Abstract
A severe pandemic of Coronavirus Disease (COVID‐19) has been sweeping the globe since 2019, and this time, it did not stop, with frequent mutations transforming into virulent strains, for instance, B.1.1.7, B.1.351, and B.1.427. In recent months, a fungal infection, mucormycosis has emerged with more fatal responses and significantly increased mortality rate. To measure the severity and potential alternative approaches against black fungus coinfection in COVID‐19 patients, PubMed, Google Scholar, World Health Organization (WHO) newsletters, and other online resources, based on the cases reported and retrospective observational analysis were searched from the years 2015–2021. The studies reporting mucormycosis with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) coinfection and/or demonstrating potential risk factors, such as a history of diabetes mellitus or suppressed immune system were included, and reports published in non‐English language were excluded. More than 20 case reports and observational studies on black fungus coinfection in COVID‐19 patients were eligible for inclusion. The results indicated that diabetes mellitus, hyperglycemic, and immunocompromised COVID‐19 patients with mucormycosis were at a higher risk. We found that it was prudent to assess the potential risk factors and severity of invasive mycosis via standardized diagnostic and clinical settings. Large‐scale studies need to be conducted to identify early biomarkers and optimization of diagnostic methods has to be established per population and geographical variation. This will not only help clinicians around the world to detect the coinfection in time but also will prepare them for future outbreaks of other potential pandemics.
Keywords: black fungus, COVID‐19, diagnosis, fungal infection, mucormycosis
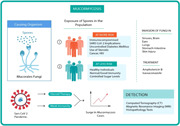
Highlights
Observational studies and case reports of Post‐COVID black fungus co‐infection highlighted in review.
Along with risk factors, radiological interventions of black fungus co‐infection in COVID‐19 patients and challenges for accurate diagnosis were elaborated.
The review discusses interconnection between fungus and SARS‐CoV‐2 co‐infection of mechanism to identify potential biomarkers.
Our conclusion will contribute to motivate researchers to design follow‐up plans for black fungus infection in COVID‐19.
1. INTRODUCTION
The outbreak of Coronavirus Disease 2019 (COVID‐19) labeled as lethal viral pneumonia, commenced as an epidemic in Wuhan, China in December 2019, and since then, 201 million active cases, 4.6 million deaths have been confirmed worldwide as of August 06, 2021. The United States, India, Brazil, Russia, and France are the majorly affected countries, where coronavirus is not only exhibiting rapid infection but also a high mortality rate. The origin of fatal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) variants and pathogenic coinfections were also reported to increase at a linear rate, especially in India. 1 , 2 , 3 Critically ill and hospitalized COVID‐19 patients were reported with a dysregulated immune system, 4 , 5 which increases the risk of coinfections, such as mucormycosis (black fungus), pulmonary aspergillosis, and candidiasis. 6 , 7 Several physicians were reporting and documenting an alarming number of black fungus coinfection among the COVID‐19 patients in earlier the year 2021.
Mucormycosis (zygomycosis) is an acute and fatal, but rare fungal infection caused by a category of molds called mucormycetes, under subphylum Mucormycotina and order Mucorales. 8 , 9 A total of 11 genera and ~27 species have been reported under order Mucorales, among them, Rhizopus is the predominant genera followed by Mucor and Lichtheimia. 10 Mucorales fungi are commonly found in hematological malignancies and stem cells or solid organ transplantation. 11 , 12 Recently, an epidemical emergency of a black fungus with an aggressive and contiguous spread in COVID‐19 patients has been reported. Patients who had a history of diabetes mellitus, immunocompromised conditions, and recently underwent bone marrow transplants were vulnerable to this coinfection. The COVID‐19 condition also provides an ideal environment, such as hypoxia, high glucose (diabetes or steroid‐induced hyperglycemia), increased ferritin, low phagocytic activity of white blood cells (WBCs) to facilitate germination of fungal spores, metabolic acidosis, and diabetes ketoacidosis induced acidic medium. 13 Additionally, the altered response of T‐helper cells, higher proinflammatory and anti‐inflammatory cytokines, and cytokine release syndrome that induce lung pathology in COVID‐19 patients also promote pulmonary microbial proliferation. 14 , 15 Although, several case reports and observational studies on mucormycosis in COVID‐19 patients have been reported, information on frequency, incidence, and susceptibility profiles of secondary infections remain scarce, even after the availability of detailed COVID‐19 data. 16 It is also challenging for clinicians to differentiate between the SARS‐CoV‐2 virus and possible superadded fungus infection.
