre | Retrospective study |
| Gupta et al., 2021 45 | 433, 3924 | USA | Multicentre | Retrospective study |
| Hermine et al., 2021 (CORIMUNO TOCI) 46 | 63, 130 | France | Multicentre | RCT |
| Hill et al., 2020 47 | 43, 88 | USA | Single centre | Retrospective study |
| Holt et al., 2020 48 | 32, 62 | USA | Single centre | Retrospective study |
| Horby et al., 2021 (RECOVERY) 15 | 2022, 4113 | UK | Multicentre | RCT |
| Ip et al., 2020 49 | 134, 547 | USA | Multicentre | Retrospective study |
| Kewan et al., 2020 50 | 28, 51 | USA | Single centre | Retrospective study |
| Klopfenstein et al., 2020 51 | 30, 206 | France | Single centre | Retrospective study |
| Lewis et al., 2020 52 | 497, 994 | USA | Multicentre | Retrospective study |
| Martínez‐Sanz et al., 2021 53 | 260, 1229 | Spain | Multicentre | Retrospective study |
| Menzella et al., 2020 54 | 41, 79 | Italy | Single centre | Retrospective study |
| Mikulska et al., 2020 55 | 130, 196 | Italy | Single centre | Prospective study |
| Moiseev et al., 2020 56 | 159, 328 | Russia | Multicentre | Retrospective study |
| Narain et al., 2021 18 | 527, 5776 | USA | Multicentre | Retrospective study |
| Nasa et al., 2020 57 | 22, 85 | UAE | Multicentre | Retrospective study |
| Okoh et al., 2021 58 | 20, 60 | USA | Single centre | Retrospective study |
| Patel et al., 2021 59 | 42, 83 | USA | Single centre | Retrospective study |
| Potere et al., 2020 60 | 10, 20 | Italy | Single centre | Retrospective study |
| Quartuccio et al., 2020 61 | 42, 111 | Italy | Single centre | Retrospective study |
| Rajendram et al., 2021 62 | 82, 164 | USA | Multicentre | Retrospective study |
| Rodríguez‐Baño et al., 2021 63 | 239, 778 | Spain | Multicentre | Retrospective study |
| Rodríguez‐Molinero et al., 2021 64 | 22, 44 | Spain | Multicentre | Retrospective study |
| Rojas‐Marte et al., 2020 65 | 96, 193 | USA | Single centre | Retrospective study |
| Rosas et al., 2021 (COVACTA) 66 | 294, 438 | Europe and North America | Multicentre | RCT |
| Rossi et al., 2020 67 | 106, 246 | France | Single centre | study |
| Rossotti et al., 2020 68 | 74, 222 | Italy | Single centre | Retrospective study |
| Roumier et al., 2021 69 | 50, 96 | France | Single centre | Prospective study |
| Ruiz‐Antorán et al., 2021 70 | 268, 506 | Spain | Multicentre | Retrospective study |
| Salama et al., 2021 (EMPACTA) 17 | 249, 377 | International b | Multicentre | RCT |
| Salvarani et al., 2021 (RCT‐TCZ‐COVID‐19) 71 | 60, 126 | Italy | Multicentre | RCT |
| Soin et al., 2021 (COVINTOC) 72 | 91, 180 | India | Multicentre | RCT |
| Somers et al., 2020 73 | 78, 174 | USA | Single centre | Retrospective study |
| Stone et al., 2020 (BACC Bay) 74 | 161, 243 | USA | Multicentre | RCT |
| Tian et al., 2021 75 | 65, 195 | China | Multicentre | Retrospective study |
| Tsai et al., 2020 76 | 66, 132 | USA | Single centre | Retrospective study |
| Veiga et al., 2021 (TOCIBRAS) 77 | 65, 129 | Brazil | Multicentre | RCT |
| Zheng et al., 2020 78 | 92, 181 | China | Single centre | Retrospective study |
Note: Sample sizes given for patients receiving intervention (n) alongside total patients included (N) in the study.
Abbreviations: COVID‐19, coronavirus disease 2019; RCT, randomized controlled trial.
UK, France, Netherlands, Australia, New Zealand, Canada, Spain and Ireland.
USA, Peru, Brazil, Kenya, South Africa and Mexico.
FIGURE 1.
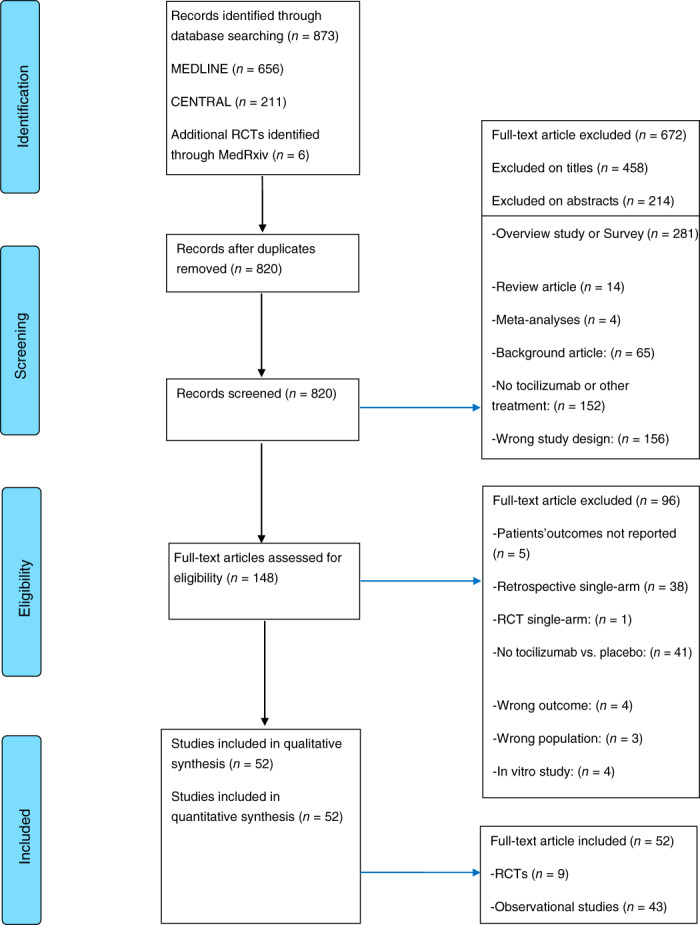
Meta‐analysis flow diagram illustrating systematic search and screening strategy, including the number of studies meeting eligibility criteria and number of excluded studies
Patients received tocilizumab either intravenously (8 mg/kg up to 800 mg, once or twice) or subcutaneously (324 mg). The total population of participants was 27,004, of whom 8048 (29.8%) received tocilizumab. The RCTs involved 6604 participants, of whom 3358 (50.8%) received tocilizumab, and observational cohort studies involved 20,400 participants, of whom 4690 (23%) received tocilizumab. In 39 studies, corticosteroids were concomitantly administered; eight of them were RCTs and 31 observational studies (Table S1 in the Supporting Information).
