Abstract
Background
Large clinical trials have demonstrated the overall safety of vaccines for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). However, reports have emerged of autoimmune phenomena, including vaccine‐associated myocarditis, immune thrombocytopenia, and immune thrombotic thrombocytopenia.
Case Presentation
Here we present a novel case of a young woman who developed life‐threatening autoimmune hemolytic anemia (AIHA) after her first dose of a SARS‐CoV‐2 mRNA vaccine. Notably, initial direct antiglobulin testing was negative using standard anti‐IgG reagents, which are “blind” to certain immunoglobulin (IgG) isotypes. Further testing using an antiglobulin reagent that detects all IgG isotypes was strongly positive and confirmed the diagnosis of AIHA. The patient required transfusion with 13 units of red blood cells, as well as treatment with corticosteroids, rituximab, mycophenolate mofetil, and immune globulin.
Conclusion
As efforts to administer SARS‐CoV‐2 vaccines continue globally, clinicians must be aware of potential autoimmune sequelae of these therapies.
Keywords: autoimmune hemolytic anemia, direct antiglobulin test, SARS‐CoV‐2 mRNA vaccine
Abbreviations
- AHG
anti‐human globulin
- AIHA
autoimmune hemolytic anemia
- ANA
anti‐nuclear antibodies
- CMV
cytomegalovirus
- DAT
direct antiglobulin test
- EBV
epstein barr birus
- HIV
human immunodeficiency virus
- IgG
immunoglobulin
- IVIG
IV immune globulin
- LDH
lactate dehydrogenase
- MCV
mean corpuscular volume
- MGH
Massachusetts General Hospital
- MMF
mycophenolate mofetil
- mRNA
messenger RNA
- RBCs
red blood cells
- RSV
respiratory syncytial virus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
1. CASE REPORT
A 41‐year‐old woman received her first dose of the SARS‐CoV‐2 mRNA‐1273 vaccine in March 2021. Seven days after vaccination, she noted fatigue and dark urine. Twenty days after vaccination, she was admitted to another hospital, where initial laboratory findings were notable for hemoglobin 7.1 g/dl, mean corpuscular volume (MCV) 93 fl, reticulocyte count 15.5%, total bilirubin 3.7 mg/dl, direct bilirubin 0.8 mg/dl, haptoglobin <8 mg/dl, and lactate dehydrogenase (LDH) 746 U/L (additional laboratory values shown in Table 1). Her baseline hemoglobin was 14 g/dl, last assessed 2 years prior. The direct antiglobulin test (DAT) was negative for IgG and C3d, although an eluate prepared from the patient's red blood cells (RBCs) was reactive against all test cells. She was transfused a total of 7 units of RBCs, started on prednisone (oral, 1 mg/kg/day), and discharged home with a hemoglobin of 7.3 g/dl.
TABLE 1.
Laboratory data
| Reference range, adult | Upon presentation to initial hospital a | Day of admission to MGH b | 4 weeks after discharge from MGH c | |
|---|---|---|---|---|
| Hematology | ||||
| Hemoglobin (g/dl) | 12.0–16.0 | 7.1 | 7.3 | 10.9 |
| Hematocrit (%) | 36.0–46.0 | 19.5 | 21.9 | 31.6 |
| Mean corpuscular volume (fl) | 80–100 | 93 | 109 | 103.9 |
| RBC distribution width (%) | 11.5–14.5 | 20.7 | 31.5 | 15.9 |
| White blood cell count (per/μl) | 4500–13,000 | 15,000 | 19,000 | 8860 |
| Platelets (per/μl) | 150,000‐400,000 | 204,000 | 142,000 | 238,000 |
| Hemolysis Labs | ||||
| Reticulocyte (%) | 0.7–2.5 | 15.5 | >30.0 | 12.4 |
| Total Bilirubin (mg/dl) | 0.0–1.0 | 3.7 | 3.9 | 0.9 |
| Direct Bilirubin (mg/dl) | 0.0–0.4 | 0.8 | 0.6 | ‐ |
| Haptoglobin (mg/dl) | >10 | <8 | <10 | <10 |
| Lactate dehydrogenase (U/L) | 100–210 | 746 | 3095 | 317 |
| Alanine aminotransferase (U/L) | 7–33 | 50 | 139 | 41 |
| Aspartate aminotransferase (U/L) | 9–32 | 40 | 181 | 23 |
| Chemistry | ||||
| Sodium (mmol/L) | 135–145 | 137 | 137 | 139 |
| Potassium (mmol/L) | 3.4–5.0 | 4.0 | 4.1 | 4.1 |
| Chloride (mmol/L) | 98–108 | 105 | 100 | 103 |
| Bicarbonate (mmol/L) | 23–32 | 26 | 24 | 26 |
| BUN (mg/dl) | 8–25 | 18 | 26 | 15 |
| Creatinine (mg/dl) | 0.60–1.50 | 0.70 | 0.77 | 0.62 |
| Glucose (mg/dl) | 70–110 | 95 | 150 | 147 |
| Calcium (mg/dl) | 8.5–10.5 | 9.0 | 9.0 | 8.5 |
| Total protein (g/dl) | 6.0–8.3 | 7.6 | 7.5 | 6.8 |
| Albumin (g/dl) | 3.3–5.0 | 4.0 | 4.5 | 4.1 |
| Alkaline phosphatase (IU/L) | 30–100 | 70 | 78 | 63 |
Note: References ranges are those used at Massachusetts General Hospital (MGH).
