The coronavirus disease 2019 (COVID‐19) pandemic has had a significant impact on the provision of maternal healthcare and maternal and fetal outcomes around the world 1 , 2 , 3 , 4 . An increase in maternal morbidity and mortality has been identified and attributed to a number of causes 5 . These include difficulties faced by healthcare systems in adapting to rapidly changing circumstances during the pandemic and inequity in service provision globally according to the income status of the country 6 .
In general, women are at increased risk of infection during pregnancy. Alterations in immune function and increased physiological demand on maternal metabolism can lead to more complicated recovery and worse outcome 7 . In particular, pregnant women are at increased risk of severe respiratory illness, for example, influenza 8 , 9 . During the COVID‐19 pandemic, the relationship between severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and maternal health has been explored in a number of large‐scale cohort studies and meta‐analyses of the current literature. These studies have highlighted an apparent link between COVID‐19 and pre‐eclampsia 10 , 11 , 12 , but it is not currently known whether this association is causal.
In 1965, the English statistician Sir Austin Bradford Hill proposed a set of nine criteria to assess the causality of the relationship between a presumed cause and an observed effect 13 . While some advocate against the exclusive use of these criteria to judge causality, arguing, for example, that scientific deduction is more powerful, they are still widely accepted and applied. The criteria are: (1) the strength of the association (effect size), i.e. the larger the association, the greater the likelihood that the relationship is causal; (2) consistency (reproducibility), i.e. consistent findings observed by different persons in different places with different samples strengthens the likelihood of an effect being causal; (3) specificity, i.e. causation is likely if there is a very specific population at a specific site and disease with no other likely explanation; (4) temporal sequence; (5) biological gradient (dose–response relationship), i.e. greater exposure should generally lead to greater incidence of the effect; (6) plausibility; (7) coherence (between epidemiological and laboratory findings); (8) experimental evidence; and (9) analogous evidence. Some authors also include reversibility, i.e. if the cause is removed, the effect should disappear. Based on the published literature, we assessed the causality of the relationship between SARS‐CoV‐2 infection in pregnancy and the development of pre‐eclampsia using the Bradford Hill criteria.
Strength of the association
A large‐scale national cohort study of 342 090 women was conducted in England between 29 May 2020 and 31 July 2021, as part of the National Maternity and Perinatal Audit 14 . The study found that women testing positive for SARS‐CoV‐2 at the time of birth had higher rates of fetal death, preterm delivery, pre‐eclampsia or eclampsia and delivery by emergency Cesarean section, as compared with women without a positive test for SARS‐CoV‐2. The rate of pre‐eclampsia or eclampsia was 3.9% in women with SARS‐CoV‐2 infection compared with 2.5% in those without (adjusted odds ratio (aOR), 1.55; 95% CI, 1.29–1.85; P < 0.001).
The INTERCOVID cohort study 10 , a large‐scale multinational study that assessed pregnancy outcome in 43 institutions across 18 countries, compared a total of 706 pregnant women diagnosed with COVID‐19 and 1424 pregnant women without COVID‐19. This study found that women with COVID‐19 were at increased risk of pre‐eclampsia, eclampsia and hemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome (8.4% vs 4.4%; relative risk (RR), 1.76; 95% CI, 1.27–2.43). Women with either asymptomatic or symptomatic SARS‐CoV‐2 infection who had risk factors for pre‐eclampsia, such as increased body mass index (BMI), diabetes, pre‐existing hypertension or other chronic comorbidities, were found to have a 4 times greater risk of developing pre‐eclampsia or eclampsia compared with women who did not have SARS‐CoV‐2 infection. Women diagnosed with COVID‐19 were also at increased risk of preterm birth (RR, 1.59; 95% CI, 1.30–1.94). The majority (83%) of preterm births in women diagnosed with COVID‐19 were medically indicated; the leading indication was pre‐eclampsia/eclampsia/HELLP syndrome (24.7%). Moreover, when the maternal morbidity and mortality index was calculated, women with symptomatic SARS‐CoV‐2 infection, compared to women with asymptomatic infection, were found to have a higher incidence of several pregnancy complications, including pregnancy‐induced hypertension, pre‐eclampsia, eclampsia, HELLP syndrome and maternal death 10 .
Conde‐Agudelo and Romero recently performed a systematic review of 28 studies that included 790 954 pregnant women across the globe, of whom 15 524 were diagnosed with SARS‐CoV‐2 infection 12 . The meta‐analysis of aORs demonstrated that SARS‐CoV‐2 infection during pregnancy was associated with a significant increase in the odds of pre‐eclampsia (pooled aOR, 1.58; 95% CI, 1.39–1.80; P < 0.0001; I 2 = 0%; 11 studies). There was also an increased risk of severe pre‐eclampsia (pooled aOR, 1.76; 95% CI, 1.18–2.63; I 2 = 58%; seven studies), eclampsia (pooled aOR, 1.97; 95% CI, 1.01–3.84; I 2 = 0%; three studies) and HELLP syndrome (pooled aOR, 2.10; 95% CI, 1.48–2.97; one study) in women with SARS‐CoV‐2 infection.
Some large cohort studies that highlighted important outcomes in pregnant women with COVID‐19, such as increased maternal morbidity in UK, USA and Mexican populations 15 , 16 , were not designed specifically to assess the incidence of pre‐eclampsia or other hypertensive disorders of pregnancy, so were not able to add data to address this question.
