Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has caused the coronavirus disease 2019 (COVID‐19) pandemic in humans since late 2019. Here, we investigated SARS‐CoV‐2 infection in dogs and cats during COVID‐19 quarantine at private veterinary hospitals in Thailand. From April to May 2021, we detected SARS‐CoV‐2 in three out of 35 dogs and one out of nine cats from four out of 17 households with confirmed COVID‐19 patients. SARS‐CoV‐2 RNA was detected from one of the nasal, oral, rectal and environmental swabs of dog‐A (15 years old, mixed breed, male dog), cat‐B (1 year old, domestic shorthair, male cat), dog‐C (2 years old, mixed breed, female dog) and dog‐D (4 years old, Pomeranian, female dog). The animals tested positive for SARS‐CoV‐2 RNA from 4 to 30 days after pet owners were confirmed to be COVID‐19 positive. The animals consecutively tested positive for SARS‐CoV‐2 RNA for 4 to 10 days. One dog (dog‐A) showed mild clinical signs, while the other dogs and a cat remained asymptomatic during quarantine at the hospitals. SARS‐CoV‐2 specific neutralizing antibodies were detected in both the dogs and cat by surrogate virus neutralization tests. Phylogenetic and genomic mutation analyses of whole genome sequences of three SARS‐CoV‐2 strains from the dogs and cat revealed SARS‐CoV‐2 of the Alpha variant (B.1.1.7 lineage). Our findings are suggestive of human‐to‐animal transmission of SARS‐CoV‐2 in COVID‐19‐positive households and contamination of viral RNA in the environment. Public awareness of SARS‐CoV‐2 infection in pet dogs and cats in close contact with COVID‐19 patients should be raised.
Keywords: cat, dog, infection, SARS‐CoV‐2, Thailand
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is a pandemic disease. As of 16 June 2021, more than 175 million confirmed human cases have been reported, with over 3.81 million deaths (WHO, 2021c). Evidence of SARS‐CoV‐2 spillover from humans to animals has been reported in dogs, cats, tigers, lions, gorillas and minks (McAloose et al., 2020; Newman et al., 2020; Sit et al., 2020). SARS‐CoV‐2 infection in domestic dogs and cats has been reported in 22 countries in America, Europe and Asia (Decaro, Balboni et al., 2021; OIE, 2021b). Cats are susceptible to SARS‐CoV‐2 infection and can show mild‐to‐moderate respiratory symptoms, while dogs developed no or mild respiratory symptoms (McAloose et al., 2020; Sailleau et al., 2020; Segalés et al., 2020). In Thailand, the current outbreak (3rd wave) of COVID‐19 started in late March 2021, and the number of confirmed human cases of SARS‐CoV‐2 infection is still rising (WHO, 2021a). During the outbreak, pet dogs and cats of COVID‐19‐positive patients were optionally quarantined at university and private veterinary hospitals. In this study, we collected swab samples (nasal, oral and rectal swabs) from 35 dogs and nine cats from COVID‐19‐positive households and examined them for SARS‐CoV‐2 infection in those animals. We identified SARS‐CoV‐2 infection in three dogs and one cat by virological testing, serological testing and viral genome analysis. This study is the first to report dogs and cats infected with SARS‐CoV‐2 in Thailand.
2. METHODS
2.1. Sample collection from dogs and cats
In this study, we investigated SARS‐CoV‐2 infection in domestic dogs and cats quarantined at private animal hospitals during the third wave of the COVID‐19 outbreak reported by Thailand's Centre for COVID‐19 Situation Administration (CCSA) (WHO, 2021a). From March to May 2021, we collected samples from dogs (n = 35) and cats (n = 9) from 17 households located in Bangkok and the vicinity (Table S1). It is noted that all animals lived indoor. The sample collection was conducted under the approval of the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Science, Chulalongkorn University, Thailand (approval No. 2031035). The sampling of dogs and cats was conducted according to the convenience or willingness for COVID‐19 testing of owners and animal hospital staff. In total, nasal swabs (n = 58), oral swabs (n = 61) and rectal swabs (n = 93) from 35 dogs and nine cats were collected. Serum samples (n = 9) were collected from SARS‐CoV‐2‐positive animals. Nasal, oral and rectal swabs were collected by using flocked nylon swabs (Copan, California, USA). Environmental samples including hair/body swabs (n = 11), water container swabs (n = 11) and floor swabs (n = 11) were collected from cages of COVID‐19 positive pets at the animal hospitals. Environmental sampling was conducted before animal sampling and daily cleaning with disinfectant. Each swab was placed in 1 ml of RNAprotect® Tissue Reagent (Qiagen, Hilden, Germany). Blood samples (1–2 ml) were collected from the cephalic or saphenous vein. The samples were transported to the laboratory of the Center of Excellence for Emerging and Re‐emerging Infectious Diseases in Animals (CUEIDAs), Chulalongkorn University, within 24 h.
2.2. Detection of SARS‐CoV‐2 RNA by real‐time RT‐PCR
The swab samples, including nasal swabs (n = 58), oral swabs (n = 61) and rectal swabs (n = 93), were subjected to RNA extraction by using the magnetic bead‐based automatic purification equipment of a GENTi™ 32 ‐ Automated Nucleic Acid Extraction System (GeneAll®, Seoul, South Korea). In brief, the swab sample was vigorously vortexed for at least 15 s before removing the swab. Next 200 μl of supernatant was mixed with 7 μl of RNA carrier and then added to an extraction tube. The RNA extraction process was performed according to the manufacturer's instructions. Finally, 50 μl of viral RNA was obtained from the RNA extraction process.
For the detection of SARS‐CoV‐2, real‐time RT‐PCR based on primers and probes specific to the E and RdRp genes following WHO recommendations was used (Corman et al., 2020), and primers and probes specific to the N1 and N2 genes following the Centers for Disease Control and Prevention recommendations were also used (CDC, 2020) (Table S2). A one‐step real‐time RT‐PCR assay was performed by using a Superscript III One‐Step RT‐PCR System with Platinum Taq Polymerase (Invitrogen™, California, USA). In brief, a total 25 μl reaction contained 2 μl of RNA, 12.5 μl of 2X reaction buffer of the SuperScript® III Platinum® One‐Step Quantitative RT‐PCR System (Invitrogen™, California, USA), 1 μl of reverse transcriptase/Platinum Taq, 0.8 mM MgSO4, 0.8 μM each primer and probe and RNase‐free water. Thermal cycling was performed at 50°C for 15 min for reverse transcription, followed by 95°C for 2 min and then 45 cycles of 95°C for 15 s, and 60°C for 30 s for the N1 and N2 genes. For the E and RdRP genes, thermal cycling was performed at 55°C for 10 min for reverse transcription, followed by 95°C for 3 min and then 45 cycles of 95°C for 15 s and 58°C for 30 s. Samples with a Ct value of <36 were considered positive, while samples with a Ct value of 36–40 were considered suspected and those with a Ct value > 40 were considered negative (CDC, 2020). In this study, we used the World Organisation for Animal Health (OIE) definition for a confirmed case of animal SARS‐CoV‐2 infection, in which at least two specific targets (genomic regions) tested positive, indicating SARS‐CoV‐2 positivity (OIE, 2021a).
2.3. Detection of SARS‐CoV‐2 antibodies by indirect enzyme‐linked immunosorbent assay (indirect ELISA) and virus neutralization test (VNT)
We used an ID Screen® SARS‐CoV‐2 Double Antigen Multispecies ELISA Kit (ID VET, Montpellier, France) to detect SARS‐CoV‐2 antibodies in serum samples. This indirect ELISA was based on the detection of anti‐SARS‐CoV‐2 nucleocapsid antibodies (IgG) in the tested animal serum and was performed according to the manufacturer's instructions (Sailleau et al., 2020). Briefly, 25 μl of each serum sample and positive and negative control samples were transferred to separate wells, diluted with 25 μl of dilution buffer, incubated at 37°C for 45 min and washed five times with 300 μl of washing buffer. After washing, 100 μl of horseradish peroxidase (HRP)‐conjugated N protein recombinant antigen was added and incubated at 25°C for 30 min. Then, the wells were washed five times with 300 μl of washing buffer. After washing, 100 μl of the substrate was added to each well and incubated at 25°C for 20 min. Then, 100 μl of stop solution was added to stop the reaction. The optical density (OD) at 450 nm of each sample was read. The OD of each sample was calculated as the S/P percentage (S/P%). Serum with S/P% > 60% was defined as positive, while serum with S/P% 50%−60% was considered suspected.