20 days after vaccination.
35 days after vaccination.
77 days after vaccination.
The patient continued to feel unwell, and 2 days after discharge from the other hospital (35 days after vaccination), she presented to Massachusetts General Hospital (MGH) with persistent fatigue and dyspnea on exertion. She had a history of central retinal vein occlusion and hypertension. Medications included aspirin (oral, 81 mg/day) and metoprolol succinate (oral, 100 mg/day); she remained on prednisone (oral, 1 mg/kg/day), initiated at the other hospital. The patient worked as an attorney and lived with her husband and three children. She had no personal or family history of autoimmune disease.
On admission to MGH, the patient was in mild distress. Vital signs were notable for temperature 96.9F, blood pressure 133/78 mm Hg, heart rate 79 beats/min, respiratory rate 18 breaths/min, and oxygen saturation 98% on room air. Laboratory values revealed hemoglobin 7.3 g/dl, MCV 109 fl, reticulocyte count >30%, total bilirubin 3.9 mg/dl, direct bilirubin 0.6 mg/dl, haptoglobin <10 mg/dl, and LDH 3095 U/L (additional laboratory values shown in Table 1). A peripheral blood film revealed numerous spherocytes, reticulocytes, and nucleated RBCs. Testing for HIV, HBV, EBV, CMV, tick‐borne illnesses, influenza, and RSV was negative. ANA was weakly positive at 1:80. Serum immunofixation and serum‐free light chains were within normal limits, and flow cytometry did not reveal a monoclonal B cell or T cell population.
Repeat DAT at MGH was again negative using routine polyspecific, IgG and C3 reagents in tube testing (Gamma‐clone, Immucor, Norcross, GA). The plasma antibody screen was also negative (MTS gel cards, Ortho Clinical Diagnostics, Pamano Beach, FL). Despite the negative DAT, antibody was eluted from the patient's RBCs that demonstrated strong (3+) panreactivity when tested in the MTS gel system. A subsequent DAT performed using MTS gel cards—rather than Gamma‐clone—was positive (2+) for IgG. Further testing demonstrated the presence of a cold autoantibody that was reactive (3+) at 37 degrees.
Prednisone (oral, 1 mg/kg per day) was continued upon admission to MGH. On hospital day 2, the patient received rituximab infusion (1000 mg). On hospital day 5, mycophenolate mofetil (MMF, oral, 1000 mg twice daily) was added (Figure 1). She received IV immune globulin (IVIG, 1 g/kg) on hospital days 7 and 8, as well as a second infusion of rituximab (1000 mg) on hospital day 11.
FIGURE 1.