Progressive coinfection of mucormycosis in COVID‐19 patients has caused new challenges for researchers, and clinicians, which includes identification and diagnosis of the pathogens. Initially, in 2021, India became a major hotspot for black fungus coinfection with SARS‐CoV‐2, and widespread use of steroids in COVID‐19 treatment increased the risk of mucormycosis. Additionally, India is reported to have ~80 times higher incidence rate of mucormycosis in comparison to developed countries, 17 therefore, the second invasion of SARS‐CoV‐2 had dramatic effects on human health and the economy. Health‐care professionals were facing problems with timely diagnosis and appropriate treatment while considering underlying comorbid conditions of the patients. Moreover, the shortage of medical resources such as beds and medications for mucormycosis‐SARS‐CoV‐2 coinfection is not only affecting health‐care systems, it also requires collecting measurable evidence to prepare the medical world for future outbursts. With all this in mind, the present systematic review focused on the detailed summary of mucormycosis cases reported in COVID‐19 patients, with a major focus on current limitations and challenges in diagnostics and pharmaceutical interventions.
2. MATERIAL AND METHODS
To design a systematic search strategy to investigate fungal infection in COVID‐19 positive patients; PubMed (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar (https://scholar.google.com/), and other online web sources were searched from the last 5 years and analyzed using terminologies such as “COVID‐19,” “SARS‐CoV‐2,” “Fungal infection,” “Mucormycete,” “Black Fungus,” “mucormycosis,” “Immune signaling,” “Immunotherapy,” “Diagnosis,” and “Treatment.” The criteria for the selection of observational or case reports as primary studies was their surveillance of (i) COVID‐19 patients of any age and gender with a fungal infection, (ii) In‐house hospitalized and discharged COVID‐19 patients with immunocompromised diseases and comorbid conditions, (iii) clinical studies or literature reviews on prescribed treatment and diagnostic methods for COVID‐19 coinfection. We excluded studies that reported clinical efficacy and mortality rate with no targeted outcomes. Unpublished data, thesis work, press releases, and literature in other languages (except English) were also excluded. Furthermore, to provide relevant information, articles focusing on potential risks of SARS‐CoV‐2 and “black fungus” in the Indian scenario and optimal risk were profoundly studied.
3. RESULTS
The preliminary screening of the literature search assimilated 237 potential results, among them 146 articles fulfilled the inclusion criteria, containing 135 published articles, and 11 online web sources. After abstract and methodology screening, 81 articles and documents were shortlisted, and 65 were excluded, due to 59 articles' specified exclusion criteria and 6 articles being in a language other than English. The flowchart of articles' selection and exclusion is illustrated in Figure 1.
Figure 1.
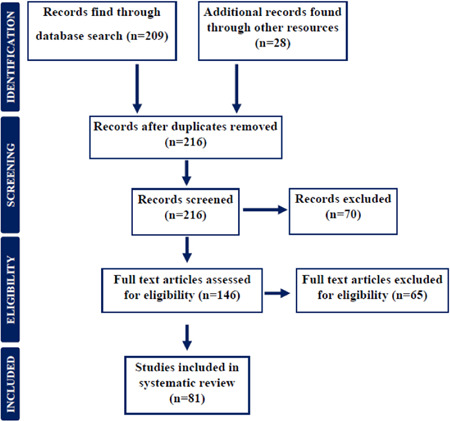
Preferred reporting flow chart for including and studying studies and case reports for systematic review
4. DISCUSSION
Respiratory viral infections predispose the patients to coinfection, which further increases disease severity and mortality. The influenza outbreak in 1918 and the H1N1 influenza pandemic in the year 2009 were initially reported to associate with subsequent bacterial infections, especially Streptococcus pneumonia (S. pneumonia). 18 , 19 Despite the demonstration of severe coinfection existence in respiratory diseases, the current situation of COVID‐19 with fungal coinfections is understudied. Fungal infections were not very common, yet were serious complications in SARS‐CoV‐2 induced pneumonia in critically ill or hospitalized patients. In our literature search, very limited articles reported on fungal coinfection in COVID‐19 patients, and very few provided details of the associated mechanism of action of pathogens. Even so, we analyzed these studies and focused on potential outcomes to control the epidemic.