To detect the presence of SARS‐CoV‐2‐neutralizing antibodies, sera of dogs and cats were subjected to sVNTs by using a cPass™ SARS‐CoV‐2 Neutralization Antibody Detection Kit (GenScript Biotech, Jiangsu, China). The assay detects neutralizing antibodies for the interaction between the virus receptor‐binding domain (RBD) and the ACE2 cell surface receptor (Tan et al., 2020). Briefly, 50 μl of each 1:10‐diluted serum sample was mixed with 50 μl of horseradish peroxidase conjugated to the SARS‐CoV‐2 spike RBD (HRP‐RBD) and incubated at 37°C for 30 min. After dilution, each mixture was added to each well precoated with ACE2 protein and incubated at 37°C for 15 min. Then, the wells were washed 4 times with 260 μl of washing buffer. After washing, TMB solution was added and incubated at 25°C for 15 min. Then, 50 μl of stop solution was added. The OD at 450 nm of each well was read. The OD of each sample was calculated as the inhibition percentage (% inhibition); serum with % inhibition above 20% was considered positive, and serum with % inhibition not exceeding 20% was considered negative (Meyer et al., 2020).
2.4. Characterization and phylogenetic analysis of SARS‐CoV‐2
We performed whole‐genome sequencing of 3 SARS‐CoV‐2 strains by Oxford Nanopore sequencing. All gene segments of SARS‐CoV‐2 were amplified by ARTICS nCoV‐2019 sequencing protocol V3 (LoCost). Briefly, 8 μl of undiluted RNA was mixed with 2 μl of LunaScript® RT SuperMix (NEB, Ipswich, MA, USA) and incubated at 25°C for 2 min, 55 °C for 10 min and 95 °C for 1 min for cDNA synthesis. The SARS‐CoV‐2 primer scheme was used to perform two pools of multiplex PCRs by using Q5® Hot Start High‐Fidelity DNA polymerase (NEB, MA, USA) according to the ARTIC protocol. For the ARTIC multiplex PCR, thermal cycling was set at 98°C for 30s and then 35 cycles of 98°C for 15s and 65°C for 5 min. After ARTIC multiplex PCR, library preparation was performed following the Oxford Nanopore rapid sequencing kit (SQK‐RAD004) manufacturer's instructions and Midnight SARS‐CoV‐2 genome sequencing protocol. In brief, PCR products of pools 1 and 2 were mixed (10 μl of pool 1 and 10 μl of pool 2) and 7.5 μl of the mixture was used for binding with 2.5 μl of fragmentation mix from an Oxford Nanopore rapid sequencing kit. After incubation at 30°C for 1 min, 80°C for 1 min and 4°C for 30 s, the product was cleaned up by AMPure XP Bead Cleanup (Beckman Coulter, CA, USA) in a 1:1 ratio and eluted with 10 mM Tris‐HCl pH 8.0. One microliter of rapid adapter was added, and the mixture was loaded into a flow cell (Oxford Nanopore MinION device) and run under MinKNOW (6) (v19.12.5) software (Baker et al., 2021). The output reads from the Oxford Nanopore MinION device were filtered using the sequencing summary file under the following parameters: minimum read length ≥ 500 nt and read quality ≥7. The reads that passed the parameters were converted from “Fast5” into “Fastq” format using the GPU version of the Nanopore Guppy basecaller (v3.4.4) tool. Genome assembly was conducted by using the genome detective program (Vilsker et al., 2019). De‐novo approach with CLC Genomics Workbench Bio assembly software v11.0.1 (CLC Bio, 2005, Denmark) was used for nucleotide data analysis. Whole‐genome nucleotide sequences of SARS‐CoV‐2 from the dogs and cat were then submitted to the GenBank database under the accession numbers MZ396818, MZ401455 and MZ414173.
Phylogenetic analysis of SARS‐CoV‐2 was performed by comparing with nucleotide sequences of 942 genomes (at least 29,000 base pairs in length) isolated from Thailand from January 2020 to May 2021. The genome sequences were selected and downloaded from the GISAID database. The 5′ and 3′ untranslated regions were trimmed with at least 95% reference genome coverage and retained (Wuhan‐Hu‐1). The dataset was aligned using the MAFFT FFT‐NS‐2 algorithm and default parameter settings (Katoh et al., 2002). A neighbour‐joining tree was constructed by using MEGA program v7.0 (Tempe, AZ, USA) with the maximum composite likelihood substitution model and bootstrapping with 1,000 replicates. Lineage classification was performed by using the Pangolin tool (Rambaut et al., 2020). Genome mutation analysis of SARS‐CoV‐2 was performed based on variant classifications and definitions (CDC) (CDC, 2021; Kumar et al., 2016). Genome positions were based on the reference genome sequence of Wuhan‐Hu‐1 (MN908947).
2.5. Data analysis
Descriptive statistics were used to describe demographic information, locations and types of samples from dogs and cats in this study. Frequencies and percentages were used to report SARS‐CoV‐2 infection in animals and households. Phylogenetic analysis was performed by the neighbour‐joining algorithm with the maximum composite likelihood substitution model and bootstrapping with 1,000 replicates by the MEGA program.
3. RESULTS
All nasal, oral and rectal swab samples from dogs and cats (n = 44) from 17 households of COVID‐19‐positive patients were screened for SARS‐CoV‐2 RNA by real‐time RT‐PCR with specific primers and probes for the N1, N2, E and RdRp genes. A total of three out of 35 dogs (8.6%) and one out of nine cats (11.1%) from four out of 17 (23.53%) households were positive for SARS‐CoV‐2 RNA confirmed by real‐time RT‐PCR. The dogs and cat were followed up for nasal, oral, rectal and environmental (hair, water containers, floor) sample collection at quarantine animal hospitals until all tests were negative (Table 1, Figure 1 and Table S1).
TABLE 1.
Description of the dogs and cat positive for SARS‐CoV‐2 and COVID‐19‐positive households
| ID | District | Location | Species | Breed | Sex | Age | Clinical signs | Date of first detection | COVID‐19‐positive household (# of owners) | SARS‐CoV‐2 sequence (bp) | GenBank accession # |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Dog‐A CU27042 |
Bang Khae | Bangkok | Dog | Mixed | Male | 15 years |
Nasal discharge, Sneezing |
3 May 21 |
Household‐A (2) A1. 50‐year‐old male A2. 45‐year‐old female |
WGS (29778 bp) |
MZ396818 |
|
Cat‐B CU27081 |
Mueang |
Samut Prakarn |
Cat |
Domestic shorthair |
Male | 1 year | Healthy | 7 May 21 |
Household‐B (3) B1. 35‐year‐old male B2. 85‐year‐old male B3. 80‐year‐old female (2 deaths: B1, B2) |
WGS (29713 bp) |
MZ401455 |
|
Dog‐C CU27184 |
Bangkok Noi | Bangkok | Dog | Mixed | Female | 2 years | Healthy | 19 May 21 |
Household‐C (3) C1. 26‐year‐old male C2. > 60‐year‐old female C3. > 80‐year‐old female (1 death; C1) |
WGS (29743 bp) |
MZ414173 |
|
Dog‐D CU27186 |
Bang Khen | Bangkok | Dog | Pomeranian | Female | 4 years | Healthy | 19 May 21 |
Household‐D (5) D1. 18‐year‐old female D2. > 40‐year‐old female D3. > 50‐year‐old male D4. > 70‐year‐old female D5. > 70‐year‐old male |
ND* | ND |
ND; not done due to low viral load Ct 32.46–36.59.