Study outcomes
Mortality
Forty‐seven studies with 25,385 participants, of whom 7814 patients were treated with tocilizumab, reported adjusted hazard ratios or crude results for overall mortality. Nine of the studies were RCTs with 6604 participants, of whom 3358 patients received tocilizumab, while 38 of the studies were observational studies with 18,781 participants, of whom 4456 patients were treated with tocilizumab. In both the RCTs and the observational studies, a meaningful survival benefit was observed in patients treated with tocilizumab. The benefit was 11% in the RCTs (RR 0.89, 95% CI 0.82 to 0.96, 95% PI 0.80 to 0.97) and 31% in observational studies (RR 0.69, 95% CI 0.58 to 0.83, 95% PI 0.28 to 1.73). RCTs presented small heterogeneity (I 2 = 0.3%), whereas observational studies presented very large heterogeneity (I 2 = 84.0%) (Figure 2). In order to assess the possible sources of heterogeneity, we performed a meta‐regression of the mortality rates, including age of participants, gender, the type of centre that the study engaged (single/multicentre) and the continent where the study was performed. All the aforementioned factors were not significant.
FIGURE 2.
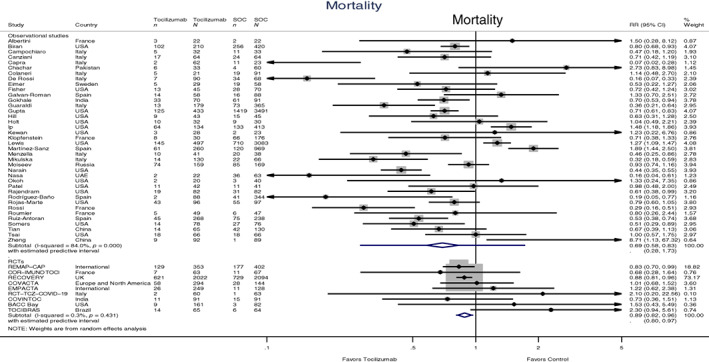
Forest plot of mortality risk ratios (RRs) comparing tocilizumab and control treatment. Sample sizes are given for participants receiving intervention and participants receiving standard of care treatment (SOC), included in the study, when data were available. Summary estimates are presented separately for observational studies and randomized controlled trials (n, deaths; N, group size)
Need for IMV
Fourteen studies with a total of 6713 participants, of whom 3285 patients were treated with tocilizumab, reported results for the need for IMV or intubation. Four of them were RCTs with a total of 4977 participants, of whom 2568 received tocilizumab. The need for IMV was reduced by 19% in patients treated with tocilizumab (RR 0.81, 95% CI 0.71 to 0.93, 95% PI 0.60 to 1.09); small heterogeneity was observed in the RCTs (I 2 = 0.0%). In the 10 observational studies with a total of 1736 participants, of whom 717 were treated with tocilizumab, there was a numerical reduction in the need for IMV by 19%; however, this did not reach statistical significance (RR 0.81, 95% CI 0.57 to 1.14, 95% PI 0.28 to 2.29). The observational studies showed large heterogeneity (I 2 = 70.2%) (Figure 3A).
FIGURE 3.
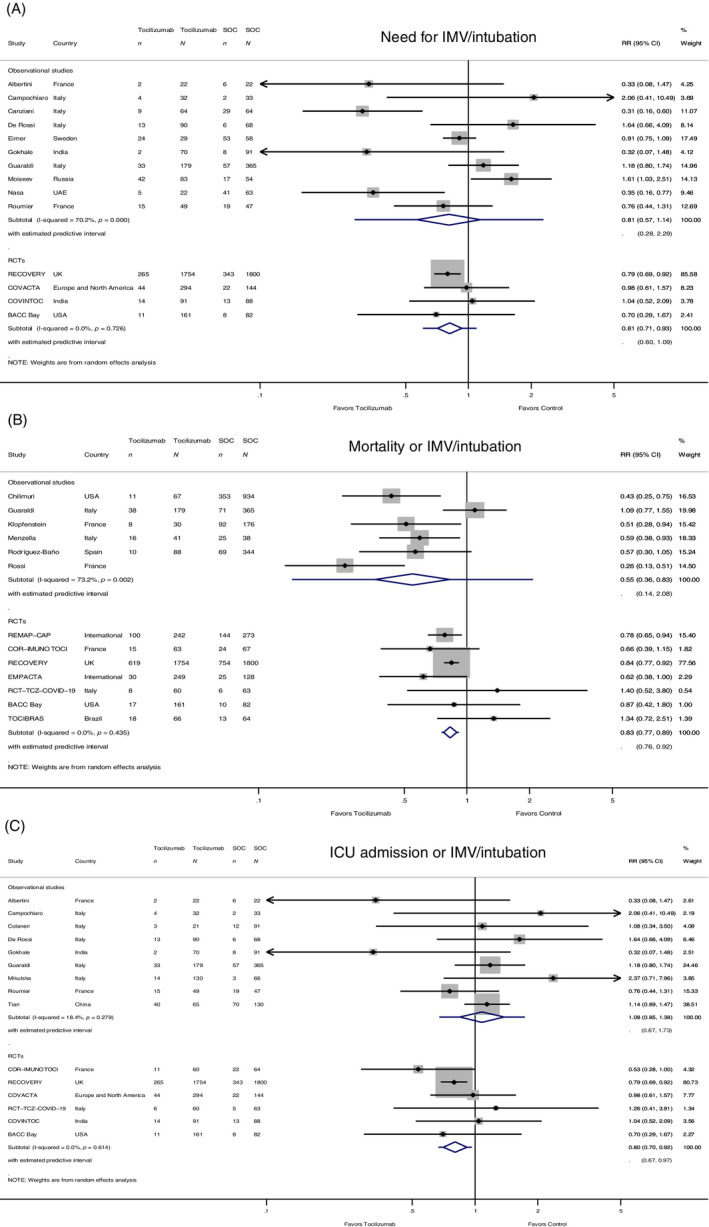
(A) Forest plot of risk ratios (RRs) for the need for invasive mechanical ventilation (IMV)/intubation comparing tocilizumab and control treatment (n, cases of need for IMV/intubation; N, group size). (B) Forest plot of the composite outcome of mortality or IMV/intubation RRs comparing tocilizumab and control treatment (n, cases of mortality or IMV/intubation; N, group size). (C) Forest plot of the composite outcome of intensive care unit (ICU) admission or IMV/intubation RRs comparing tocilizumab and control treatment (n, cases of ICU admission or IMV/intubation; N, group size). Sample sizes are given for participants receiving intervention and participants receiving standard of care treatment (SOC), included in the study, when data were available. Summary estimates are presented separately for observational studies and randomized controlled trials
Composite endpoint of mortality or IMV
Thirteen studies with a total of 9064 participants, of whom 3655 patients received tocilizumab, reported results on the composite outcome of mortality or IMV. In the seven RCTs (5986 participants; 2973 received tocilizumab), the composite adverse outcome was reduced by 17% in patients treated with tocilizumab (RR 0.83, 95% CI 0.77 to 0.89, 95% PI 0.76 to 0.92); small heterogeneity was observed (I 2 = 0.0%, p = 0.435). In the six observational studies (3087 participants; 682 received tocilizumab), the composite adverse outcome was however reduced by 45% (RR 0.55, 95% CI 0.36 to 0.83, 95% PI 0.14 to 2.08), with large heterogeneity among studies (I 2 = 73.2%) (Figure 3B).