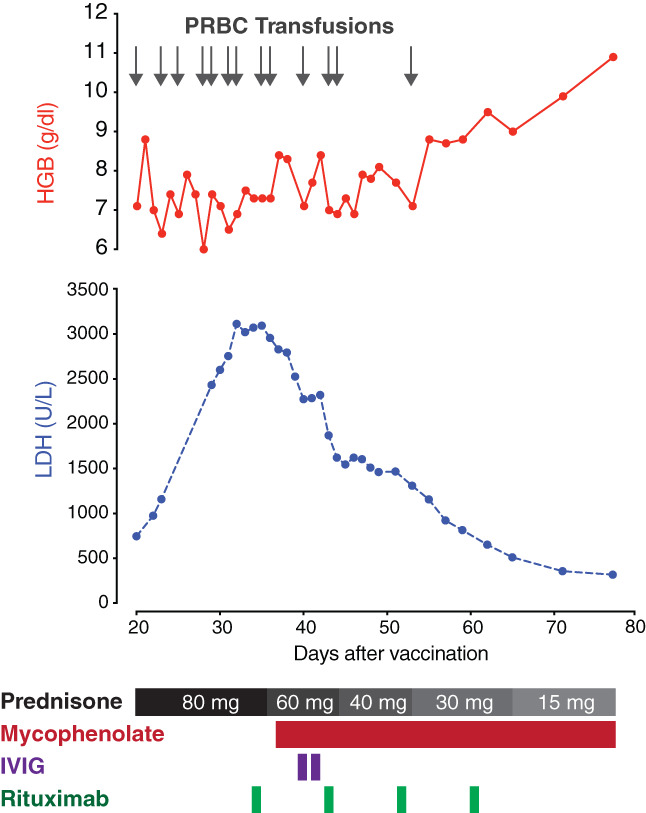
Hemoglobin and LDH trends, and response to treatment. The X‐axis represents the number of days following receipt of the first dose of the SARS‐CoV‐2 mRNA‐1273 vaccine. The Y‐axes represent hemoglobin (above) and LDH concentrations (below). Gray arrows represent RBC transfusions. Black and gray rectangles indicate the prednisone taper. MMF, IVIG, and rituximab are depicted in red, purple, and green, respectively. IVIG, intravenous immune globulin; LDH, lactate dehydrogenase; MMF, mycophenolate mofetil; pRBCs, red blood cells [Color figure can be viewed at wileyonlinelibrary.com]
On day 8 of admission to MGH, the patient's LDH began to fall, and her hemoglobin stabilized at 8 g/dl after receiving an additional 6 units of RBCs (Figure 1). She was discharged home from MGH on hospital day 14 (49 days after receiving the vaccine). She was given two additional weekly doses of rituximab (1000 mg) as an outpatient, and corticosteroids and MMF were tapered. At an office visit 4 weeks after discharge from MGH and 11 weeks after receipt of the vaccine, her hemoglobin was 10.9 g/dl (no RBC transfusion in the preceding 3 weeks) and LDH was 317 U/L. A second dose of the vaccine was deferred. Testing for antibodies to the SARS‐CoV‐2 virus, performed 11 weeks after receipt of a single dose of the vaccine, was negative.
Autoimmune hemolytic anemia (AIHA) is a rare disorder characterized by the production of autoantibodies against RBC antigens, leading to hemolysis. AIHA can either be primary or occur secondary to rheumatologic conditions, lymphoproliferative disorders, infection, or medications. 1 In the present case, there was a strong clinical suspicion that the SARS‐CoV‐2 mRNA vaccine was the inciting event for the AIHA based on the chronology of events, the atypical autoantibody profile, and the absence of other causes. 2 , 3 , 4 , 5
Diagnosis of AIHA is typically made based on clinical presentation, with a DAT confirming the presence of autoantibody on patient RBCs. False‐negative DATs may occur if a patient makes an uncommon immunoglobulin isotype that is not recognized by the anti‐human globulin (AHG) reagent used. 6 In our case, the patient's initial DAT was negative when tested with Gamma‐clone (Immucor) anti‐IgG reagent, which does not recognize IgG4 or some types of IgG3, but positive when a reagent that detects all IgG isotypes (MTS gel system, Ortho) was used during the elution studies. These unusual findings suggest that the warm (IgG) autoantibody consisted of an atypical subtype of either IgG4 or IgG3. Workup also revealed the presence of a cold autoantibody with a thermal amplitude of 37°C, indicating that our patient may have developed a mixed warm/cold AIHA, which comprise approximately 8% of cases of AIHA. 7 It is difficult to discern the precise contribution of the cold autoantibody to this patient's illness given that the DAT did not detect complement; this could suggest a clinically irrelevant cold autoantibody or one that is potent and resulted in brisk hemolysis and a false‐negative DAT.