FIGURE 1.
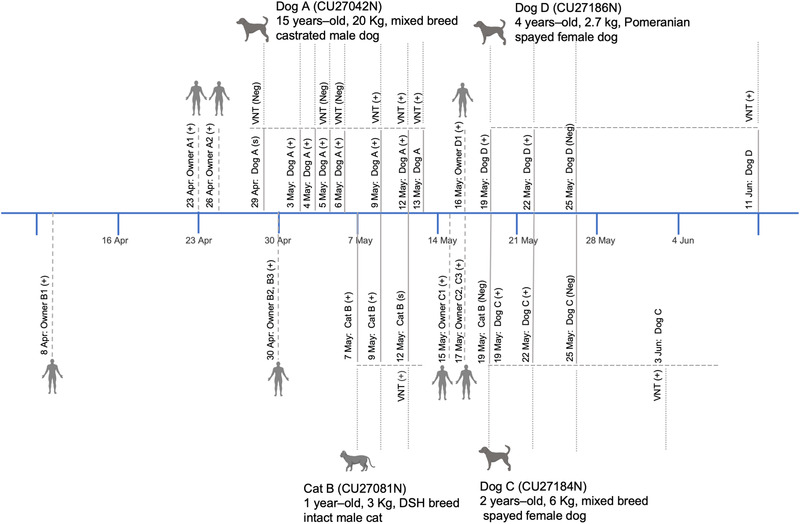
Timeline of SARS‐CoV‐2 detection in dogs and cats in the study
Dog‐A (CU27042) is a 15‐year‐old, 20 kg, mixed breed, castrated male dog. The animal had pre‐existing clinical disorders, including degenerative mitral value disease (stage B1), multiple hepatic masses and fibrosarcoma at the right metatarsus. The owners of dog‐A were a 50‐year‐old man and 45‐year‐old woman who were diagnosed with COVID‐19 on 23 April 2021 (owner A1) and 26 April 2021 (owner A2). On 29 April 2021, dog‐A visited Chulalongkorn University Small Animal Hospital for surgery for right hind limb amputation. Nasal, oral and rectal swabs were collected, and the animal was transferred to a private animal hospital for post‐operative care. Additional sample collection for SARS‐CoV‐2 detection was carried out on six further occasions at the private animal hospital. A blood sample was collected on six occasions for serological testing. During quarantine, the dog developed mild clinical signs, including nasal discharge, sneezing and laboured breathing (on 5–8 May 2021) (Table S3). We detected SARS‐CoV‐2 RNA from nasal and oral swabs of dog‐A by real‐time RT‐PCR in six consecutive sample collections between 3 and 12 May 2021. A high viral load (low Ct value) was observed in nasal and oral swabs of the dog on 4–9 May 2021 (Ct 15.67–34.59) and in all environmental samples (hair, water container and floor) between 4 and 12 May 2021 (Ct 26.32–35.58) (Table 2).
TABLE 2.
SARS‐CoV‐2 detection in the dogs and cat by realtime RT‐PCR specific to N1, N2, E and RdRp
| Dog‐A | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Realtime RT‐PCR (Ct value) | ||||||||||||
| Nasal swab | Oral swab | Rectal swab | ||||||||||
| Date | N1 a | N2 a | E b | RdRp b | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp |
| 29 Apr 21 | – | – | – | – | s (37.30) | – | – | – | s (37.18) | – | – | – |
| 3 May 21 | + (32.39) | s (38.14) | – | – | + (27.88) | + (33.11) | + (32.02) | + (33.05) | – | – | – | – |
| 4 May 21 | + (15.67)* | + (18.45)* | + (18.87)* | + (22.19)* | + (25.56) | + (31.04) | + (28.77) | + (31.41) | – | – | – | – |
| 5 May 21 | + (15.59) | + (16.77) | + (19.10) | + (21.67) | + (28.39) | + (33.30) | + (33.20) | + (33.44) | – | – | – | – |
| 6 May 21 | + (26.61) | + (30.68) | + (29.01) | + (28.04) | + (29.73) | + (34.54) | + (31.59) | + (31.25) | – | – | – | – |
| 9 May 21 | + (30.66) | s (36.09) | + (32.62) | + (33.06) | + (34.59) | – | + (34.44) | – | – | – | – | – |
| 12 May 21 | + (31.93) | s (38.82) | – | – | – | – | – | – | – | – | – | – |
| Hair/body swab | Water container swab | Floor swab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | |
| 29 Apr 21 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 3 May 21 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 4 May 21 | + (29.11) | s (38.20) | + (31.81) | + (34.37) | + (33.44) | – | + (33.97) | – | + (27.95) | + (33.38) | + (31.81) | + (34.01) |
| 5 May 21 | + (29.29) | s (38.33) | + (32.48) | + (33.87) | + (30.27) | + (34.72) | + (33.08) | + (34.86) | + (26.32) | + (32.45) | + (30.64) | + (32.57) |
| 6 May 21 | + (29.86) | s (37.35) | + (32.17) | – | s (36.13) | – | – | – | s (38.73) | – | – | – |
| 9 May 21 | + (34.04) | s (39.47) | + (34.43) | – | – | – | – | – | + (35.58) | – | + (35.13) | – |
| 12 May 21 | + (31.99) | – | – | – | – | – | – | – | s (37.19) | – | – | – |
| Cat‐B | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Realtime RT‐PCR (Ct value) | ||||||||||||
| Nasal swab | Oral swab | Rectal swab | ||||||||||
| Date | N1 a | N2 a | E b | RdRp b | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp |
| 7 May 21 | + (26.69) | + (31.06) | + (28.86) | + (29.75) | + (33.60) | s (38.95) | + (32.71) | – | + (30.90) | – | – | – |
| 9 May 21 | + (32.37) | – | + (34.0) | + (34.73) | s (37.80) | s (39.72) | – | – | – | – | – | – |
| 12 May 21 | s (36.39) | – | – | – | – | – | – | – | – | – | – | – |
| 19 May 21 | – | – | – | – | – | – | – | – | – | – | – | – |
| Hair/body swab | Water container swab | Floor swab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | |
| 7 May 21 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 9 May 21 | – | – | – | – | s (37.28) | – | – | – | – | – | s (36.73) | – |
| 12 May 21 | s (37.38) | – | – | – | + (35.71) | – | – | – | – | – | – | – |
| 19 May 21 | – | – | – | – | – | – | – | – | – | – | – | – |
| Dog‐C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Realtime RT‐PCR (Ct value) | ||||||||||||
| Nasal swab | Oral swab | Rectal swab | ||||||||||
| Date | N1 a | N2 a | E b | RdRp b | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp |
| 19 May 2021 | + (28.46) | s (38.03) | + (31.85) | + (35.05) | – | – | – | – | – | – | – | – |
| 22 May 2021 | + (28.18) | s (39.21) | s (36.02) | s (36.38) | + (31.22) | – | – | – | – | – | – | – |
| 25 May 2021 | – | – | – | – | – | – | – | – | – | – | – | – |
| Hair/body swab | Water container swab | Floor swab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | |
| 19 May 2021 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 22 May 2021 | + (34.01) | – | – | – | – | – | – | – | + (32.88) | – | – | – |
| 25 May 2021 | – | – | – | – | – | – | – | – | – | – | – | – |
| Dog‐D | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Realtime RT‐PCR (Ct value) | ||||||||||||
| Nasal swab | Oral swab | Rectal swab | ||||||||||
| Date | N1 a | N2 a | E b | RdRp b | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp |
| 19 May 2021 | + (32.46) | – | + (34.42) | s (36.59) | – | – | – | – | – | – | – | – |
| 22 May 2021 | – | – | – | – | + (33.94) | – | – | – | – | – | – | – |
| 25 May 2021 | – | – | – | – | – | – | – | – | – | – | – | – |
| Hair/body swab | Water container swab | Floor swab | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | N1 | N2 | E | RdRp | |
| 19 May 2021 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 22 May 2021 | – | – | – | – | – | – | – | – | – | – | – | – |
| 25 May 2021 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Primers and probes specific to the N1 gene and N2 gene of SARS‐CoV‐2 following the Centers for Disease Control and Prevention recommendations (CDC, 2020).