4.1. Fungal coinfection in COVID‐19 patients
Similar to other respiratory diseases, such as influenza, in which ~25% of older adult patients acquire secondary coinfections, COVID‐19 patients were also reporting similar fungal coinfection. 20 However, to date very limited data are available regarding the impact of fungal coinfection and relevant clinical outcomes for COVID‐19 patients. However, the severity of hospitalized patients with steroid medications and comorbid conditions toward fungal coinfection is higher than home‐quarantine SARS‐CoV‐2‐positive patients. Therefore, there is a clinical demand for a vigorous investigation into coinfection with COVID‐19.
In this direction, Zhang et al. performed a single‐center, retrospective case series of 55 severe and 166 nonsevere COVID‐19‐positive patients and concluded that 3.2% of 221 patients had fungal coinfection. 21 Similarly, a retrospective study published in The Lancet also confirmed coinfection of Aspergillus flavus (A. flavus), Candida glabrata (C. glabrata), and Candida albicans (C. albicans) in COVID‐19 patients. These patients demonstrated fever, cough, shortness of breath, sore throat, muscle ache, confusion, and headache during coinfection. A total of 17 out of 99 patients developed Acute Respiratory Distress Syndrome (ARDS) and out of that 17, 11 patients died. In this study, COVID‐19 infection was reported to raise body temperature, breathlessness, osmolarity, and hypoxic condition during and post‐SARS‐CoV‐2 infection. 22 These conditions are favorable to fungus invasion, growth, and pathogenic development inside the human body. Additionally, Salehi et al. also found coinfected COVID‐19 patients with C. albicans (70.7%), C. glabrata (10.7%), C. dubliniensis (9.2%), C. parapsilosis sensu stricto (4.6%), C. tropicalis (3%), and C. krusei (1.5%) in 53 hospitalized COVID‐19 patients. 23 Patients majorly reported respiratory distress and prolonged fever followed by lymphopenia, leukopenia, and leukocytosis. In summary, overlapped symptoms and masked laboratory tests in the above studies are indicating the possibility of certain interactions between fungi and SARS‐CoV‐2, which is yet to be explored at the molecular level. However, potential association and risk factors in SARS‐CoV‐2 and black fungus coinfection are illustrated in Figure 2.
Figure 2.
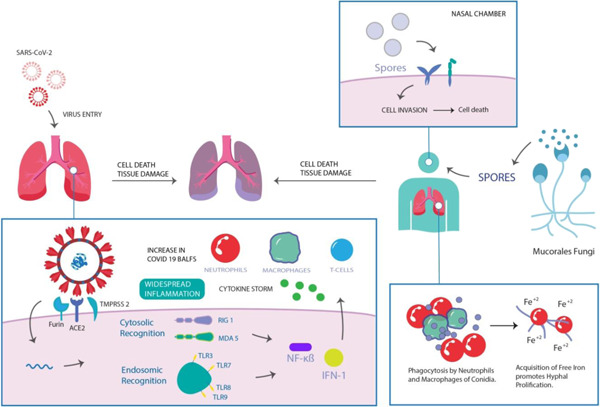
Representation of the potential association between SARS‐CoV‐2 and mucormycosis
Major fungi with SARS‐CoV‐2 coinfection belong to the category of Aspergillus, Candida, and Klebsiella, 24 which were reported as common secondary infections. Unlike last year, “black fungus” coinfection was a major cause of death in COVID‐19 patients, especially in India. 25 , 26 Looking back on the SARS epidemic in the year 2003, China‐based studies stated that 14.8%–27% of SARS patients were coinfected with fungus and the mortality rate was exaggerated by 25%–73.7%, which was higher than that of severely ill patients (up to 21.9%–33%). 27 , 28 In a similar direction, studies demonstrated 5.0% 29 and 5.8% 30 COVID‐19 positive critically ill patients with fungal coinfection. However, the low infection rate did not alarm the health authorities at the time. Moreover, additional shared risk factors were studied in COVID‐19 patients to further explore the potential association between the pathogens. For this, not limited to the severity of coinfections in the intensive care unit (ICU) hospitalized COVID‐19 patients, the following characteristics were also recognized 14 , 31 , 32 :
-
(1)
Profound immunosuppression due to disease severity or genetic inheritance.
-
(2)
Cytokine storm during hematopoietic transplantation.
-
(3)
Heavily mechanically ventilated during long‐term hospitalization.
-
(4)
Corticosteroid medications during diabetes mellitus.
-
(5)
Broad‐spectrum antibacterial drugs during severe SARS‐CoV‐2 infection.