Composite endpoint of ICU admission or IMV
Fifteen studies with a total of 6804 participants, of whom 3350 patients received tocilizumab, reported results on the composite endpoint of ICU admission or IMV. In the six RCTs (5233 participants; 2691 received tocilizumab), the adverse outcome was reduced by 20% in patients treated with tocilizumab (RR 0.80, 95% CI 0.70 to 0.92, 95% PI 0.67 to 0.97), with no heterogeneity (I 2 = 0.0%). In the nine observational studies (1571 participants; 659 patients were treated with tocilizumab), there was no significant difference between the two treatment groups (RR 1.08, 95% CI 0.85 to 1.38, 95% PI 0.67 to 1.73) (Figure 3C).
Duration of hospitalization
Four RCTs and 14 observational studies reported results for the duration of hospitalization (in days) for a total of 4653 participants, of whom 2202 patients received tocilizumab. From the studies reporting results for the median duration of hospitalization, in the three RCTs (1680 participants; 896 received tocilizumab), there was however a numerical reduction in the length of hospitalization between the two groups (−1.06 days, 95% CI −2.18 to 0.07, 95% PI −15.54 to 13.43) without reaching statistical significance, whereas in the nine observational studies (2161 participants; 941 received tocilizumab) there was no significant difference between the two treatment groups (−0.15 days, 95% CI −0.80 to 0.50, 95% PI −2.65 to 2.34). In both types of studies, there was very large heterogeneity (I 2 = 99.0% and 97.5% for RCTs and observational studies, respectively) (Figure S1 in the Supporting Information).
Subgroup analysis
Differences in mortality in non‐ICU and ICU‐treated patients
Of the nine RCTs reporting mortality data, only one was performed in the ICU setting. Of the 38 observational studies reporting mortality, 24 with a total of 11,630 participants of whom 2681 received tocilizumab were conducted in non‐ICU patients, whereas 14 studies with a total of 6956 participants of whom 1775 received tocilizumab were conducted in ICU patients. The use of tocilizumab was associated with an improvement in mortality, both in non‐ICU and in ICU patients (RR 0.69 and 0.75, respectively) (Figure S2 in the Supporting Information). In the only RCT that was performed in the ICU setting (REMAP‐CAP), 16 the survival benefit was significant (RR 0.83, 95% CI 0.70 to 0.99), with an effect comparable to that of all nine RCTs (RR 0.89) (Figure 2).
Differences in mortality in patients according to the concomitant use of systemic corticosteroids
Two RCTs and three observational studies reported mortality data separately in patients who received systemic corticosteroids as part of the standard of care regimen and those who did not receive corticosteroids.
In the two RCTs, there was no significant difference in mortality in the tocilizumab versus the control group (RR 0.99, 95% CI 0.79 to 1.24); this was evident both in patients who received corticosteroids (RR 0.92, 95% CI 0.67 to 1.24) and in those who did not receive corticosteroids (RR 1.12, 95% CI 0.93 to 1.34) (Figure 4A).
FIGURE 4.
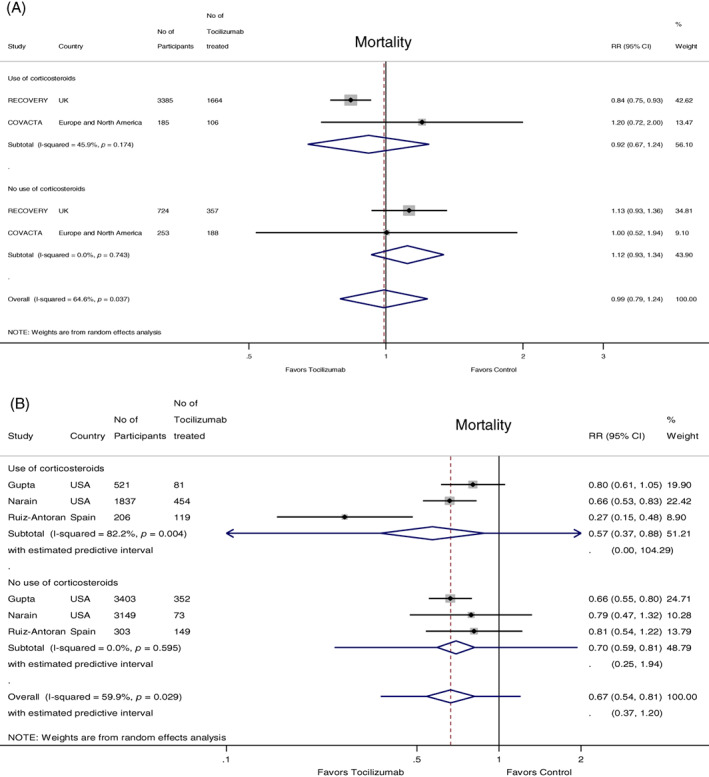
(A) Forest plot of mortality risk ratios (RRs) comparing the concomitant effect of tocilizumab versus control treatment in patients receiving or not receiving systemic corticosteroids in randomized controlled trials. (B) Forest plot of mortality RRs comparing the concomitant effect of tocilizumab versus control treatment in patients receiving or not receiving systemic corticosteroids in observational studies. Sample sizes are given for the total number of participants and the participants receiving intervention in the respective groups
In the observational studies (n = 3), the use of tocilizumab was associated with a lower mortality risk (RR 0.67, 95% CI 0.54 to 0.81); this effect was evident both in the patients who received corticosteroids (RR 0.57, 95% 0.37 to 0.88) and in those who did not receive corticosteroids (RR 0.70, 95% CI 0.59 to 0.81), with a trend for a greater benefit in the group of patients who received systemic corticosteroids (Figure 4B).