There have been cases of AIHA reported following vaccination to other pathogens. 8 Vaccines activate both the humoral and cell‐mediated arms of the adaptive immune system by production of effector and memory cells. The mechanism underpinning vaccine‐induced autoimmunity is not clear, but it may be due to molecular mimicry and the development of auto‐reactive T cells in a susceptible host. 9
The cornerstone of treatment for AIHA is immunosuppression, with corticosteroids serving as first‐line therapy in warm and mixed AIHA. 1 Second‐line therapy for AIHA includes rituximab, a monoclonal antibody directed at CD20. In a meta‐analysis of 409 patients with AIHA refractory to corticosteroids, the overall response rate to rituximab was 73%. 10 Third‐line agents include azathioprine, cyclosporine, cyclophosphamide, and MMF. IVIG may be used in combination with other treatments, especially in cases of severe hemolysis. Splenectomy remains an option as well, with an approximately 80% response rate, with 20%–50% of patients achieving sustained response. 1 Clinical trials are ongoing evaluating the safety and efficacy of novel treatments for AIHA, including the Syk inhibitor fostamatinib (clinicaltrials.gov NCT04138927), Bruton tyrosine kinase inhibitor ibrutinib (NCT04398459), and neonatal Fc receptors (NCT04119050).
Our patient developed a robust AIHA 1 week after receiving her first dose of the SARS‐CoV‐2 mRNA vaccine. Although causation cannot be proven, the temporal relationship in the absence of an alternative cause argues in favor of de novo vaccine‐induced AIHA.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
INFORMED CONSENT
The patient provided written consent for publication of this case.
DISCLOSURES
H. Al‐Samkari declares the following universal disclosures: Research funding to institution (Agios, Dova, Amgen); Consultancy (Agios, Dova, Argenx, Rigel, Sobi, Novartis, Moderna). J. Lo has served as a consultant for Viiv Healthcare and Gilead Sciences. All other authors have no relevant conflicts of interest to disclose.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Walter Dzik and Mandakolathur Murali for their help in the laboratory evaluation of this patient.
Gadi SRV, Brunker PAR, Al‐Samkari H, Sykes DB, Saff RR, Lo J, et al. Severe autoimmune hemolytic anemia following receipt of SARS‐CoV‐2 mRNA vaccine. Transfusion. 2021;61:3267–3271. 10.1111/trf.16672
REFERENCES
- 1. Barcellini W, Fattizzo B. How I treat warm autoimmune hemolytic anemia. Blood. 2021;137(10):1283–94. 10.1182/blood.2019003808 [DOI] [PubMed] [Google Scholar]
- 2. Advisory Committee on Immunization Practices (ACIP) COVID‐19 VaST Work Group Technical Report – May 17, 2021. 2021.
- 3. Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, et al. Thrombocytopenia following Pfizer and Moderna SARS‐CoV‐2 vaccination. Am J Hematol. 2021;96(5):534–7. 10.1002/ajh.26132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund‐Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384 2124–30. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384 2092–101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howie HL, Delaney M, Wang X, Er LS, Vidarsson G, Stegmann TC, et al. Serological blind spots for variants of human IgG3 and IgG4 by a commonly used anti‐immunoglobulin reagent. Transfusion. 2016;56(12):2953–62. 10.1111/trf.13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barcellini W, Fattizzo B, Zaninoni A, Radice T, Nichele I, Di Bona E, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–6. 10.1182/blood-2014-06-583021 [DOI] [PubMed] [Google Scholar]
- 8. Seltsam A, Shukry‐Schulz S, Salama A. Vaccination‐associated immune hemolytic anemia in two children. Transfusion. 2000;40(8):907–9. 10.1046/j.1537-2995.2000.40080907.x [DOI] [PubMed] [Google Scholar]
- 9. Segal Y, Shoenfeld Y. Vaccine‐induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–94. 10.1038/cmi.2017.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reynaud Q, Durieu I, Dutertre M, Ledochowski S, Durupt S, Michallet A‐S, et al. Efficacy and safety of rituximab in auto‐immune hemolytic anemia: a meta‐analysis of 21 studies. Autoimmun Rev. 2015;14(4):304–13. 10.1016/j.autrev.2014.11.014 [DOI] [PubMed] [Google Scholar]