Consistency (reproducibility)
Of the 28 studies included in the systematic review and meta‐analysis by Conde‐Agudelo and Romero 12 , 14 were conducted in North America, six in Europe, five in Asia and two in Latin America. The remaining study was performed across 18 countries. This meta‐analysis assessed heterogeneity among studies by visually inspecting forest plots and by estimating I 2. Significant heterogeneity was predefined as an I 2 value ≥ 30%. The prespecified subgroups analyzed to explore potential sources of heterogeneity were defined according to the severity of SARS‐CoV‐2 infection (asymptomatic vs symptomatic), study design (retrospective cohort vs prospective cohort vs cross‐sectional), assessment of the association as a primary vs secondary aim, whether confounding factors were controlled for (yes vs no), geographic location (North America vs Europe vs Asia vs Latin America vs multiregion), sample size (< 200 vs 200–999 vs 1000–5000 vs > 5000), test used for diagnosing SARS‐CoV‐2 infection (reverse‐transcription polymerase chain reaction (RT‐PCR) vs RT‐PCR or antigens vs antibodies in serum vs mixed/unclear) and timing of the diagnosis of SARS‐CoV‐2 infection (at any time during pregnancy vs at admission for delivery). The impact of risk of bias on the results was also examined by performing a sensitivity analysis that included only studies with a low risk of bias.
The analysis demonstrated that the direction and magnitude of the effect of SARS‐CoV‐2 infection during pregnancy on the risk of pre‐eclampsia was consistent across most prespecified subgroup and sensitivity analyses. However, smaller studies (< 200 women), those with a retrospective design that did not adjust for confounding factors and those from Asia, reported slightly higher ORs than larger, cross‐sectional studies that did adjust for confounding factors.
It should be recognized that the meta‐analysis was dominated by two large cross‐sectional studies, one from the UK 14 and the other from the USA 17 , which collectively contributed 748 526 (94.6%) of the 790 954 pregnant women included in the meta‐analysis, which could potentially temper the conclusions drawn regarding reproducibility among different countries and ethnicities. However, the UK study 14 , which included white (76.3%), Asian (12.2%) and black (4.6%) pregnant women, found that the association between SARS‐CoV‐2 and pre‐eclampsia persisted even after multiple regression adjusting for maternal age, ethnicity, parity, pre‐existing diabetes mellitus, pre‐existing hypertension and socioeconomic deprivation measured using the index of multiple deprivation 2019.
In summary, there is good evidence of the consistency of the association between SARS‐CoV‐2 infection in pregnancy and pre‐eclampsia, but further evidence is needed.
Specificity
Pre‐eclampsia is a disease specific to pregnancy. The risk factors for the development of pre‐eclampsia are well‐documented and include hypertensive disease in previous pregnancy, chronic hypertension, chronic renal disease, diabetes mellitus or autoimmune disease, nulliparity, maternal age ≥ 40 years, BMI ≥ 35 kg/m2, family history of pre‐eclampsia, interpregnancy interval > 10 years and conception by in‐vitro fertilization 18 , 19 , 20 . Low‐dose aspirin, started before 15 weeks' gestation, reduces the risk of pre‐eclampsia in high‐risk women 21 .
It is not clear whether pregnant women are at increased risk of contracting SARS‐CoV‐2 infection, but the risk factors for developing more severe COVID‐19 in pregnancy are similar to those in non‐pregnant individuals, namely being of black, Asian or minority ethnicity, being overweight/obese and having a chronic comorbidity (in particular, asthma and hypertension) 22 , 23 . The overlap in risk factors for pre‐eclampsia and severe COVID‐19 highlights the potential for confounding of the association between the two conditions.
The INTERCOVID study 24 found that women who were overweight at the first antenatal visit and who were subsequently diagnosed with COVID‐19 had the highest risk of pre‐eclampsia/eclampsia (RR, 2.62; 95% CI, 1.57–4.36), which suggests that being overweight modifies the effect of COVID‐19 exposure.
The 28 studies included in the meta‐analysis performed by Conde‐Agudelo and Romero 12 varied significantly in the maternal factors for which analyses were adjusted, but most adjusted for maternal age, BMI, pre‐existing comorbidities and race/ethnicity. Fourteen studies did not adjust for any confounders or perform any matching of variables. Four of the studies were designed specifically to evaluate the association between SARS‐CoV‐2 infection during pregnancy and pre‐eclampsia 10 , 25 , 26 , 27 . Of these, one 25 did not adjust for any confounding factors, one 26 adjusted for race and parity, one 27 adjusted for race, BMI, use of low‐dose aspirin and chronic hypertension, and one 10 adjusted for maternal age, parity, cigarette smoking, being overweight/obese, history of diabetes, cardiac disease, hypertension or renal disease, and history of adverse pregnancy outcome. The unadjusted OR (95% CI) for the association between SARS‐CoV‐2 infection during pregnancy and pre‐eclampsia in these four studies were, respectively, 1.94 (1.09–3.46), 1.33 (0.64–2.75), 1.76 (1.01–3.05) and 1.93 (1.34–2.78). These are comparable to the pooled OR of 1.62 (95% CI, 1.45–1.82) among all 28 studies included in the meta‐analysis 12 . It is clear that adjustment for the known risk factors for pre‐eclampsia has been incomplete at best, but these results suggest that the relationship between SARS‐CoV‐2 infection in pregnancy and subsequent pre‐eclampsia is maintained even after adjustment for some of these potential confounding factors.