Publication bias and small study effect
Egger's test was not statistically significant when mortality was assessed as outcome for both RCTs (p = 0.155) and observational studies (p = 0.095), suggesting no such bias (Figure S3 in the Supporting Information). When ICU admission or IMV and only IMV were assessed as outcomes, Egger's test was also not significant for both RCTs and observational studies (p = 0.709 and p = 0.65, respectively, for ICU admission or IMV; p = 0.435 and p = 0.456, respectively, for IMV) suggesting no such bias (Figures S4 and S5 in the Supporting Information). Egger's test was statistically significant when mortality or IMV was assessed as an outcome for observational studies (p = 0.016), suggestive of bias. No such bias was observed when RCTs were considered (Egger's test p‐value = 0.885) (Figure S6 in the Supporting Information). Finally, no such bias was observed when median hospitalization days were assessed as outcome when observational studies (Egger's test p‐value = 0.676) were considered (Figure S7 in the Supporting Information).
DISCUSSION
In this meta‐analysis of 52 studies (nine RCTs and 43 observational studies that included 27,004 patients with COVID‐19, of whom 8048 were treated with tocilizumab), a significant survival benefit of tocilizumab versus usual care in both RCTs and observational studies was shown. In secondary analyses, there was a benefit regarding tocilizumab use both in the ICU and non‐ICU settings. In the studies providing data on the concomitant use of tocilizumab with systemic corticosteroids, there was no reduction in mortality with the concomitant use in the RCTs, while the reduction in mortality was evident in observational studies regardless of systemic corticosteroids use. Both RCTs and observational studies illustrated the positive effect of tocilizumab on the risk for intubation/IMV and in the composite outcome of mortality or IMV. Finally, we observed a benefit in favour of tocilizumab only in the RCTs providing data for the composite outcome of ICU admission or IMV.
The analysis of RCTs demonstrated an 11% reduction in mortality, despite some negative results from earlier small RCTs. 46 , 71 The mortality benefit was driven mainly by the large RECOVERY trial that included patients both in ICU and non‐ICU settings with progressive COVID‐19 (those with an oxygen saturation lower than 92% on room air or receiving oxygen therapy and evidence of systemic inflammation as expressed by CRP levels ≥75 mg/L), 15 and the REMAP‐CAP trial that included critically ill patients receiving organ support in intensive care. 16 A plausible explanation for this disagreement is that the RECOVERY and REMAP‐CAP trials involved more patients with severe or critical illness who were likely to have entered the CRS where anti‐inflammatory therapy is likely to be more beneficial. 79 Interestingly, it seems that the positive result of tocilizumab on survival in RECOVERY is likely a synergistic effect with corticosteroids, as shown by the RRs of 0.84 and 1.13 in patients who received and did not receive corticosteroids, respectively. Moreover, in REMAP‐CAP, steroids were used in 82% of participants (and 93.3% for patients enrolled post 17 June 2020). In contrast, in the early small RCTs, steroid use was likely less frequent. Based on this observation, we performed a secondary analysis of the concomitant steroid use in the RECOVERY 15 and COVACTA 66 trials that provided such data, where the observed trend for a benefit in favour of the combined treatment versus corticosteroids alone (RR 0.92) was driven mainly by the RECOVERY data. A similar effect was observed irrespective of the use of systemic corticosteroids in the analysis of observational studies. As all patients in need of oxygen supplementation are receiving systemic corticosteroids as standard of care nowadays, the additional benefit shown with the use of tocilizumab in patients treated with corticosteroids is of importance.
Similar findings were demonstrated in a recent meta‐analysis performed by the WHO of 27 RCTs (nine published and 18 unpublished) estimating the association between administration of IL‐6 antagonists (tocilizumab, sarilumab and siltuximab) compared with usual care or placebo and 28‐day all‐cause mortality. 80 In our meta‐analysis, we have additionally included 43 observational studies in support of the RCT data, and searched the utility of tocilizumab in the need for IMV, composite endpoints of mortality or IMV and ICU admission or IMV, length of hospitalization and differences in mortality in subgroups (ICU and non‐ICU patients and patients receiving or not receiving concomitant corticosteroids).
Observational studies also showed a beneficial survival effect with 31% mortality reduction. These studies are characterized by significant heterogeneity regarding participants' characteristics, study protocols, drug dosage and route of administration, standard of care regimens and, most importantly, a plausible selection bias in the decision for administration of tocilizumab. Notably, a large proportion of patients received concomitant corticosteroids (up to 60% overall; Table S1 in the Supporting Information) in these studies, and possibly in some instances in higher doses as life‐saving treatment. 81 In our secondary analysis, a reduction in mortality was observed irrespective of the use of systemic corticosteroids; however, combination therapy had a more pronounced effect on mortality (RR 0.57 in the corticosteroids group vs. 0.70 in the no‐corticosteroids group). The data from observational studies, despite their significant heterogeneity, overall support the observation in RCTs for a beneficial effect of tocilizumab on mortality, which may be more prominent in addition to systemic corticosteroids that anyway represent the cornerstone of treatment of patients with COVID‐19 and respiratory failure.
The beneficial effect of tocilizumab was also demonstrated by a significant reduction in the need for IMV by 19% in RCTs, with a similar trend in observational studies. The higher benefit in observational studies versus RCTs in the composite endpoint of mortality or IMV (45% vs. 17%) likely represents the higher mortality benefit in observational studies. Nevertheless, both these endpoints (mortality and the need for IMV) are relatively ‘hard’ endpoints, clearly reflecting the patients' needs. In contrast, we had contradictory results in the composite endpoint of the need for ICU admission or IMV, with a 20% reduction in the risk in RCTs and no beneficial effect in the observational studies. However, no difference was observed in hospitalization days in both types of studies. These contradictory results may reflect the setting of the studies, as ICU availability and admission criteria, as well as hospital discharge criteria, may differ in different parts of the world, and this is also reflected in the expected heterogeneity in the observational trials data.