Primers and probes specific to the E and RdRp genes of SARS‐CoV‐2 following the WHO recommendations (Corman et al., 2020).
NA; Not available.
Cat‐B (CU27081) is a 1‐year‐old, 3 kg, domestic‐shorthair intact male cat. The animal was healthy and transferred to a private animal hospital on 7 May 2021. There were three members in household B. The owner of cat‐B was a 35‐year‐old male (owner B1) who tested positive for SARS‐CoV‐2 on 8 April 2021. An additional two members, an 85‐year‐old male (owner B2) and 80‐year‐old female (owner B3), were diagnosed with COVID‐19 on 30 April 2021. Owners B1 and B2 passed away after hospitalization in April and in May 2021, respectively. Swab samples from cat‐B were collected on four occasions between 7 and 19 May 2021. During quarantine, the cat did not show any clinical signs. Nasal, oral and rectal swabs tested positive for SARS‐CoV‐2 RNA on the first sample collection (7 May 2021) (Ct 26.69–33.60). A nasal swab was tested positive on the next sample collection (9 May 2021) (Ct 32.37–34.73). High Ct values (low viral loads) were detected from water container swabs on 12 May 2021 (Ct 35.71) (Table 2).
Dog‐C (CU27184) is a 2‐year‐old, 6 kg, mixed breed spayed female dog. The dog was transferred to a private animal hospital on 19 May 2021, after the owner (owner C1), a 26‐year‐old male, tested positive for COVID‐19 on 15 May 2021. Other members of household C, two adult females (owners C2 and C3), were diagnosed with COVID‐19 on 17 May 2021. Patient C1 was hospitalized and died in May 2021. Specimens were collected from dog‐C on three occasions between 19 and 25 May 2021. Dog‐C was healthy during the quarantine period. Nasal swabs tested positive at the first two visits (19 and 22 May 2021) (Ct 28.18–35.05). Environmental samples (hair and floor) showed high Ct values on 22 May 2021 (Ct 32.88–34.01) (Table 2).
Dog‐D (CU27186) is a 4‐year‐old, 2.75 kg, Pomeranian, spayed female dog. The dog was transferred to a private animal hospital on 19 May 2021. There were five members in household D. The owner of the dog (owner D1), an 18‐year‐old female, tested positive for COVID‐19 on 16 May 2021. All members of household D, adult males and females (owners D2‐D5) were also diagnosed with COVID‐19. Swab samples were collected from dog‐D on three occasions between 19 and 25 May 2021. Nasal swabs tested positive with a high Ct value (32.46–34.42) at the first visit (19 May 2021) and oral swab was positive (Ct 33.94) at the second visit (22 May 2021). Rectal and environmental samples tested negative (Table 2).
Serum samples collected from the dogs and cat positive for SARS‐CoV‐2 were tested for anti‐SARS‐CoV‐2 antibodies by indirect multispecies ELISA (indirect ELISA) and surrogate virus neutralization test (sVNT). Serum from dog‐A was positive by indirect ELISA at 79.66%S/P and 70.97%S/P (20–21 days after owner‐A1 tested positive, respectively). Dog‐A developed neutralizing antibodies with 42.39%−60.55% inhibition (17 days after owner‐A1 tested positive). Serum from Cat‐B, collected on 12 May 2021, was positive by both indirect ELISA (87.20%) and sVNT (90.15%) (35 days after owner B1 tested positive). Dog‐C had neutralizing antibodies with 37.20% inhibition (20 days after owner C1 tested positive). Dog‐D had neutralizing antibodies with 32.17% inhibition (27 days after owner D1 tested positive). Control dog and cat sera, pre‐COVID‐19 sera, feline coronavirus (FCoV)‐positive sera and sera from animals positive for canine respiratory coronavirus (CRCoV) and canine enteric coronavirus (CECoV) tested negative by both indirect ELISA and sVNT (Table S4).
In this study, we characterized three SARS‐CoV‐2 isolates from two dogs and a cat. Viral RNA from the nasal swabs of dog‐A (Ct 15.67‐22.19) on 4 May 2021, cat‐B (Ct 26.69‐29.75) on 7 May 2021 and dog‐C (Ct 28.46‐35.05) on 19 May 2021 was subjected to whole‐genome sequencing by Nanopore sequencing using the ARTIC primer set. The RNA from dog‐D (Ct 32.46‐36.59) was unsuccessfully sequenced due to the low viral titre. The SARS‐CoV‐2 genome sequences were obtained from dog‐A (29,778 nt), cat‐B (29,713 nt) and dog‐C (29,743 nt) and submitted to the GenBank database under the accession numbers MZ396818, MZ401455 and MZ414173, respectively (Table S5). We compared the whole genome sequences of SARS‐CoV‐2 from the dogs and cat with 942 of full‐length sequences of the viruses from Thailand. The Thai‐SARS‐CoV‐2 clustered with the viruses of the Alpha variant (B.1.1.7 lineage) which is the predominant lineage of the recent third wave of COVID‐19 outbreaks in Thailand (Figure 2). Analysis of genomic mutations of SARS‐CoV‐2 showed that the viruses from the dogs and cat contained mutations resembling the viruses of the B.1.1.7 lineage but differing from those of different lineages (B.1.36.16, A.6 and Wuhan‐Hu‐1) (Table 3).
FIGURE 2.
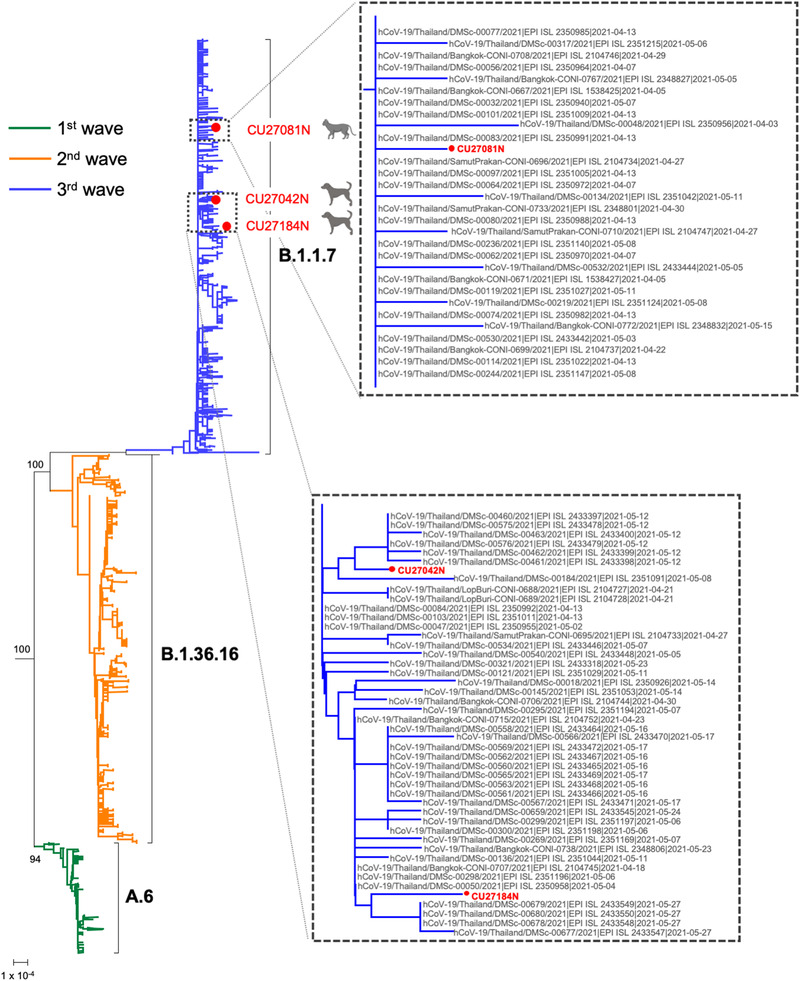
Phylogenetic tree of 942 full‐length SARS‐CoV‐2 isolates from Thailand retrieved from the GISAID database and three whole genome sequences of SARS‐CoV‐2 from the dogs and cat. The viruses isolated during the first, second and third waves are presented as green lines [first wave; n = 144], orange lines [second wave; n = 2550] and blue lines [third wave; n = 543]. The brackets indicate the lineages as A.6, B.1.36.16 and B.1.1.7. The tree was rooted by using the Wuhan‐Hu‐01 isolate. The scale bar indicates nucleotide substitutions per site
TABLE 3.