-
(6)
Prolonged trauma, neutropenia, and Human Immunodeficiency Virus infection/Acquired Immune Deficiency Syndrome (HIV/AIDS) (Cluster of Differentiation 4 (CD4) < 200 cells/μl).
The differential symptoms of SARS‐CoV‐2 and fungal coinfection are yet to be discovered, and the balance between underlying disease and SARS‐CoV‐2 infection as a potential risk factor for fungal infection has not been identified to date. 31 Therefore, surveys at a large scale and sufficient descriptions of symptoms during patients' registration should be made mandatory.
4.2. Diagnostic methods for mucormycosis in COVID‐19 coinfection
Gradual increment in mucormycosis in COVID‐19 patients across the globe is causing an additional challenge in the identification and diagnosis of fungal infection on time. In this direction, the French High Council for Public Health has recommended systematic screening of mucormycosis coinfection in COVID‐19 patients. 31 The symptoms of mucormycosis are not specific, however, ophthalmoplegia, headache, watery eyes, blackish appearance on the skin or mucosa, and fever are reported in mucormycosis and SARS‐CoV‐2 coinfection. 33 , 34 , 35 Fungal coinfection in COVID‐19 patients is generally suspected on the basis of direct microscopical imaging or plus fluorescent brighteners in biological samples such as sputum, skin lesions, and Bronchoalveolar Lavage Fluid (BALF), unlike COVID‐19 detection. 36 , 37 , 38 Radiographic features, such as pansinusitis or ethmoid sinusitis, nodular lesions, and reverse halo signs are also helpful in invasive mucormycosis detection in COVID‐19 patients. 36
However, in terms of laboratory tests, mucormycosis shows negative results in galactomannan index and beta 1, 3‐d‐glucan (BDG) assays, unlike Aspergillus, whereas R. oryzae is known to have beta‐glucan synthase to synthesize beta‐glucan. 39 Hence, this assay needs to be modified and revised as per biomarkers associated with SARS‐CoV‐2 coinfection, as it was negative in one case report. 40 Additionally, antigen‐antibody‐based serological tests, such as Enzyme‐Linked Immunosorbent Assay (ELISA), immunoblots, and immunodiffusion tests can be also used to detect mucormycosis coinfection in COVID‐19 patients. 37 , 41 In this direction, a monoclonal antibody (namely, 2DA6) that recognizes α−1,6‐linked mannose, can be used, which is conserved in both Zygomycota and Ascomycota. 37 Therefore, the proposed immunodiagnostic method cannot be considered to differentiate specific black fungus in COVID‐19 patients. Molecular‐based assays include amplification methods such as Polymerase Chain Reaction (PCR), Restriction Fragment Length Polymorphism (RFLP), real‐time PCR, and sequencing to identify mucormycosis. 37 , 42 These methods mainly target Internal Transcribed Spacer (ITS) or 18 S rRNA gene of the pathogens, however, ITS is highly specific for Mucorales detection, unlikely to detect other fungi which come under Mucormycetes. 41 Additionally, these in‐house assays are not widely studied, and clinical evaluation is still missing. Therefore, Matrix‐Assisted Laser Desorption Ionization‐Time of Flight Mass Spectrometry (MALDI‐TOF MS), metabolomics, or culture‐based additional detection tools could have been recommended. 37 But, MALDI‐TOF does not have access to a robust mold spectral database of filamentous fungi, this could limit their use in black fungus detection 43 and false‐negative results can be obtained in the case of mucormycosis in SARS‐CoV‐2 coinfection.
Currently, microscopic examinations and culture of mucormycosis and SARS‐CoV‐2 coinfection are recommended for reliable detection. The histopathological examination in a study published in BMJ Case Report confirmed broad aseptate hyphae at an obtuse angle of the ethmoid sinus in fungal colonies, and further culture of the sample confirmed the presence of Rhizopus spp. based mucormycosis. 44 In another study, brown‐colored sporangiospores and nodal rhizoids in hyphae were also identified in COVID‐19 patients with a history of diabetes mellitus. 45 To further confirm the diagnosis, hematoxylin‐eosin (HE), periodic acid‐Schiff (PAS), or Grocott methenamine silver (GMS) stains can be used to show tissue invasion of nonpigmented hyphae. 41 However, microscopical examinations and cell culture techniques require technical expertise to perform and analyze the results. Additionally, laboratories with higher bio‐safety level are the primary requirement in SARS‐CoV‐2 coinfection detection. Therefore, reliable and feasible alternatives, for instance, lab‐on‐chip or advanced Artificial Neural Network (ANN) associated radiology techniques are suggested to be adopted in future diagnostics.