Our data provide further support to guidelines that recommend the use of tocilizumab in combination with corticosteroids in hospitalized COVID‐19 patients recently admitted to the ICU or those outside ICU who are exhibiting rapid respiratory decompensation. 82 The aforementioned data were verified in the recent WHO meta‐analysis, demonstrating that the effect of tocilizumab is amplified when synchronously administered with corticosteroids. 80 Tocilizumab is likely effective when inflammatory, rather than infectious, mechanisms are predominant, modulating the levels of proinflammatory IL‐6 or its effects, thus reducing the duration and/or severity of COVID‐19 illness. Although the mechanisms of hyperinflammation and lung injury in COVID‐19 are still not entirely clear, cytokine storm in severely ill patients includes, besides elevated IL‐6, an increase in a wide spectrum of cytokines and inflammatory agents, including IL‐1β, IP‐10, TNF‐α, interferon‐γ, macrophage inflammatory protein 1α and 1β and Vascular Endothelial Growth Factor (VEGF). 83 Thus, it is plausible that combination therapy with corticosteroids will provide an effective anti‐inflammatory treatment umbrella, with IL‐6 blockade representing a central weapon, as higher IL‐6 levels were strongly associated with shorter survival in patients with COVID‐19. 84
Our analysis cannot clearly support the optimal timing for tocilizumab administration, as we showed that the drug was beneficial both in moderate‐to‐severe patients treated in non‐ICU settings, as well as in critical disease managed in the ICU. The latter finding is supported by the REMAP‐CAP RCT, 16 as well as by observational studies showing a 25% survival benefit in ICU, compared to 31% in non‐ICU patients. The cornerstone for the right timing for tocilizumab administration is the appropriate selection of the patients with moderate‐to‐severe disease, based on their clinical, radiological and inflammatory profile.
To the best of our knowledge, this is the first meta‐analysis investigating the efficacy of tocilizumab on multiple outcomes in patients hospitalized with COVID‐19, while previous analyses have reported only mortality events and ICU admissions. 85 , 86 , 87 This is also the largest meta‐analysis on this topic so far, involving 8048 patients in the tocilizumab group and 18,956 patients in the control group, with data representing worldwide findings, providing diversity in ethnic background. An additional strength of our systematic review is that we evaluated both observational studies and RCTs, in order to evaluate the efficacy and effectiveness of tocilizumab on various outcomes, both in its use in settings of regular clinical care and in the controlled settings of clinical trials, further strengthening the generalizability of our results. Observational studies are not designed to replace or oppose RCTs but to complement them and provide new insights into the use and outcomes possible with available therapies when used in a non‐RCT population and/or follow‐up setting. Although observational studies are not able to achieve the high internal validity of a registration RCT, when the analyses are performed in a wider population of everyday clinical practice, they can provide useful complementary data, helping to answer questions that RCTs do not or are unable to address. Small heterogeneity was observed in the outcomes of RCTs; however, large or very large heterogeneity was detected among observational studies, plausibly attributed to study design and time of randomization, disease severity, tocilizumab dosage and route of administration, the patients' inflammatory profile and the concomitant use of corticosteroids. Our study also has some limitations. First, we did not perform subgroup analyses according to the dosage and route of tocilizumab administration, due to the lack of specific data. Second, we did not analyse safety events from tocilizumab treatment, including thrombotic events or major bleeding, and bacterial or fungal infections, as this was not the aim of our study. Third, we decided not to include observational studies in preprint format from medRxiv. The methodological quality of COVID‐19 clinical research has overall been lower than similar non‐COVID‐19 publications. 88 Therefore, results of the analyses of observational studies should be evaluated with caution, due to their heterogeneity and often retrospective design; however, they are overall in agreement with the more robust results from RCTs, thus they build on the body of evidence and further support current treatment guidelines. Fourth, despite the fact that Egger's tests suggested no evidence of small study effect, except from the analysis of ICU admission or IMV/intubation, for observational studies, we observed evidence of asymmetry in some of the funnel plots. The evident asymmetry could arise due to reasons other than small study effect, such as differences in methodological quality, heterogeneity in intervention effects, variability in clinical settings and different protocols of the trials. Lastly, we cannot comment on the optimal timing for the use of tocilizumab and the patients who are most likely to benefit from its use, as the data available would not allow us to perform such analyses.
In conclusion, this systematic review and meta‐analysis of nine RCTs and 43 observational studies provides the most up‐to‐date and complete evidence for the role of tocilizumab in the management of COVID‐19. We demonstrated that the use of tocilizumab is associated with lower mortality and risk of intubation or need for mechanical ventilation in hospitalized COVID‐19 patients, with its benefit magnified when administered concomitantly with systemic corticosteroids. The optimal timing of administration and the patients who will benefit the most need to be evaluated in future appropriately designed trials.
CONFLICT OF INTEREST
None declared.
HUMAN ETHICS APPROVAL DECLARATION
Not applicable. The protocol for this systematic review was registered with PROSPERO (CRD42021247188 at https://www.crd.york.ac.uk/prospero/; for protocol details, see Appendix S2 in the Supporting Information).
AUTHOR CONTRIBUTIONS
Christos Kyriakopoulos: Conceptualization; investigation; methodology; project administration; validation; visualization; writing – original draft. Georgios Ntritsos: Data curation; formal analysis; investigation; methodology; resources; software; visualization; writing – original draft. Athena Gogali: Data curation; investigation; methodology; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Charalampos Milionis: Conceptualization; formal analysis; methodology; project administration; supervision; writing – original draft; writing – review and editing. Evangelos Evangelou: Data curation; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing – review and editing. Konstantinos Kostikas: Conceptualization; data curation; methodology; project administration; supervision; validation; writing – original draft; writing – review and editing.
Supporting information
Appendix S1. Search strategies.
Table S1. Studies with concomitant administration of corticosteroids and sample size for corticosteroids treatment (n) and total patients included (N) in the tocilizumab and standard of care treatment arm.
Figure S1. Forest plot of the median duration of hospitalization (in days) comparing tocilizumab and control treatment.
Figure S2. Forest plot of mortality risk ratios (RRs) comparing tocilizumab and control treatment in non‐ICU and ICU patients in observational studies.