Genomic mutations of SARS‐CoV‐2 from the dogs and cat compared to those of SARS‐CoV‐2 in Thailand during the first, second and third waves. CU27042N, CU27081N and CU27184N are the SARS‐CoV‐2 isolates characterized in this study. DMSc‐00376/2021, DMSc‐00181/2021 and DMSc‐00478/2021 represent viruses from the third wave. CU‐617/2021, CU‐632/2021 and CU‐646/2021 represent viruses from the second wave. Bangkok‐CONI‐0109/2020, Bangkok‐CONI‐0290/2020 and Bangkok‐CONI‐0304/2020 represent viruses in the first wave
| ORF1a | ORF1b | Spike | ORF8 | N | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Host | Lineage | 1001* | 1708 | 2230 | 3675‐3677 | 314 | 69‐70 | 144 | 501 | 570 | 614 | 681 | 716 | 982 | 1118 | 27 | 52 | 68 | 73 | 3 | 203‐204 | 235 |
| Wuhan‐Hu‐1 | Human | B | T | A | I | SGF | P | HV | S | N | A | D | P | T | S | D | Q | R | K | Y | D | RG | S |
| CU27042N | Dog | B.1.1.7 | I | D | T | deletion | L | deletion | deletion | Y | D | G | H | I | A | H | stop | I | stop | C | L | KR | F |
| CU27081N | Cat | B.1.1.7 | I | D | T | deletion | L | deletion | deletion | Y | D | G | H | I | A | H | stop | I | stop | C | L | KR | F |
| CU27184N | Dog | B.1.1.7 | I | D | T | deletion | L | deletion | deletion | Y | D | G | H | I | A | H | stop | I | stop | C | L | KR | F |
| DMSc‐00376/2021 | Human | B.1.1.7 | I | D | T | deletion | L | deletion | deletion | Y | D | G | H | I | A | H | stop | I | stop | C | D | KR | F |
| DMSc‐00181/2021 | Human | B.1.1.7 | I | D | T | deletion | L | deletion | deletion | Y | D | G | H | I | A | H | stop | I | stop | C | L | KR | F |
| DMSc‐00478/2021 | Human | B.1.1.7 | I | D | T | deletion | L | deletion | deletion | Y | D | G | H | I | A | H | stop | I | stop | C | L | KR | F |
| CU‐617/2021 | Human | B.1.36.16 | T | A | I | SGF | L | HV | S | N | A | G | P | T | S | D | Q | R | K | Y | D | RG | S |
| CU‐632/2021 | Human | B.1.36.16 | T | A | I | SGF | L | HV | S | N | A | G | P | T | S | D | Q | R | K | Y | D | RG | S |
| CU‐646/2021 | Human | B.1.36.16 | T | A | I | SGF | L | HV | S | N | A | G | P | T | S | D | Q | R | K | Y | D | RG | S |
| Bangkok‐CONI‐0109/2020 | Human | A.6 | T | A | I | SGF | P | HV | S | N | A | D | P | T | S | D | Q | R | K | Y | D | RG | S |
| Bangkok‐CONI‐0290/2020 | Human | A.6 | T | A | I | SGF | P | HV | S | N | A | D | P | T | S | D | Q | R | K | Y | D | RG | S |
| Bangkok‐CONI‐0304/2020 | Human | A.6 | T | A | I | SGF | P | HV | S | N | A | D | P | T | S | D | Q | R | K | Y | D | RG | S |
Genome positions are based on the reference genome sequence of Wuhan‐Hu‐1 (MN908947).
4. DISCUSSION
We reported SARS‐CoV‐2 infection in three dogs and one cat in Thailand. One animal (dog‐A) showed illness with mild respiratory signs, but the other animals did not display any clinical symptoms and did not show any important blood chemistry abnormalities (Table S6). Similar to other studies, the infected dogs and cat showed non‐specific and mild respiratory signs such as nasal discharge, sneezing, coughing and inappetence (Calvet et al., 2021; Klaus et al., 2021). During quarantine and follow‐up visits, no animal died from viral infection, even though dog‐A had pre‐existing underlying diseases. It remains unclear whether infected dogs can transmit the virus to other animals, while cat to cat transmission has been observed in an experimental setting (Bosco‐Lauth et al., 2020; Shi et al., 2020). It should be noted that our results showed a high viral load (low Ct value) in environmental samples from dog‐A during quarantine at the hospital. Thus, contamination of SARS‐CoV‐2 in the environment and possible transmission from contaminated areas should not be ignored. Contamination of SARS‐CoV‐2 from animals to the environment, such as the fur and floor, has been reported in some studies (Klaus et al., 2021; Oreshkova et al., 2020). Stability of SARS‐CoV‐2 on the surface have been reported that viable virus could be detected up to 72 h on the surfaces (van Doremalen et al., 2020). Unfortunately, in this recent study, virus isolation was not performed due to the limitation of laboratory facility and the permission on live‐virus propagation. Thus, shedding of infectious or viable virus from dogs and cats could not be confirmed.
This study is the first to report SARS‐CoV‐2 infection in domestic dogs and cats in Thailand. Our results suggested that dogs and cats can acquire viral infections from households with SARS‐CoV‐2‐infected humans. Similarly, in previous reports, SARS‐CoV‐2 infection in dogs and cats in households with COVID‐19 patients has been reported in many countries (Barrs et al., 2020; Calvet et al., 2021; Gaudreault et al., 2020; Musso et al., 2020; Newman et al., 2020; Ruiz‐Arrondo et al., 2020; Sailleau et al., 2020; Segalés et al., 2020; Sit et al., 2020). In this study, the frequency of SARS‐CoV‐2 positivity in dogs (8.6%), cats (11.1%) and households (23.5%) was lower than that in previous studies in Brazil, China and the United States based on similar diagnostic assays (Barrs et al., 2020; Calvet et al., 2021; Hamer et al., 2020), but higher than that reported in some countries (Ruiz‐Arrondo et al., 2020; Sailleau et al., 2020; Sit et al., 2020). There was a limitation that only rectal swabs could be collected from some households, which could have resulted in missing some infected animals in our investigation. In contrast, serial sample collection from animals provided more opportunity to detect SARS‐CoV‐2. For example, dog‐A showed suspected results in oral and rectal swabs at the first visit, but after six additional visits, the nasal, oral and environmental swabs tested positive for SARS‐CoV‐2. Our findings support the importance of longitudinal sample collection for the investigation of SARS‐CoV‐2 infection in pet animals. Regarding the persistence of SARS‐CoV‐2 RNA, the dogs consecutively tested positive for SARS‐CoV‐2 RNA for 4 to 10 days (dog‐A, 10 days; dog‐C, 4 days and dog‐D, 4 days), while cats consecutively tested positive for 6 days (cat‐B, 6 days). In contrast, in previous studies, SARS‐CoV‐2 RNA has been observed for 14 to 31 days (in Brazil), 13 days (in China) and 25 days (in the United States) after the first positive sample (Calvet et al., 2021; Hamer et al., 2020; Shi et al., 2020; Sit et al., 2020). The dogs and cat in this study tested positive for SARS‐CoV‐2 RNA from 5 to 30 days after the index COVID‐19 owner tested positive, which is comparable to that in studies in Brazil (11 to 51 days), China (28 days) and the United States (32 days) (Calvet et al., 2021; Hamer et al., 2020; Shi et al., 2020; Sit et al., 2020).