Temporal sequence
The systematic review by Conde‐Agudelo and Romero 12 included studies performed in women with a diagnosis of SARS‐CoV‐2 infection at any point in pregnancy. Of the 28 studies, 15 included women diagnosed with SARS‐CoV‐2 infection at any point during pregnancy; the other 13 studies included women in whom infection was diagnosed at the time of admission for delivery. These latter 13 studies included the two studies from the UK 14 and USA 17 that collectively contributed 94.6% of the 790 954 pregnant women included in the meta‐analysis. It is, therefore, unlikely that any meaningful information on the temporal relationship between SARS‐CoV‐2 infection and the development of pre‐eclampsia can be drawn from these 13 studies.
Few studies have focused on women in whom pre‐eclampsia preceded a diagnosis of SARS‐CoV‐2 infection. In one study that did 28 , among 1223 SARS‐CoV‐2‐positive pregnant women, there were 51 cases of pre‐eclampsia, of which 21 were diagnosed before SARS‐CoV‐2 infection, seven at the same gestational age as SARS‐CoV‐2 infection and 23 after SARS‐CoV‐2 infection. When the 21 women in whom pre‐eclampsia was diagnosed before SARS‐CoV‐2 infection were compared with those who did not develop pre‐eclampsia, there was a trend towards an increased risk of subsequently developing moderate or severe COVID‐19 (unadjusted RR, 2.28 (95% CI, 0.92–5.61) (P = 0.07); adjusted RR (aRR), 1.96 (95% CI, 0.8–4.84) (P = 0.14)).
In the study 28 , among the 23 cases of pre‐eclampsia diagnosed after SARS‐CoV‐2, the median interval from diagnosis of SARS‐CoV‐2 infection to diagnosis of pre‐eclampsia was 16 (interquartile range (IQR), 7–61) days. Only one other study 26 reported on the time from diagnosis of SARS‐CoV‐2 infection to diagnosis of pre‐eclampsia. In the study, the median interval was 3.79 (IQR, 0.43–13.0) weeks. The hazard ratio for this association was 2.88 (95% CI, 1.20–6.93) for infection diagnosed before 32 weeks and 2.74 (95% CI, 0.98–7.71) for infection diagnosed at or after 32 weeks' gestation.
In the absence of prospective cohort studies of pregnant women with and without a diagnosis of SARS‐CoV‐2 infection evaluating subsequent development of pre‐eclampsia, there is likely to be significant under‐reporting of women who had SARS‐CoV‐2 infection but did not go on to develop pre‐eclampsia. In most women included in studies in the literature, the diagnosis of SARS‐CoV‐2 infection was made in the third trimester; given that the pathophysiology of pre‐eclampsia is thought to originate in the first and early second trimesters, it might be expected that any causal relationship with SARS‐CoV‐2 infection would be more readily established at these earlier gestational ages.
In conclusion, the temporal relationship between COVID‐19 and pre‐eclampsia has been suggested by studies but has not been confirmed.
Biological gradient (dose–response relationship)
In the systematic review by Conde‐Agudelo and Romero 12 , both asymptomatic and symptomatic SARS‐CoV‐2 infection significantly increased the odds of pre‐eclampsia. However, the association was stronger in patients with symptomatic infection (OR, 2.11; 95% CI, 1.59–2.81) than in those with asymptomatic infection (OR, 1.59; 95% CI, 1.21–2.10).
A meta‐analysis of 1219 pregnant patients giving birth in one of 33 hospitals in the USA used the National Institutes of Health (NIH) criteria for classifying the severity of SARS‐CoV‐2 infection as either asymptomatic, mild, moderate, severe or critical 29 . On adjusted analysis, women with severe‐to‐critical COVID‐19, compared to asymptomatic women, had an increased risk of hypertensive disorders of pregnancy (40.4% vs 18.8%; aRR, 1.61; 95% CI, 1.18–2.20). However, mild‐to‐moderate COVID‐19 was not associated with adverse perinatal outcome, as compared with asymptomatic infection.
The INTERCOVID study reported that longer duration of symptomatic COVID‐19 was associated with an increased RR of pre‐eclampsia, eclampsia or HELLP syndrome 10 .
A retrospective observational study of 1223 pregnant women in the UK compared the severity of SARS‐CoV‐2 infection in pregnant women and the likelihood of subsequent pre‐eclampsia 28 . Patients were classified into four groups according to disease severity based on NIH criteria: asymptomatic, mild, moderate and severe. The model included adjustment for the prior risk of pre‐eclampsia based on maternal characteristics and medical history, using a competing‐risks model. Women in whom the diagnosis of pre‐eclampsia was made before the diagnosis of SARS‐CoV‐2 infection were excluded from the analysis. Compared with a background (expected) risk of pre‐eclampsia of around 1%, the observed incidence of pre‐eclampsia in those with asymptomatic, mild, moderate and severe SARS‐CoV‐2 infection was 1.9%, 2.2%, 5.7%, and 11.1%, respectively. This monotonic relationship was statistically significant (chi‐square test for trend P = 0.0017). After adjusting for differences in the prior risk of pre‐eclampsia, as determined by the competing‐risks model, severe COVID‐19 disease was associated with an almost 5‐fold higher risk of pre‐eclampsia than was asymptomatic infection (aRR, 4.9; 95% CI, 1.56–15.38). Moderate or severe COVID‐19 was also associated with a greater risk of pre‐eclampsia compared with asymptomatic or mild infection (aRR, 3.3; 95% CI, 1.48–7.38). The authors argued that their finding that the risk of pre‐eclampsia was greater for more severe SARS‐CoV‐2 infection supports the hypothesis of a causal relationship.