Figure S3. Egger's test assessing mortality for RCTs and observational studies.
Figure S4. Egger's test assessing IMV/intubation for RCTs and observational studies.
Figure S5. Egger's test assessing ICU admission or IMV/intubation for RCTs and observational studies.
Figure S6. Egger's test assessing mortality or IMV/intubation for RCTs and observational studies.
Figure S7. Egger's test assessing median hospitalization days for observational studies.
Appendix S2. PROSPERO protocol registration.
Visual Abstract Tocilizumab administration for the treatment of hospitalized patients with COVID‐19: A systematic review and meta‐analysis.
Kyriakopoulos C, Ntritsos G, Gogali A, Milionis H, Evangelou E, Kostikas K. Tocilizumab administration for the treatment of hospitalized patients with COVID‐19: A systematic review and meta‐analysis. Respirology. 2021;26:1027–1040. 10.1111/resp.14152
Associate Editor: Conroy Wong Senior Editor: Paul King
REFERENCES
- 1. Zhou P, Yang X‐L, Wang X‐G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns Hopkins University of Medicine . COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021. [cited 2021 Apr 30]. Available from: https://coronavirus.jhu.edu/map.html
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR‐T cell therapy. Biomark Res. 2018;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384:693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, Dib SM, et al. Beneficial effects of colchicine for moderate to severe COVID‐19: a randomised, double‐blinded, placebo‐controlled clinical trial. RMD Open. 2021;7:e001455. 10.1136/rmdopen-2020-001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barkas F, Ntekouan SF, Kosmidou M, Liberopoulos E, Liontos A, Milionis H. Anakinra in hospitalized non‐intubated patients with coronavirus disease 2019: a systematic review and metaanalysis. Rheumatology. 2021. 10.1093/rheumatology/keab447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calabrese LH, Calabrese C. Baricitinib and dexamethasone for hospitalized patients with COVID‐19. Cleve Clin J Med. 2021. 10.3949/ccjm.88a.ccc073 [DOI] [PubMed] [Google Scholar]
- 9. Gremese E, Cingolani A, Bosello SL, Alivernini S, Tolusso B, Perniola S, et al. Sarilumab use in severe SARS‐CoV‐2 pneumonia. EClinicalMedicine. 2020;27:100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apaydın ÇB, Çınar G, Cihan‐Üstündağ G. Small‐molecule antiviral agents in ongoing clinical trials for COVID‐19. Curr Drug Targets. 2021. 10.2174/1389450122666210215112150 [DOI] [PubMed] [Google Scholar]
- 11. Vanhove B, Duvaux O, Rousse J, Royer P‐J, Evanno G, Ciron C, et al. High neutralizing potency of swine glyco‐humanized polyclonal antibodies against SARS‐CoV‐2. Eur J Immunol. 2021;51:1412–22. 10.1002/eji.202049072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77:1865–79. 10.1007/s40265-017-0829-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell‐induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–22. 10.1080/1744666X.2019.1629904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RECOVERY Collaborative Group . Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. Lancet. 2021;397:1637–45. 10.1016/S0140-6736(21)00676-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gordon AC, Mouncey PR, Al‐Beidh F, Rowan KM, Nichol AD, Arabi YM, et al. Interleukin‐6 receptor antagonists in critically ill patients with Covid‐19. N Engl J Med. 2021;384:1491–502. 10.1056/NEJMoa2100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid‐19 pneumonia. N Engl J Med. 2021;384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative survival analysis of immunomodulatory therapy for coronavirus disease 2019 cytokine storm. Chest. 2021;159:933–48. 10.1016/j.chest.2020.09.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davis JS, Ferreira D, Paige E, Gedye C, Boyle M. Infectious complications of biological and small molecule targeted immunomodulatory therapies. Clin Microbiol Rev. 2020;33:e00035‐19. 10.1128/CMR.00035-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Angriman F, Ferreyro BL, Burry L, Fan E, Ferguson ND, Husain S, et al. Interleukin‐6 receptor blockade in patients with COVID‐19: placing clinical trials into context. Lancet Respir Med. 2021;9:655–64. 10.1016/S2213-2600(21)00139-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). London: Cochrane; 2021. Available from: http://www.training.cochrane.org/handbook [Google Scholar]
- 24. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 25. Higgins JTP, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. Higgins JTP, Thomas J, Cochrane handbook for systematic reviews of interventions. [cited 2021 Aug 24]. Available from: London: Cochrane; 2021. https://training.cochrane.org/handbook/current/chapter-06 [Google Scholar]
- 26. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6:e010247. 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kulinskaya E, Dollinger MB. An accurate test for homogeneity of odds ratios based on Cochran's Q‐statistic. BMC Med Res Methodol. 2015;15:49. 10.1186/s12874-015-0034-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albertini L, Soletchnik M, Razurel A, Cohen J, Bidegain F, Fauvelle F, et al. Observational study on off‐label use of tocilizumab in patients with severe COVID‐19. Eur J Hosp Pharm Sci Pract. 2021;28:22–7. 10.1136/ejhpharm-2020-002414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, et al. Tocilizumab among patients with COVID‐19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–12. 10.1016/S2665-9913(20)30277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Campochiaro C, Della‐Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, et al. Efficacy and safety of tocilizumab in severe COVID‐19 patients: a single‐centre retrospective cohort study. Eur J Intern Med. 2020;76:43–9. 10.1016/j.ejim.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Canziani LM, Trovati S, Brunetta E, Testa A, De Santis M, Bombardieri E, et al. Interleukin‐6 receptor blocking with intravenous tocilizumab in COVID‐19 severe acute respiratory distress syndrome: a retrospective case‐control survival analysis of 128 patients. J Autoimmun. 2020;114:102511. 10.1016/j.jaut.2020.102511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Capra R, De Rossi N, Mattioli F, Romanelli G, Scarpazza C, Sormani MP, et al. Impact of low dose tocilizumab on mortality rate in patients with COVID‐19 related pneumonia. Eur J Intern Med. 2020;76:31–5. 10.1016/j.ejim.2020.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chachar AZK, Khan KA, Iqbal J, Shahid AH, Asif M, Fatima SA, et al. ‘Tocilizumab‐an option for patients with COVID‐19 associated cytokine release syndrome: a single center experience’, a retrospective study‐original article. Ann West Med Surg. 2021;63:102165. 10.1016/j.amsu.2021.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chilimuri S, Sun H, Alemam A, Kang KS, Lao P, Mantri N, et al. Tocilizumab use in patients with moderate to severe COVID‐19: a retrospective cohort study. J Clin Pharm Ther. 2021;46:440–6. 10.1111/jcpt.13303 [DOI] [PubMed] [Google Scholar]