Infected dogs and cats develop antibodies against SARS‐CoV‐2 as early as 7 to 14 days post infection. The seropositivity of dogs in a previous study varied but was higher than that in cats (Barrs et al., 2020; Hamer et al., 2020; Patterson et al., 2020). In this study, we used the parameter of the first pet owner positive by real‐time PCR as day 1 (index case). Dog‐A and cat‐B developed anti‐N IgG antibodies according to indirect ELISA, and 100% of animals developed neutralizing antibodies according to sVNT. Dog‐A and cat‐B had neutralizing antibodies as early as 17 days and 35 days, respectively. For dog‐C and dog‐D, serum samples were collected 20 to 27 days after the owner C1 and D1 positive for SARS‐CoV‐2, and we detected anti‐SARS‐CoV‐2 antibodies in the serum as expected. Cat‐B had the highest antibody level among the animals tested (87.20% by ELISA and 90.15% by VNT), which reflects the timing for serum sample collection in which the animal might have been exposed to COVID‐19 owners since 8 April 2021 (35 days after the index case, owner B1 positive). In addition, felines can develop high antibody titres, as demonstrated in previous studies (Barrs et al., 2020; Hamer et al., 2020; Patterson et al., 2020). It should be noted that the discrepancy between the result of ELISA and sVNT had been observed. It has been reported that the N‐protein‐based ELISA is less correlated with the neutralization assay (Decaro, Grassi et al., 2021; Decaro, Vaccari et al., 2021; Folegatti et al., 2020; Ni et al., 2020; Okba et al., 2020). Therefore, it was not unexpected that the negative serum of dog‐C (35.82%) and dog‐D (2.96%) based on N protein‐based ELISA had neutralizing activity (dog‐C; 37.20% and dog‐D; 32.17%).
Phylogenetic analysis was performed on three whole genome sequences from the dogs (dog‐A, dog‐C) and cat (cat‐B). Unfortunately, the limitation of this study is that the samples from SARS‐CoV‐2‐positive pet owners were not available due to limits on access of human sample collection at state quarantine facilities. Instead, the phylogenetic analysis included the full‐length sequences of SARS‐CoV‐2 from Thailand (n = 942), which are publicly available on the Global Initiative on Sharing All Influenza Data (GISAID) database. The whole genome sequences of dog‐A, cat‐B and dog‐C were clustered with human SARS‐CoV‐2 of the Alpha variant (B.1.1.7 lineage). The phylogenetic tree clearly demonstrated that the B.1.1.7 lineage was a predominant lineage of the recent third wave of COVID‐19 outbreaks in Thailand. According to the genome comparison of SARS‐CoV‐2, the SARS‐CoV‐2 isolates from the dogs and cat showed all mutations in agreement with human SARS‐CoV‐2 of the B.1.1.7 lineage (DMSc‐00376/21, DMSc‐00181/21, DMSc‐00478/21). All genomic mutations (21 positions) in the ORF1a, ORF1b, spike, ORF8 and N genes were identical for canine, feline and human isolates of the B.1.1.7 viruses lineage analyzed in the study. The mutations in the spike gene conformed to the CDC classification and definitions of the B.1.1.7 lineage (alpha was proposed for the WHO label on 15 June 2021), which is a variant of concern (CDC, 2021; WHO, 2021b). In other studies, SARS‐CoV‐2 infection in pet dogs and cats from various SARS‐CoV‐2 lineages has been reported such as the B.1.1.39 lineage in cats in Switzerland (Klaus et al., 2021), the B.1.177 lineage in a dog in Italy (Decaro, Vaccari et al., 2021) and clades G, GH and GR in dogs and cats in the United States (Hamer et al., 2020).
In summary, this study provides evidence of SARS‐CoV‐2 infection in domestic dogs and a cat from COVID‐19‐positive households during quarantine at the private animal hospitals in Thailand. The role of dogs and cats in SARS‐CoV‐2 transmission among species or across species from animals to humans is still unclear. However, SARS‐CoV‐2 RNA was observed in environmental samples, thus possible transmission from contaminated areas should not be ignored. This study supports the role of the One Health approach in the mitigation and control of emerging infectious diseases, such as COVID‐19, for improving human and animal health.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
ETHICAL APPROVAL
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Science, Chulalongkorn University, Thailand (IACUC No. 2031035). This study complies with the ARRIVE guidelines. Verbal consent was obtained from all pet owners and private animal hospital staff after explaining the objectives and benefits of the study during sample collection.
AUTHOR CONTRIBUTION
WJ, KC and AA designed the study and drafted the manuscript. AA edited and approved the manuscript. WJ, KC, EC, KU, NB, SB, RTt and NT investigated SARS‐CoV‐2‐ positive households and collected samples from animals. WJ, KC, NB, SB, SS and RTw curated demographic information of dogs, cats and pet owners. WJ, KC, EC, KU, SC and NT performed molecular diagnosis, serological diagnosis and viral whole‐genome sequencing. WJ, KC, EC, KU, KS and AA performed viral sequence analysis. All authors provided critical review and approved the manuscript.
Supporting information
Supporting information.
Supplement Table 1. List of COVID‐19‐positive households from which dogs and cats were sampled in this study
Supplement Table 2. List of the primers and probes used for SARS‐CoV‐2 detection in this study
Supplement Table 3. Clinical signs of a SARS‐CoV‐2‐infected dog (dog‐A) in this study
Supplement Table 4. Anti‐N IgG antibodies and neutralizing antibodies in the SARS‐CoV‐2‐ infected dogs and cat
Supplement Table 5. Detail information of Oxford Nanopore MinION sequencer result of SARS‐CoV‐2 in this study.
Supplement Table 6. Complete blood count and blood chemistry profiles of the SARS‐CoV‐2‐infected dogs and cat in this study
ACKNOWLEDGEMENTS
We would like to thank the Chulalongkorn University animal hospital and private animal hospitals for their collaboration on sample collection. We thank the pet owners and the office of Disease Prevention and Control, Department of Disease Control, Ministry of Public Health for their support on data curation. Chulalongkorn University supported the Ph.D. scholarship of the second century fund (C2F) to the first author (WJ). Chulalongkorn University supported the Center of Excellence for Emerging and Re‐emerging Infectious Diseases in Animals (CUEIDAs) and the One Health Research cluster. This research was partially funded by Chulalongkorn University's TSRI Fund (CU_FRB640001_01_31_1), the Agricultural Research Development Agency (ARDA) (PRP6405031220) and the office of National Higher Education Science Research and Innovation Policy Council (NXPO‐PMU‐B) (B17F640011).
Jairak, W. , Charoenkul, K. , Chamsai, E. , Udom, K. , Chaiyawong, S. , Bunpapong, N. , Boonyapisitsopa, S. , Tantilertcharoen, R. , Techakriengkrai, N. , Surachetpong, S. , Tangwangvivat, R. , Suwannakarn, K. , & Amonsin, A. (2022). First cases of SARS‐CoV‐2 infection in dogs and cats in Thailand. Transboundary and Emerging Diseases, 69, e979–e991. 10.1111/tbed.14383
DATA AVAILABILITY STATEMENT
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files. The data that support the findings of this study have been deposited at the GenBank with accession numbers MZ396818, MZ401455 and MZ414173.