4.3. SARS‐CoV‐2 and mucormycosis (black fungus) coinfection
The emergence of the second wave of SARS‐CoV‐2 and fatal viral variants across the globe has presented a formidable challenge to clinicians and health professionals. Many decisions were made based on limited clinical sources and scientific evidence for hospitalized COVID‐19 treatment. Bacteria, especially S. pneumonia and fungal coinfections have common complications, which were also present in other pandemics. 46 , 47 However, information on fungal coinfections in COVID‐19 patients, their incidence, and clinical approaches for treatment have been scarce. Therefore, our study gathered information and epidemiology of COVID‐19 and fungal coinfections, especially mucormycosis (black fungus) to provide potential guidelines.
A life‐threatening condition of mucormycosis is an invasive and progressive fungal infection with a 50% mortality rate. 48 However, a majority of 70% of cases from all mucormycosis is caused by R. arrhizus, which is recently reported to be linked with SARS‐CoV‐2 coinfection. 49 , 50 The main risk factor of mucormycosis is reported higher in COVID‐19 patients with comorbid conditions of rhino‐orbital‐cerebral, uncontrolled diabetes mellitus with ketoacidosis, gastric and pulmonary diseases, hematological malignancy, and long‐term corticosteroid usage. 40 , 51 , 52 These factors confirmed the potential synergistic effect between black fungus and SARS‐CoV‐2 pathogens. The major overlapping potential mechanisms involved in the proposed coinfection might be as follows:
-
(1)
Fungal coinfection in COVID‐19 was reported to increase the degree of systemic inflammation, hence, inflation in disease severity, and mortality rate were observed in the patients. To evaluate this concept, Tan et al. reported an increased level of proinflammatory cytokines, especially Interleukin‐6 (IL‐6), which was associated with severe lung injury in coinfected patients. 53 Additionally, lymphocytes (such as B‐cells, T‐cells, and natural killer cells [NK]) were reported to be altered and affected host immune functions to provide a suitable environment for coinfection. 54 , 55
-
(2)
Patients with diabetes mellitus also have dysfunctional immune system components due to severe inflammatory states. Low level of C4 complement protein along with altered functionality of neutrophils, truncated response to cytokines and reduced IL‐10 production was reported as the result of increased glycosylation in diabetic patients. 56 , 57 These immune responses along with reduced polymorphonuclear leukocyte mobilization, chemotaxis, and phagocytic activity in relevant patients are the results of lower Major Histocompatibility Complex I (MHC‐I) cell expression in the relevant patients. 58 , 59 , 60 These dysregulations of immune cells in hyperglycemic condition can get worse by endothelial cell disruption, which may lead to multiorgan damage in SARS‐CoV‐2 patients. The above circumstances create favorable conditions for the successful invasion and attachment of Mucorales hyphae inside the human body.
-
(3)
The immunocompromised patients were reported to release iron through sequestering proteins in host cells, 61 which was consumed by the fungi, that is, Mucorales, via high‐affinity iron permease 62 and they gradually grow in favorable conditions in the host. In hyperglycemic conditions, SARS‐CoV‐2 coronavirus also contributes to iron metabolism dysfunction and posing a risk of black‐fungus coinfection. 63 High ferritin levels in COVID‐19 patients were reported to release reactive oxygen species (ROS) and damage nearby tissues. 64 , 65 Additionally, diabetic ketoacidosis condition in COVID‐19 patients also increases acidic environment and ultimately enhances the free ferric ion level to provide momentum for black fungus growth.
-
(4)
In terms of COVID‐19 medications, mainly steroids and antibiotics are under clinical prescription in the current pandemic. 10 Although, steroids reduce inflammation in SARS‐CoV‐2 infection, they also lower immune system activity, which alters WBCs and T‐helper cells production. 66 This immunocompromised state opens the door for Mucorales invasion and multiplies growth at a rapid rate.