- 38. Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe COVID‐19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8:695. 10.3390/microorganisms8050695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Rossi N, Scarpazza C, Filippini C, Cordioli C, Rasia S, Mancinelli CR, et al. Early use of low dose tocilizumab in patients with COVID‐19: a retrospective cohort study with a complete follow‐up. EClinicalMedicine. 2020;25:100459. 10.1016/j.eclinm.2020.100459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eimer J, Vesterbacka J, Svensson AK, Stojanovic B, Wagrell C, Sönnerborg A, et al. Tocilizumab shortens time on mechanical ventilation and length of hospital stay in patients with severe COVID‐19: a retrospective cohort study. J Intern Med. 2021;289:434–6. 10.1111/joim.13162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fisher MJ, Marcos Raymundo LA, Monteforte M, Taub EM, Go R. Tocilizumab in the treatment of critical COVID‐19 pneumonia: a retrospective cohort study of mechanically ventilated patients. Int J Infect Dis. 2021;103:536–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galván‐Román JM, Rodríguez‐García SC, Roy‐Vallejo E, Marcos‐Jiménez A, Sánchez‐Alonso S, Fernández‐Díaz C, et al. IL‐6 serum levels predict severity and response to tocilizumab in COVID‐19: an observational study. J Allergy Clin Immunol. 2021;147:72–80.e8. 10.1016/j.jaci.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gokhale Y, Mehta R, Kulkarni U, Karnik N, Gokhale S, Sundar U, et al. Tocilizumab improves survival in severe COVID‐19 pneumonia with persistent hypoxia: a retrospective cohort study with follow‐up from Mumbai, India. BMC Infect Dis. 2021;21:241. 10.1186/s12879-021-05912-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guaraldi G, Meschiari M, Cozzi‐Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–84. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID‐19. JAMA Intern Med. 2021;181:41–51. 10.1001/jamainternmed.2020.6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hermine O, Mariette X, Tharaux PL, Resche‐Rigon M, Porcher R, Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID‐19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. 10.1001/jamainternmed.2020.6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hill JA, Menon MP, Dhanireddy S, Wurfel MM, Green M, Jain R, et al. Tocilizumab in hospitalized patients with COVID‐19: clinical outcomes, inflammatory marker kinetics, and safety. J Med Virol. 2021;93:2270–80. 10.1002/jmv.26674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holt GE, Batra M, Murthi M, Kambali S, Santos K, Bastidas MVP, et al. Lack of tocilizumab effect on mortality in COVID19 patients. Sci Rep. 2020;10:17100. 10.1038/s41598-020-74328-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ip A, Berry DA, Hansen E, Goy AH, Pecora AL, Sinclaire BA, et al. Hydroxychloroquine and tocilizumab therapy in COVID‐19 patients – an observational study. PLoS One. 2020;15:e0237693. 10.1371/journal.pone.0237693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kewan T, Covut F, Al‐Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B. Tocilizumab for treatment of patients with severe COVID‐19: a retrospective cohort study. EClinicalMedicine. 2020;24:100418. 10.1016/j.eclinm.2020.100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klopfenstein T, Zayet S, Lohse A, Selles P, Zahra H, Kadiane‐Oussou NJ, et al. Impact of tocilizumab on mortality and/or invasive mechanical ventilation requirement in a cohort of 206 COVID‐19 patients. Int J Infect Dis. 2020;99:491–5. 10.1016/j.ijid.2020.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lewis TC, Adhikari S, Tatapudi V, Holub M, Kunichoff D, Troxel AB, et al. A propensity‐matched cohort study of tocilizumab in patients with coronavirus disease 2019. Crit Care Explor. 2020;2:e0283. 10.1097/CCE.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martínez‐Sanz J, Muriel A, Ron R, Herrera S, Pérez‐Molina JA, Moreno S, et al. Effects of tocilizumab on mortality in hospitalized patients with COVID‐19: a multicentre cohort study. Clin Microbiol Infect. 2021;27:238–43. 10.1016/j.cmi.2020.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Menzella F, Fontana M, Salvarani C, Massari M, Ruggiero P, Scelfo C, et al. Efficacy of tocilizumab in patients with COVID‐19 ARDS undergoing noninvasive ventilation. Crit Care. 2020;24:589. 10.1186/s13054-020-03306-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with COVID‐19 pneumonia. PLoS One. 2020;15:e0237831. 10.1371/journal.pone.0237831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moiseev S, Avdeev S, Tao E, Brovko M, Bulanov N, Zykova A, et al. Neither earlier nor late tocilizumab improved outcomes in the intensive care unit patients with COVID‐19 in a retrospective cohort study. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-219265 [DOI] [PubMed] [Google Scholar]
- 57. Nasa P, Singh A, Upadhyay S, Bagadia S, Polumuru S, Shrivastava PK, et al. Tocilizumab use in COVID‐19 cytokine‐release syndrome: retrospective study of two centers. Indian J Crit Care Med. 2020;24:771–6. 10.5005/jp-journals-10071-23566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. Tocilizumab use in COVID‐19‐associated pneumonia. J Med Virol. 2021;93:1023–8. 10.1002/jmv.26471 [DOI] [PubMed] [Google Scholar]
- 59. Patel K, Gooley TA, Bailey N, Bailey M, Hegerova L, Batchelder A, et al. Use of the IL‐6R antagonist tocilizumab in hospitalized COVID‐19 patients. J Intern Med. 2021;289:430–3. 10.1111/joim.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Potere N, Di Nisio M, Rizzo G, La Vella M, Polilli E, Agostinone A, et al. Low‐dose subcutaneous tocilizumab to prevent disease progression in patients with moderate COVID‐19 pneumonia and hyperinflammation. Int J Infect Dis. 2020;100:421–4. 10.1016/j.ijid.2020.07.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Quartuccio L, Sonaglia A, McGonagle D, Fabris M, Peghin M, Pecori D, et al. Profiling COVID‐19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129:104444. 10.1016/j.jcv.2020.104444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rajendram P, Sacha GL, Mehkri O, Wang X, Han X, Vachharajani V, et al. Tocilizumab in coronavirus disease 2019‐related critical illness: a propensity matched analysis. Crit Care Explor. 2021;3:e0327. 