REFERENCES
- Baker, D. J. , Aydin, A. , Le‐Viet, T. , Kay, G. L. , Rudder, S. , De Oliveira Martins, L. , Tedim, A. P. , Kolyva, A. , Diaz, M. , Alikhan, N. ‐. F. , Meadows, L. , Bell, A. , Gutierrez, A. V. , Trotter, A. J. , Thomson, N. M. , Gilroy, R. , Griffith, L. , Adriaenssens, E. M. , Stanley, R. , … O'grady, J. (2021). CoronaHiT: High‐throughput sequencing of SARS‐CoV‐2 genomes. Genome Medicine, 13(1), 21. 10.1186/s13073-021-00839-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs, V. R. , Peiris, M. , Tam, K. W. S. , Law, P. Y. T. , Brackman, C. J. , To, E. M. W. , Yu, V. Y. T. , Chu, D. K. W. , Perera, R. A. P. M. , & Sit, T. H. C. (2020). SARS‐CoV‐2 in quarantined domestic cats from COVID‐19 households or close contacts, Hong Kong, China. Emerging Infectious Diseases, 26(12), 3071–3074. 10.3201/eid2612.202786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , Vandewoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences of the USA, 117(42), 26382–26388. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet, G. A. , Pereira, S. A. , Ogrzewalska, M. , Pauvolid‐Corrêa, A. , Resende, P. C. , Tassinari, W. D. S. , Costa, A. D. P. , Keidel, L. O. , Da Rocha, A. S. B. , Da Silva, M. F. B. , Dos Santos, S. A. , Lima, A. B. M. , De Moraes, I. C. V. , Mendes Junior, A. A. V. , Souza, T. D. C. , Martins, E. B. , Ornellas, R. O. , Corrêa, M. L. , Antonio, I. M. D. S. , … Menezes, R. C. (2021). Investigation of SARS‐CoV‐2 infection in dogs and cats of humans diagnosed with COVID‐19 in Rio de Janeiro, Brazil. PLoS One, 16(4), e0250853. 10.1371/journal.pone.0250853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . (2020). Research use only 2019‐novel coronavirus (2019‐nCoV) real‐time RT‐PCR primers and probes . https://www.cdc.gov/coronavirus/2019‐ncov/lab/rt‐pcr‐panel‐primer‐probes.html
- CDC . (2021). SARS‐CoV‐2 variant classifications and definitions . https://www.cdc.gov/coronavirus/2019‐ncov/variants/variant‐info.html
- Corman, V. M. , Landt, O. , Kaiser, M. , Molenkamp, R. , Meijer, A. , Chu, D. K. , Bleicker, T. , Brünink, S. , Schneider, J. , Schmidt, M. L. , Mulders, D. G. , Haagmans, B. L. , van der Veer, B. , van den Brink, S. , Wijsman, L. , Goderski, G. , Romette, J. ‐ L. , Ellis, J. , Zambon, M. , … Drosten, C. (2020). Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveillance, 25(3). 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Balboni, A. , Bertolotti, L. , Martino, P. A. , Mazzei, M. , Mira, F. , & Pagnini, U. (2021). SARS‐CoV‐2 infection in dogs and cats: Facts and speculations. Frontiers in Veterinary Science, 8, 619207. 10.3389/fvets.2021.619207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Grassi, A. , Lorusso, E. , Patterson, E. I. , Lorusso, A. , Desario, C. , Anderson, E. R. , Vasinioti, V. , Wastika, C. E. , Hughes, G. L. , Valleriani, F. , Colitti, B. , Ricci, D. , Buonavoglia, D. , Rosati, S. , Cavaliere, N. , Paltrinieri, S. , Lauzi, S. , Elia, G. , & Buonavoglia, C. (2021). Long‐term persistence of neutralizing SARS‐CoV‐2 antibodies in pets. Transboundary and Emerging Diseases, 10.1111/tbed.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Vaccari, G. , Lorusso, A. , Lorusso, E. , De Sabato, L. , Patterson, E. I. , Di Bartolo, I. , Hughes, G. L. , Teodori, L. , Desario, C. , Colitti, B. , Ricci, D. , Buonavoglia, D. , Rosati, S. , Martella, V. , Cammà, C. , Agrimi, U. , & Elia, G. (2021). Possible human‐to‐dog transmission of SARS‐CoV‐2, Italy, 2020. Emerging Infectious Diseases, 27(7), 1981–1984. 10.3201/eid2707.204959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folegatti, P. M. , Ewer, K. J. , Aley, P. K. , Angus, B. , Becker, S. , Belij‐Rammerstorfer, S. , Bellamy, D. , Bibi, S. , Bittaye, M. , Clutterbuck, E. A. , Dold, C. , Faust, S. N. , Finn, A. , Flaxman, A. L. , Hallis, B. , Heath, P. , Jenkin, D. , Lazarus, R. , Makinson, R. , … Yau, Y. (2020). Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: A preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet, 396(10249), 467–478. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreault, N. N. , Trujillo, J. D. , Carossino, M. , Meekins, D. A. , Morozov, I. , Madden, D. W. , Indran, S. V. , Bold, D. , Balaraman, V. , Kwon, T. , Artiaga, B. L. , Cool, K. , García‐Sastre, A. , Ma, W. , Wilson, W. C. , Henningson, J. , Balasuriya, U. B. R. , & Richt, J. A. (2020). SARS‐CoV‐2 infection, disease and transmission in domestic cats. Emerging Microbes & Infections, 9(1), 2322–2332. 10.1080/22221751.2020.1833687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, S. A. , Pauvolid‐Correa, A. , Zecca, I. B. , Davila, E. , Auckland, L. D. , Roundy, C. M. , Tang, W. , Torchetti, M. , Killian, M. L. , Jenkins‐Moore, M. , Mozingo, K. , Akpalu, Y. , Ghai, R. R. , Spengler, J. R. , Behravesh, C. B. , Fischer, R. S. B. , & Hamer, G. L. (2020). Natural SARS‐CoV‐2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID‐19 cases in Texas, USA. bioRxiv, 10.1101/2020.12.08.416339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , Misawa, K. , Kuma, K. , & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14), 3059–3066. 10.1093/nar/gkf436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus, J. , Meli, M. L. , Willi, B. , Nadeau, S. , Beisel, C. , Stadler, T. , & Hofmann‐Lehmann, R. (2021). Detection and genome sequencing of SARS‐CoV‐2 in a domestic cat with respiratory signs in Switzerland. Viruses, 13(3). 10.3390/v13030496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7), 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcaloose, D. , Laverack, M. , Wang, L. , Killian, M. L. , Caserta, L. C. , Yuan, F. , Mitchell, P. K. , Queen, K. , Mauldin, M. R. , Cronk, B. D. , Bartlett, S. L. , Sykes, J. M. , Zec, S. , Stokol, T. , Ingerman, K. , Delaney, M. A. , Fredrickson, R. , Ivančić, M. , Jenkins‐Moore, M. , … Diel, D. G. (2020). From people to Panthera: Natural SARS‐CoV‐2 infection in tigers and lions at the Bronx zoo. MBio, 11(5). 10.1128/mBio.02220-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Reimerink, J. , Torriani, G. , Brouwer, F. , Godeke, G. ‐ J. , Yerly, S. , Hoogerwerf, M. , Vuilleumier, N. , Kaiser, L. , Eckerle, I. , & Eckerle, I. (2020). Validation and clinical evaluation of a SARS‐CoV‐2 surrogate virus neutralisation test (sVNT). Emerging Microbes & Infections, 9(1), 2394–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso, N. , Costantino, A. , La Spina, S. , Finocchiaro, A. , Andronico, F. , Stracquadanio, S. , Liotta, L. , Visalli, R. , & Emmanuele, G. (2020). New SARS‐CoV‐2 infection detected in an Italian pet cat by RT‐qPCR from deep pharyngeal swab. Pathogens, 9(9), 746. 10.3390/pathogens9090746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A. , Smith, D. , Ghai, R. R. , Wallace, R. M. , Torchetti, M. K. , Loiacono, C. , Murrell, L. S. , Carpenter, A. , Moroff, S. , Rooney, J. A. , & Barton Behravesh, C. (2020). First reported cases of SARS‐CoV‐2 infection in companion animals—New York, March–April 2020. Morbidity and Mortality Weekly Report, 69(23), 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, L. , Ye, F. , Cheng, M.‐L.i , Feng, Y.u , Deng, Y. ‐. Q. , Zhao, H. , Wei, P. , Ge, J. , Gou, M. , Li, X. , Sun, L. , Cao, T. , Wang, P. , Zhou, C. , Zhang, R. , Liang, P. , Guo, H. , Wang, X. , Qin, C. ‐. F. , … Dong, C. (2020). Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity, 52(6), 971‐977.e3. 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . (2021a). Considerations for sampling, testing, and reporting of SARS‐CoV‐2 in animals . https://www.oie.int/fileadmin/Home/MM/A_Sampling_Testing_and_Reporting_of_SARS‐CoV‐2_in_animals_3_July_2020.pdf