Plausibility
Several mechanisms have been proposed by which SARS‐CoV‐2 infection might cause systemic complications, such as high blood pressure, liver injury and thrombocytopenia, as well as the respiratory disease typical of COVID‐19 30 . One theory proposes involvement of the angiotensin‐converting enzyme 2 (ACE2) receptor. Activation of the renin–angiotensin–aldosterone system ultimately leads to cleavage of angiotensin I by angiotensin‐converting enzyme (ACE), converting it into angiotensin II. Angiotensin II, via a number of mechanisms (potent arteriolar vasoconstriction, increased renal tubular sodium reabsorption, increased aldosterone secretion and increased antidiuretic hormone secretion) leads to an increase in blood pressure 31 . ACE2 counterbalances the actions of ACE by cleaving and hydrolyzing angiotensin II, converting it into angiotensin (1–7), which is a vasodilator.
It has been demonstrated that SARS‐CoV‐2 enters cells in the lungs and other organs via the ACE2 receptor 32 . The spike S1 protein of SARS‐CoV‐2 binds to the enzymatic domain of ACE2 receptor on the cell surface, resulting in translocation of the virus into the cell 33 . The binding of the virus to ACE2 causes downregulation of this enzyme, resulting in reduced conversion of angiotensin II to angiotensin (1–7), allowing angiotensin II to act relatively unopposed. The ACE2 receptor is also expressed in both the syncytiotrophoblast and the cytotrophoblast 34 , 35 in the placenta, where it plays an important role in trophoblast proliferation, angiogenesis and arterial blood pressure regulation during pregnancy. Downregulation of ACE2 in the placenta by SARS‐CoV‐2 may lead to placental oxidative stress and the release of antiangiogenic factors, including soluble fms‐like tyrosine kinase‐1 (sFlt‐1) 36 , and a reduction in proangiogenic factors, leading to the characteristic features of pre‐eclampsia and HELLP syndrome 37 , 38 , 39 , 40 , 41 , 42 , 43 (Figure 1). One study 44 examined the potential role of SARS‐CoV‐2 infection in pre‐eclampsia by assessing differentially expressed genes from clinical and experimental datasets. SARS‐CoV‐2 infection was found to upregulate sFlt‐1 and endoglin (both of which are antiangiogenic factors that cause vasoconstriction), nitric oxide modulators and prothrombotic‐related molecules.
Figure 1.
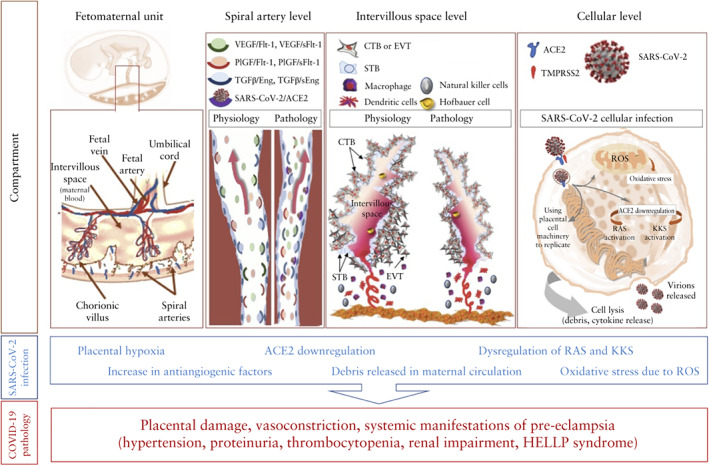
Mechanism of development of pre‐eclampsia in women with COVID‐19. ACE2, angiotensin‐converting enzyme 2; CTB, cytotrophoblast; EVT, extravillous trophoblast; HELLP, hemolysis, elevated liver enzymes and low platelet count; KKS, kinin–kallikrein system; PlGF, placental growth factor; RAS (or RAAS), renin–angiotensin system (or renin–angiotensin–aldosterone system); ROS, reactive oxygen species; (s)Eng, (soluble) endoglin; (s)Flt‐1, (soluble) fms‐like tyrosine kinase‐1; STB, syncytiotrophoblast; TGFβ, transforming growth factor beta; TMPRSS2, transmembrane protease serine 2; VEGF, vascular endothelial growth factor.
There are, therefore, several plausible mechanisms by which SARS‐CoV‐2 infection could lead to the development of pre‐eclampsia.
Coherence (between epidemiological and laboratory findings)
The abovementioned laboratory evidence that demonstrates downregulation of ACE2 and increased production of antiangiogenic factors, nitric oxide modulators and prothrombotic molecules as a result of SARS‐CoV‐2 infection is consistent with the epidemiological data.
Some histopathological studies have also identified placental lesions in COVID‐19 45 , 46 . Many viruses are known to cause histopathological changes in placental morphology, with characteristic changes seen in some cases of antenatal zika virus and cytomegalovirus infection 47 , 48 . Some reports suggest that, when compared with controls, placentae of women with severe COVID‐19 showed histopathological changes associated with poor maternal vascular perfusion 49 . This included decidual arteriopathy, peripheral and central villous infarction and villous agglutination. It is currently unknown what impact asymptomatic or mild SARS‐CoV‐2 infection might have on the placenta. Another study found that microvasculopathy was the most common finding in the placenta of SARS‐CoV‐2‐positive women 50 , suggesting that placental histopathological changes differ according to the timing of delivery in relation to COVID‐19 progression, i.e. whether the infection is in the acute stage or viral clearance has already been achieved. The placenta of a patient with symptomatic COVID‐19 at the time of delivery was found to have prominent lymphohistiocytic villitis and was one of two placentae that showed maternal malperfusion changes. This may indicate that placental changes are most likely to occur during the acute phase of the disease 50 , 51 .