10.1097/CCE.0000000000000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rodríguez‐Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, et al. Treatment with tocilizumab or corticosteroids for COVID‐19 patients with hyperinflammatory state: a multicentre cohort study (SAM‐COVID‐19). Clin Microbiol Infect. 2021;27:244–52. 10.1016/j.cmi.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rodríguez‐Molinero A, Pérez‐López C, Gálvez‐Barrón C, Miñarro A, Macho O, López GF, et al. Matched cohort study on the efficacy of tocilizumab in patients with COVID‐19. One Health. 2021;12:100214. 10.1016/j.onehlt.2021.100214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rojas‐Marte G, Khalid M, Mukhtar O, Hashmi AT, Waheed MA, Ehrlich S, et al. Outcomes in patients with severe COVID‐19 disease treated with tocilizumab: a case‐controlled study. QJM. 2020;113:546–50. 10.1093/qjmed/hcaa206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid‐19 pneumonia. N Engl J Med. 2021;384:1503–16. 10.1056/NEJMoa2028700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rossi B, Nguyen LS, Zimmermann P, Boucenna F, Dubret L, Baucher L, et al. Effect of tocilizumab in hospitalized patients with severe COVID‐19 pneumonia: a case‐control cohort study. Pharmaceuticals. 2020;13:317. 10.3390/ph13100317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rossotti R, Travi G, Ughi N, Corradin M, Baiguera C, Fumagalli R, et al. Safety and efficacy of anti‐il6‐receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect. 2020;81:e11–7. 10.1016/j.jinf.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roumier M, Paule R, Vallée A, Rohmer J, Ballester M, Brun AL, et al. Tocilizumab for severe worsening COVID‐19 pneumonia: a propensity score analysis. J Clin Immunol. 2021;41:303–14. 10.1007/s10875-020-00911-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ruiz‐Antorán B, Sancho‐López A, Torres F, Moreno‐Torres V, de Pablo‐López I, García‐López P, et al. Combination of tocilizumab and steroids to improve mortality in patients with severe COVID‐19 infection: a Spanish, multicenter, cohort study. Infect Dis Ther. 2021;10:347–62. 10.1007/s40121-020-00373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID‐19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID‐19‐associated cytokine release syndrome (COVINTOC): an open‐label, multicentre, randomised, controlled, phase 3 trial. Lancet Respir Med. 2021;9:511–21. 10.1016/S2213-2600(21)00081-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Somers EC, Eschenauer GA, Troost JP, Golob JL, Gandhi TN, Wang L, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID‐19. Clin Infect Dis. 2020;73:e445–54. 10.1093/cid/ciaa954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stone JH, Frigault MJ, Serling‐Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383:2333–44. 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tian J, Zhang M, Jin M, Zhang F, Chu Q, Wang X, et al. Repurposed tocilizumab in patients with severe COVID‐19. J Immunol. 2021;206:599–606. 10.4049/jimmunol.2000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tsai A, Diawara O, Nahass RG, Brunetti L. Impact of tocilizumab administration on mortality in severe COVID‐19. Sci Rep. 2020;10:19131. 10.1038/s41598-020-76187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Veiga VC, Jagg P, Farias DLC, Rosa RG, Dourado LK, Zampieri FG, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. 10.1136/bmj.n84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zheng KL, Xu Y, Guo YF, Diao L, Kong XY, Wan XJ, et al. Efficacy and safety of tocilizumab in COVID‐19 patients. Aging. 2020;12:18878–88. 10.18632/aging.103988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid‐19. N Engl J Med. 2020;383:1757–66. 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 80. The WHO Rapid Evidence Appraisal for COVID‐19 Therapies (REACT) Working Group , Domingo P, Mur I, Mateo GM, del Mar Gutierrez M, Pomar V, et al. Association between administration of IL‐6 antagonists and mortality among patients hospitalized for COVID‐19: a meta‐analysis. JAMA. 2021;326:499–518. 10.1001/jama.2021.11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gogali A, Kyriakopoulos C, Kostikas K. Corticosteroids in COVID‐19: one size does not fit all. Eur Respir J. 2021;57:2100224. 10.1183/13993003.00224-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. NICE guideline [NG191]. COVID‐19 | Topic | NICE. [cited 2021 Apr 30]. Available from: https://www.nice.org.uk/guidance/conditions-and-diseases/respiratory-conditions/covid19
- 83. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. The COVID‐19 Treatment Guidelines Panel. Information on COVID‐19 Treatment, Prevention and Research. [cited 2021 Apr 26]. Available from: https://www.covid19treatmentguidelines.nih.gov/
- 85. Lan S‐H, Lai C‐C, Huang H‐T, Chang S‐P, Lu L‐C, Hsueh P‐R. Tocilizumab for severe COVID‐19: a systematic review and meta‐analysis. Int J Antimicrob Agents. 2020;56:106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kaye AG, Siegel R. The efficacy of IL‐6 inhibitor tocilizumab in reducing severe COVID‐19 mortality: a systematic review. PeerJ. 2020;8:e10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhao M, Lu J, Tang Y, Dai Y, Zhou J, Wu Y. Tocilizumab for treating COVID‐19: a systemic review and meta‐analysis of retrospective studies. Eur J Clin Pharmacol. 2021;77:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jung RG, Di Santo P, Clifford C, Prosperi‐Porta G, Skanes S, Hung A, et al. Methodological quality of COVID‐19 clinical research. Nat Commun. 2021;12:943. 10.1038/s41467-021-21220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search strategies.
Table S1. Studies with concomitant administration of corticosteroids and sample size for corticosteroids treatment (n) and total patients included (N) in the tocilizumab and standard of care treatment arm.
Figure S1. Forest plot of the median duration of hospitalization (in days) comparing tocilizumab and control treatment.
Figure S2. Forest plot of mortality risk ratios (RRs) comparing tocilizumab and control treatment in non‐ICU and ICU patients in observational studies.
Figure S3. Egger's test assessing mortality for RCTs and observational studies.
Figure S4. Egger's test assessing IMV/intubation for RCTs and observational studies.
Figure S5. Egger's test assessing ICU admission or IMV/intubation for RCTs and observational studies.
Figure S6. Egger's test assessing mortality or IMV/intubation for RCTs and observational studies.
Figure S7. Egger's test assessing median hospitalization days for observational studies.
Appendix S2. PROSPERO protocol registration.
Visual Abstract Tocilizumab administration for the treatment of hospitalized patients with COVID‐19: A systematic review and meta‐analysis.