- OIE . (2021b). SARS‐CoV‐2 in animals. Situation report 1. OIE. [Google Scholar]
- Okba, N. M. A. , Müller, M. A. , Li, W. , Wang, C. , Geurtsvankessel, C. H. , Corman, V. M. , Lamers, M. M. , Sikkema, R. S. , De Bruin, E. , Chandler, F. D. , Yazdanpanah, Y. , Le Hingrat, Q. , Descamps, D. , Houhou‐Fidouh, N. , Reusken, C. B. E. M. , Bosch, B. ‐. J. , Drosten, C. , Koopmans, M. P. G. , & Haagmans, B. L. (2020). Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease patients. Emerging Infectious Diseases, 26(7), 1478–1488. 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova, N. , Molenaar, R. J. , Vreman, S. , Harders, F. , Oude Munnink, B. B. , Hakze‐Van Der Honing, R. W. , Gerhards, N. , Tolsma, P. , Bouwstra, R. , Sikkema, R. S. , Tacken, M. G. , De Rooij, M. M. , Weesendorp, E. , Engelsma, M. Y. , Bruschke, C. J. , Smit, L. A. , Koopmans, M. , Van Der Poel, W. H. , & Stegeman, A. (2020). SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveillance, 25(23). 10.2807/1560-7917.ES.2020.25.23.2001005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. I. , Elia, G. , Grassi, A. , Giordano, A. , Desario, C. , Medardo, M. , Smith, S. L. , Anderson, E. R. , Prince, T. , Patterson, G. T. , Lorusso, E. , Lucente, M. S. , Lanave, G. , Lauzi, S. , Bonfanti, U. , Stranieri, A. , Martella, V. , Solari Basano, F. , Barrs, V. R. , … Decaro, N. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. Nature Communications, 11(1), 6231. 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut, A. , Holmes, E. C. , O'toole, Á. , Hill, V. , Mccrone, J. T. , Ruis, C. , Du Plessis, L. , & Pybus, O. G. (2020). A dynamic nomenclature proposal for SARS‐CoV‐2 lineages to assist genomic epidemiology. Nature Microbiol, 5(11), 1403–1407. 10.1038/s41564-020-0770-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz‐Arrondo, I. , Portillo, A. , Palomar, A. M. , Santibanez, S. , Santibanez, P. , Cervera, C. , & Oteo, J. A. (2020). Detection of SARS‐CoV‐2 in pets living with COVID‐19 owners diagnosed during the COVID‐19 lockdown in Spain: A case of an asymptomatic cat with SARS‐CoV‐2 in Europe. Transboundary and Emerging Diseases, 68(2), 973–976. 10.1111/tbed.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailleau, C. , Dumarest, M. , Vanhomwegen, J. , Delaplace, M. , Caro, V. , Kwasiborski, A. , Hourdel, V. , Chevaillier, P. , Barbarino, A. , Comtet, L. , Pourquier, P. , Klonjkowski, B. , Manuguerra, J. ‐. C. , Zientara, S. , & Le Poder, S. (2020). First detection and genome sequencing of SARS‐CoV‐2 in an infected cat in France. Transboundary and Emerging Diseases, 67(6), 2324–2328, 10.1111/tbed.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalés, J. , Puig, M. , Rodon, J. , Avila‐Nieto, C. , Carrillo, J. , Cantero, G. , Terrón, M. T. , Cruz, S. , Parera, M. , Noguera‐Julián, M. , Izquierdo‐Useros, N. , Guallar, V. , Vidal, E. , Valencia, A. , Blanco, I. , Blanco, J. , Clotet, B. , & Vergara‐Alert, J. (2020). Detection of SARS‐CoV‐2 in a cat owned by a COVID‐19− affected patient in Spain. Proceedings of the National Academy of Sciences, 117(40), 24790–24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , Sun, Z. , Zhao, Y. , Liu, P. , Liang, L. , Cui, P. , Wang, J. , Zhang, X. , Guan, Y. , Tan, W. , Wu, G. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS‐coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. C. , Brackman, C. J. , Ip, S. M. , Tam, K. W. S. , Law, P. Y. T. , To, E. M. W. , Yu, V. Y. T. , Sims, L. D. , Tsang, D. N. C. , Chu, D. K. W. , Perera, R. A. P. M. , Poon, L. L. M. , & Peiris, M. (2020). Infection of dogs with SARS‐CoV‐2. Nature, 586, 776‐778, 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, C. W. , Chia, W. N. , Qin, X. , Liu, P. , Chen, M. I. .‐ C. , Tiu, C. , Hu, Z. , Chen, V. C.‐.W. , Young, B. E. , Sia, W. R. , Tan, Y. ‐. J. , Foo, R. , Yi, Y. , Lye, D. C. , Anderson, D. E. , & Wang, L. ‐ F. (2020). A SARS‐CoV‐2 surrogate virus neutralization test based on antibody‐mediated blockage of ACE2‐spike protein‐protein interaction. Nature Biotechnology, 38(9), 1073–1078. 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- Van Doremalen, N. , Bushmaker, T. , Morris, D. H. , Holbrook, M. G. , Gamble, A. , Williamson, B. N. , Tamin, A. , Harcourt, J. L. , Thornburg, N. J. , Gerber, S. I. , Lloyd‐Smith, J. O. , De Wit, E. , & Munster, V. J. (2020). Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. New England Journal of Medicine, 382(16), 1564–1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsker, M. , Moosa, Y. , Nooij, S. , Fonseca, V. , Ghysens, Y. , Dumon, K. , Pauwels, R. , Alcantara, L. C. , Vanden Eynden, E. , Vandamme, A. ‐. M. , Deforche, K. , & De Oliveira, T. (2019). Genome Detective: An automated system for virus identification from high‐throughput sequencing data. Bioinformatics, 35(5), 871–873. 10.1093/bioinformatics/bty695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2021a). Coronavrus disease 2019 (COVID‐19) WHO Thailand situation report No.187 . Thailand Situation Update No. 187 https://reliefweb.int/report/thailand/coronavirus‐disease‐2019‐covid‐19‐who‐thailand‐situation‐report‐187‐10‐june‐2021
- WHO . (2021b). Tracking SARS‐CoV‐2 variants. 15 June 2021 . https://www.who.int/en/activities/tracking‐SARS‐CoV‐2‐variants/
- WHO . (2021c). WHO Coronavirus (COVID‐19) dashboard. 2 July 2021 . https://covid19.who.int/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supplement Table 1. List of COVID‐19‐positive households from which dogs and cats were sampled in this study
Supplement Table 2. List of the primers and probes used for SARS‐CoV‐2 detection in this study
Supplement Table 3. Clinical signs of a SARS‐CoV‐2‐infected dog (dog‐A) in this study
Supplement Table 4. Anti‐N IgG antibodies and neutralizing antibodies in the SARS‐CoV‐2‐ infected dogs and cat
Supplement Table 5. Detail information of Oxford Nanopore MinION sequencer result of SARS‐CoV‐2 in this study.
Supplement Table 6. Complete blood count and blood chemistry profiles of the SARS‐CoV‐2‐infected dogs and cat in this study
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files. The data that support the findings of this study have been deposited at the GenBank with accession numbers MZ396818, MZ401455 and MZ414173.