Experimental evidence
Prospective cohort studies would potentially provide valuable evidence regarding the nature of the relationship between SARS‐CoV‐2 infection and pre‐eclampsia. These studies should compare pregnant women with and without SARS‐CoV‐2 infection and include measurements of those hematological, biochemical and immunological factors associated with COVID‐19 and pre‐eclampsia, as well as placental histopathological examination. Clearly, a randomized trial would be neither feasible nor ethical.
Analogous evidence
A meta‐analysis identified a higher incidence of pre‐eclampsia in pregnant women with a coronavirus‐spectrum infection (including severe acute respiratory syndrome, Middle East respiratory syndrome and COVID‐19) than in the general pregnant population 11 .
Reversibility
If SARS‐CoV‐2 infection can cause pre‐eclampsia, then vaccination against COVID‐19, antiviral therapies and COVID‐19 pandemic mitigation measures would be expected to reduce the risk of pre‐eclampsia. In a study that compared pregnancy outcome between women who were vaccinated and those who were unvaccinated against COVID‐19 52 , vaccination was found to protect against SARS‐CoV‐2 infection prior to delivery (1.4% vs 11.3%; RR, 0.13; 95% CI, 0.03–0.50; P = 0.003) and was also associated with a non‐significant decrease in the incidence of pre‐eclampsia (0.7% vs 1.2%; RR, 0.58; 95% CI, 0.08–4.25; P = 0.59). Ongoing randomized placebo‐controlled trials of COVID‐19 vaccination in pregnancy will establish whether vaccination reduces the risk of SARS‐CoV‐2 infection and adverse pregnancy outcomes, including pre‐eclampsia 53 .
Association or causation: are the Bradford Hill criteria still applicable in the 21st century?
Even though the currently available evidence would support the proposed hypothesis that COVID‐19 in pregnancy could potentially cause pre‐eclampsia, there are several limitations and more research is needed to address the remaining questions before this assertion is made. The reported 1.5 times increased risk of pre‐eclampsia in pregnant women with compared to those without SARS‐CoV‐2 infection (as compared to, for example, the 200‐fold increase in the risk of cancer in chimney sweepers, as cited by Bradford Hill) would be considered too small for a proven causal link and would more conceivably be attributed to other underlying contributors (i.e. bias or confounding). Moreover, the Bradford Hill criteria have been questioned following advancements in genetics, exposure science and statistics in the 21st century 54 , which have improved our analytical capabilities for exploring potential cause‐and‐effect relationships and our ability to appreciate the complexity of onset and progression of disease. These advancements in science and our understanding of disease origin led some researchers to question the Bradford Hill criteria when considering multifactorial causality 49 .
Conclusions
There is growing evidence that the association between SARS‐CoV‐2 infection in pregnancy and pre‐eclampsia is causal, particularly in relation to the biological gradient and plausibility. Clearly, however, more evidence is needed to bolster the other criteria, particularly in relation to temporal sequence, which is perhaps the only criterion which epidemiologists universally agree is essential to causal inference 54 . It is possible that a causal link is mediated through placental or cardiovascular pathology, but further studies are required to understand these potential mechanisms.
Since publication, in 1965, of the Bradford Hill criteria for determining the causality of observed epidemiological associations, there have been seismic advances in a range of scientific fields (for example, molecular genetics, genomics, molecular toxicology and genotoxicology) and technology (for example, computers, software, statistics and analytical methods). These disciplines can be used to ‘peer into the black box’ (as it was known at the time) between exposure and disease 54 . This means that the cause–effect relationship can often be established with a degree of certainty, leading some to argue that, in these instances, reliance on the Bradford Hill criteria becomes less relevant. Others, however, argue that application of the Bradford Hill criteria for analysis of causality can be enhanced by integrating new techniques into each of the criteria, making conclusions about causality more robust 54 . It should also be acknowledged that, in the case of SARS‐CoV‐2 infection and pre‐eclampsia, we are still just beginning to shine some light onto this particular ‘black box’ between exposure and disease.
Healthcare professionals should be aware that SARS‐CoV‐2 infection in pregnant women, even in those who remain asymptomatic, is a risk factor for subsequent development of pre‐eclampsia. They should also be cognisant of the additive effect of the combination of these two conditions on adverse pregnancy outcome. Pregnant women who test positive for SARS‐CoV‐2 will benefit from close monitoring of blood pressure and liver and renal function in order to allow early diagnosis of pre‐eclampsia and HELLP syndrome 55 . Performing a swab to test for SARS‐CoV‐2 in women presenting with pre‐eclampsia but non‐classical biochemical markers may be useful in settings in which SAR‐CoV‐2 testing on admission is not universal 55 .
REFERENCES
- 1. Kc A, Gurung R, Kinney MV, Sunny AK, Moinuddin M, Basnet O, Paudel P, Bhattarai P, Subedi K, Shrestha MP, Lawn JE, Målqvist M. Effect of the COVID‐19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health 2020; 8: e1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumari V, Mehta K, Choudhary R. COVID‐19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob Health 2020; 8: e1116–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. RECOVERY Collaborative Group . Convalescent plasma in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised controlled, open‐label, platform trial. Lancet 2021; 397: 2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Relph S, Jardine J, Magee LA, von Dadelszen P, Morris E, Ross‐Davie M, Draycott T, Khalil A. Authors' reply re: Maternity services in the UK during the coronavirus disease 2019 pandemic: a national survey of modifications to standard care. BJOG 2021; 128: 937–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chmielewska B, Barratt I, Townsend R, Kalafat E, van der Meulen J, Gurol‐Urganci I, O'Brien P, Morris E, Draycott T, Thangaratinam S, Le Doare K, Ladhani S, von Dadelszen P, Magee L, Khalil A. Effects of the COVID‐19 pandemic on maternal and perinatal outcomes: a systematic review and meta‐analysis. Lancet Glob Health 2021; 9: e759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kotlar B, Gerson E, Petrillo S, Langer A, Tiemeier H. The impact of the COVID‐19 pandemic on maternal and perinatal health: a scoping review. Reprod Health 2021; 18: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen M, Zeng J, Liu X, Sun G, Gao Y, Liao J, Yu J, Luo X, Qi H. Changes in physiology and immune system during pregnancy and coronavirus infection: A review. Eur J Obstet Gynecol Reprod Biol 2020; 255: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vousden N, Bunch K, Knight M, Brocklehurst P, Kurinczuk JJ, O'Brien P, Quigley, M . Incidence, risk factors and impact of seasonal influenza in pregnancy: A national cohort study. PLoS One 2021; 16: e0244986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dawood FS, Kittikraisak W, Patel A, Rentz Hunt D, Suntarattiwong P, Wesley MG, Thompson MG, Soto G, Mundhada S, Arriola CS, Azziz‐Baumgartner E, Brummer T, Cabrera S, Chang HH, Deshmukh M, Ellison D, Florian R, Gonzales O, Kurhe K, Kaoiean S, Rawangban B, Lindstrom S, Llajaruna E, Mott JA, Saha S, Prakash A, Mohanty S, Sinthuwattanawibool C, Tinoco Y. Incidence of influenza during pregnancy and association with pregnancy and perinatal outcomes in three middle‐income countries: a multisite prospective longitudinal cohort study. Lancet Infect Dis 2021; 21: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, Roggero P, Prefumo F, Silva do Vale M, Cardona‐Perez JA, Maiz N, Cetin I, Savasi V, Deruelle P, Easter SR, Sichitiu J, CPS Conti, Ernawati E, Mhatre M, Teji JS, Liu B, Capelli C, Oberto M, Salazar L, Gravett MG, Cavoretto PI, Nachinab WV, Galadanci H, Oros D, Ayede AI, Sentilhes L, Bako B, Savorani M, Cena H, Garcia‐May PK, Etuk S, Casale R, Abd‐Elsalam S, Ikenoue S, Aminu MB, Vecciarelli C, Duro EA, Usman MA, John‐Akinola Y, Nieto R, Ferrazi E, Bhutta ZA, Langer A, Kennedy SH, Papageorghiou AT. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID‐19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021; 175: 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, Vecchiet J, Nappi L, Scambia G, Berghella V, D'Antonio F. Outcome of coronavirus spectrum infections (SARS, MERS, COVID‐19) during pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM 2020; 2: 100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conde‐Agudelo A, Romero R. SARS‐COV‐2 infection during pregnancy and risk of preeclampsia: a systematic review and meta‐analysis. Am J Obstet Gynecol 2021. DOI: 10.1016/j.ajog.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill AB. The environment and disease: association or causation? 1965. J R Soc Med 2015; 108: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gurol‐Urganci I, Jardine JE, Carroll F, Draycott T, Dunn G, Fremeaux A, Harris T, Hawdon J, Morris E, Muller P, Waite L, Webster K, van der Meulen J, Khalil A. Maternal and perinatal outcomes of pregnant women with SARS‐CoV‐2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol 2021; 225: 522.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez‐Portilla RJ, Sotiriadis A, Chatzakis C, Torres‐Torres J, Espino Y Sosa S, Sandoval‐Mandujano K, Castro‐Bernabe DA, Medina‐Jimenez V, Monarrez‐Martin JC, Figueras F, Poon LC. Pregnant women with SARS‐CoV‐2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx). Ultrasound Obstet Gynecol 2021; 57: 224–231. [DOI] [PubMed] [Google Scholar]
- 16. Mullins E, Hudak ML, Banerjee J, Getzlaff T, Townson J, Barnette K, Playle R, Perry A, Bourne T, Lees CC; PAN‐COVID investigators and the National Perinatal COVID‐19 Registry Study Group . Pregnancy and neonatal outcomes of COVID‐19: coreporting of common outcomes from PAN‐COVID and AAP‐SONPM registries. Ultrasound Obstet Gynecol 2021; 57: 573–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, Greene MF, Solomon SD. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID‐19. JAMA Intern Med 2021; 181: 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Collaborating Centre for Women's and Children's Health (UK). Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. RCOG Press: London, 2010. [PubMed] [Google Scholar]
- 19. ACOG . Committee Opinion No. 638: First‐trimester risk assessment for early‐onset preeclampsia. Obstet Gynecol 2015; 126: e25–27. [DOI] [PubMed] [Google Scholar]
- 20. Sotiriadis A, Hernandez‐Andrade E, da Silva Costa F, Ghi T, Glanc P, Khalil A, Martins WP, Odibo AO, Papageorghiou AT, Salomon LJ, Thilaganathan B; ISUOG CSC Pre‐eclampsia Task Force. ISUOG Practice Guidelines: role of ultrasound in screening for and follow‐up of pre‐eclampsia. Ultrasound Obstet Gynecol 2019; 53: 7–22. [DOI] [PubMed] [Google Scholar]
- 21. Rolnik DL, Wright D, Poon LC, O'Gorman N, Syngelaki A, de Paco Matallana C, Akolekar R, Cicero S, Janga D, Singh M, Molina FS, Persico N, Jani JC, Plasencia W, Papaioannou G, Tenenbaum‐Gavish K, Meiri H, Gizurarson S, Maclagan K, Nicolaides KH. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017; 377: 613–622. [DOI] [PubMed] [Google Scholar]
- 22. Torres‐Torres J, Martinez‐Portilla RJ, Espino‐Y‐Sosa S, Estrada‐Gutierrez G, Solis‐Paredes JM, Villafan‐Bernal JR, Medina‐Jimenez V, Rodriguez‐Morales AJ, Rojas‐Zepeda L, Poon LC. Comorbidity, poverty and social vulnerability as risk factors for mortality in pregnant women with confirmed SARS‐CoV‐2 infection: analysis of 13 062 positive pregnancies including 176 maternal deaths in Mexico. Ultrasound Obstet Gynecol 2022; 59: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, Balaji R, Lee SI, Qiu X, Yuan M, Coomar D, Sheikh J, Lawson H, Ansari K, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Tiberi S, Brizeula V, Broutet N, Kara E, Kim CR, Thorson A, Escuriet R, Oladapo OT, Mofenson L, Zamora J, Thangaratinam S. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta‐analysis. BMJ 2020; 370: m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papageorghiou AT, Deruelle P, Gunier RB, Rauch S, García‐May PK, Mhatre M, Usman, MA , Abd‐Elsalam S, Etuk S, Simmons LE, Napolitano R, Deantoni S, Liu B, Prefumo F, Savasi V, Silva do Vale M, Baafi E, Zainab G, Nieto R, Maiz N, Aminu MB, Cardona‐Perez JA, Craik R, Winsey A, Tavchioska G, Bako B, Oros D, Rego A, Benski AC, Hassan‐Hanga F, Savorani M, Giuliani F, Sentilhes L, Risso M, Takahashi K, Vencchiarelli C, Ikenoue S, Thiruvengadam R, CPS Conti, Ferrazzi E, Cetin I, Nachinab VB, Ernwati E, Duro EA, Kholin A, Firlit ML, Easter SR, Sichitiu J, Firlit ML, Easter SR, Sichitiu J, Bowale A, Casale R, Cerbo RM, Cavoretto PI, Eskenazi B, Thornton JG, Bhutta ZA, Kennedy SH, Villar J. Preeclampsia and COVID‐19: results from the INTERCOVID prospective longitudinal Study. Am J Obstet Gynecol 2021; 225: 289.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madden N, Emeruwa U, Polin M, Bejerano S, Gyamfi‐Bannerman C, Booker WA. COVID‐19 and new hypertensive disease in pregnancy. Am J Obstet Gynecol 2021; 224 (Suppl): S23–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenbloom JI, Raghuraman N, Carter EB, Kelly JC. Coronavirus disease 2019 infection and hypertensive disorders of pregnancy. Am J Obstet Gynecol 2021; 224: 623–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chornock R, Iqbal SN, Wang T, Kodama S, Kawakita T, Fries M. Incidence of hypertensive disorders of pregnancy in women with COVID‐19. Am J Perinatol 2021; 38: 766–772. [DOI] [PubMed] [Google Scholar]
- 28. Lai J, Romero R, Tarca AL, Iliodromiti S, Rehal A, Banerjee A, Yu C, Peeva G, Palaniappan V, Tan L, Mehta M, Nicolaides KH. SARS‐CoV‐2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose‐response relationship supporting causality. Am J Obstet Gynecol 2021; 225: 689–693.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, Manuck TA, Miodovnik M, Sowles A, Clark K, Gyamfi‐Bannerman C, Mendez‐Figueroa H, Sehdev HM, Rouse DJ, Tita ATN, Bailit J, Costantine MM, Simhan HN, Macones GA; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal‐Fetal Medicine Units (MFMU) Network . Disease Severity and Perinatal Outcomes of Pregnant Patients With Coronavirus Disease 2019 (COVID‐19). Obstet Gynecol 2021; 137: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coronado‐Arroyo JC, Concepción‐Zavaleta MJ, Zavaleta‐Gutiérrez FE, Concepción‐Urteaga LA. Is COVID‐19 a risk factor for severe preeclampsia? Hospital experience in a developing country. Eur J Obstet Gynecol Reprod Biol 2021; 256: 502–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin‐Converting Enzyme 2: SARS‐CoV‐2 Receptor and Regulator of the Renin‐Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ Res 2020; 126: 1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhalla V, Blish CA, South AM. A historical perspective on ACE2 in the COVID‐19 era. J Hum Hypertens 2020; 35: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Djomkam ALZ, Olwal CO, Sala TB, Paemka L. Commentary: SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Front Oncol 2020; 10: 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cui D, Liu Y, Jiang X, Ding C, Poon LC, Wang H, Yang H. Single‐cell RNA expression profiling of SARS‐CoV‐2‐related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet Gynecol 2021; 57: 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hecht JL, Quade B, Deshpande V, Mino‐Kenudson M, Ting DT, Desai N, Dygulska B, Heyman T, Salafia C, Shen D, Bates SV, Roberts DJ. SARS‐CoV‐2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID‐19‐positive mothers. Mod Pathol 2020; 33: 2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Torres‐Torres J, Espino‐y‐Sosa S, Poon LC, Solis‐Paredes JM, Estrada‐Gutierrez G, Espejel‐Nuñez A, Juarez‐Reyes A, Etchegaray‐Solana A, Alfonso‐Guillen Y, Aguilar‐Andrade L, Hernández‐Pacheco JA, Villafan‐Bernal JR, Martinez‐Portilla RJ. Increased levels of soluble fms‐like tyrosine kinase‐1 are associated with adverse outcome in pregnant women with COVID‐19. Ultrasound Obstet Gynecol 2022; 59: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Todros T, Masturzo B, De Francia S. COVID‐19 infection: ACE2, pregnancy and preeclampsia. Eur J Obstet Gynecol Reprod Biol 2020; 253: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bloise E, Zhang J, Nakpu J, Hamada H, Dunk CE, Li S, Imperio GE, Nadeem L, Kibschulll M, Phetcharawan Lye, Matthews SG, Lye JS. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal‐fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol 2021; 224: 298.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ouyang Y, Bagalkot T, Fitzgerald W, Sadovsky E, Chu T, Martínez‐Marchal A, Brieño‐Enríquez M, Su JE, Margolis L, Sorkin A, Sadovsky Y. Term human placental trophoblasts express SARS‐CoV‐2 entry factors ACE2, TMPRSS2, and Furin. mSphere 2021; 6: e00250‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taglauer E, Benarroch Y, Rop K, Barnett E, Sabharwal V, Yarrington C, Wachman EM. Consistent localization of SARS‐CoV‐2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID‐19 positive maternal‐fetal dyads. Placenta 2020; 100: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Zhang T, Uhl S, Lubor BC, Chandar V, Gil C, Zhang W, Dodson B, Bastiaans J, Prabhu M, Salvatore CM, Yang YJ, Baergen RN, tenOever BR, Landau NR, Chen S, Schwartz RE, Stuhlmann. SARS‐CoV‐2 infects syncytiotrophoblast and activates inflammatory responses in the placenta. bioRxiv [Preprint]. 2021. DOI: 10.1101/2021.06.01.446676. [DOI] [Google Scholar]
- 42. Verma S, Joshi CS, Silverstein RB, He M, Carter EB, Mysorekar IU. SARS‐CoV‐2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin‐angiotensin system. Med (N Y) 2021; 2: 575–590.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res 2019; 124: 1094–1112. [DOI] [PubMed] [Google Scholar]
- 44. Beys‐da‐Silva WO, da Rosa RL, Santi L, Tureta EF, Terraciano PB, Guimarães JA, Passos EP, Berger M. The risk of COVID‐19 for pregnant women: evidences of molecular alterations associated with preeclampsia in SARS‐CoV‐2 infection. Biochim Biophys Acta Mol Basis Dis 2021; 1867: 165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mongula JE, Frenken MWE, van Lijnschoten G, Arents NLA, de Wit‐Zuurendonk LD, Schimmel‐de Kok APA, van Runnard Heimel PJ, Porath MM, Goossens SMTA. COVID‐19 during pregnancy: non‐reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet Gynecol 2020; 56: 773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Girolamo R, Khalil A, Alameddine S, D'Angelo E, Galliani C, Matarrelli B, Buca D, Liberati M, Rizzo G, D'Antonio F. Placental histopathology after SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis. Am J Obstet Gynecol MFM 2021; 3: 100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA. Placental Pathology of Zika Virus: Viral Infection of the Placenta Induces Villous Stromal Macrophage (Hofbauer Cell) Proliferation and Hyperplasia. Arch Pathol Lab Med 2017; 141: 43–48. [DOI] [PubMed] [Google Scholar]
- 48. Lee JK, Oh SJ, Park H, Shin OS. Recent Updates on Research Models and Tools to Study Virus‐Host Interactions at the Placenta. Viruses 2019; 12: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental Pathology in COVID‐19. Am J Clin Pathol 2020; 154: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Menter T, Mertz KD, Jiang S, Chen H, Monod C, Tzankov A, Waldvogel S, Schulzke SM, Hösli I, Bruder E. Placental Pathology Findings during and after SARS‐CoV‐2 Infection: Features of Villitis and Malperfusion. Pathobiology 2021; 88: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Linehan L, O'Donoghue K, Dineen S, White J, Higgins JR, Fitzgerald B. SARS‐CoV‐2 placentitis: An uncommon complication of maternal COVID‐19. Placenta 2021; 104: 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS‐CoV‐2 vaccination in pregnancy. Am J Obstet Gynecol MFM 2021; 3: 100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben‐David A, Asraf K, Doolman R, Levin EG, Regev Yochay G, Yinon Y. Short‐term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID‐19 vaccine. Ultrasound Obstet Gynecol 2021; 58: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol 2015; 12: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ahmed I, Eltaweel N, Antoun L, Rehal A. Severe pre‐eclampsia complicated by acute fatty liver disease of pregnancy, HELLP syndrome and acute kidney injury following SARS‐CoV‐2 infection. BMJ Case Rep 2020; 13: e237521. [DOI] [PMC free article] [PubMed] [Google Scholar]