4.4. Category of mucormycosis and SARS‐CoV‐2 coinfection
4.4.1. COVID‐19 and pulmonary mucormycosis
Pulmonary mucormycosis in COVID‐19 coinfection generally occurs after inhalation of fungal sporangiospores, and being an angioinvasive agent, it causes infarction of tissues. 67 A study published in CMAJ in April 2020 indicated involvement of fungal infection in a COVID‐19 patient (40 years, female) with a history of diabetic ketoacidosis. 36 The right lower‐lobe cavitary was observed in chest Computed Tomography (CT), whereas sputum microscopy indicated pauci‐septate filamentous fungus, which suggested the presence of Rigidoporus microporus. However, the patient showed no symptoms of mucormycosis on the skin, no necrotic lesion was observed, and she recovered after intravenous liposomal amphotericin B administration. In a similar study, a COVID‐19 patient (44 years, female) with a history of diabetes also showed diffused Ground‐Glass Opacities (GGOs) and several cavitary lesions in the chest to identify SARS‐CoV‐2, whereas bronchoscopy confirmed gray‐colored necrotic lesion near the left upper lobe and lingual. 68 The cell culture results revealed the presence of pauci‐septate hyphae consistent with zygomycetes, however, it could not differentiate among C. albicans, C. glabrata, and C. krusei along with A. flavus and A. niger. These studies indicate the difficulty in mucormycosis detection; therefore, clinicians should start fungal treatment in COVID‐19 patients with uncontrolled diabetes mellitus and compromised immune systems along with hypoxemia and fever.
The current scenario of mucormycosis is not restricted to simultaneous SARS‐CoV‐2 coinfection, patients with post‐COVID also triggered the risk for fatal fungal infection. A case study of post‐COVID infection of the patient (72 years, male), with a history of diabetes mellitus, showed a nodule in the right upper lobe, the posterior segment with central cavitating necrosis in Positron Emission Tomography (PET)‐CT imaging. 69 The radiographical features of the right arm and thigh of the patient also exhibited hypermetabolic mediastinal nodes and hypermetabolic soft tissue nodules. These alterations were visually confirmed by histopathology images through the presence of nonseptate fungal hyphae, as shown in Figure 3. These studies confirmed more prominent COVID‐19 and mucormycosis coinfection in the hyperglycemic and immunocompromised patients, who also stayed longer in ICU.
Figure 3.
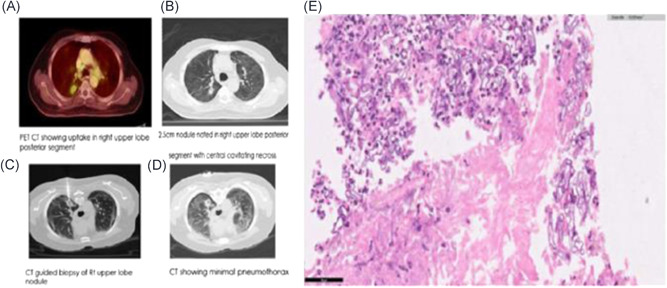
Identification of mucormycosis in COVID‐19 patients: (A), (B) Positron Emission Tomography (PET)‐Computed tomography (CT) imaging demonstrating nodule in right upper lobe posterior segment along with cavitating necrosis. (C), (D) Hypermetabolic mediastinal nodes with soft tissue nodules in right arms and thigh. (E) Nonseptate fungal hyphae, suggestive of mucormycosis via microscopy/histopathological imaging representation. Reproduced from (69), Copyright 2021, with permission from IP Indian Journal of Immunology and Respiratory Medicine. COVID‐19, coronavirus disease 2019
4.4.2. COVID‐19 and mucormycosis coinfection severity in diabetes mellitus
Though mucormycosis infection is rare, it is reported in several COVID‐19 patients and cases reported published to guide clinicians and researchers for appropriate treatment. An article published in Cambridge University Press demonstrated a prospective observational study to analyze possible associations between mucormycosis and post‐COVID‐19 conditions in an India‐based population. 33 The CT images demonstrated paranasal sinuses with intracranial involvement in 8.69% of the cases, and Magnetic Resonance Imaging (MRI) confirmed the intra‐orbital extension of mucormycosis in 43.47% of the cases, as shown in Figure 4. These results also confirmed the risk factor of diabetes mellitus in 98% of cases (21 out of 23), where the patients had a history of steroid medications during COVID‐19 treatment. A multicentric retrospective study in India also showed a major risk factor in diabetes mellitus (75%) in COVID‐19 and mucormycosis coinfection in comparison to chronic kidney disease (12.5%), chronic liver disease (6.25%), pulmonary tuberculosis (TB) (6.25%), and patients on immunosuppressive agents (6.25%). 70
Figure 4.
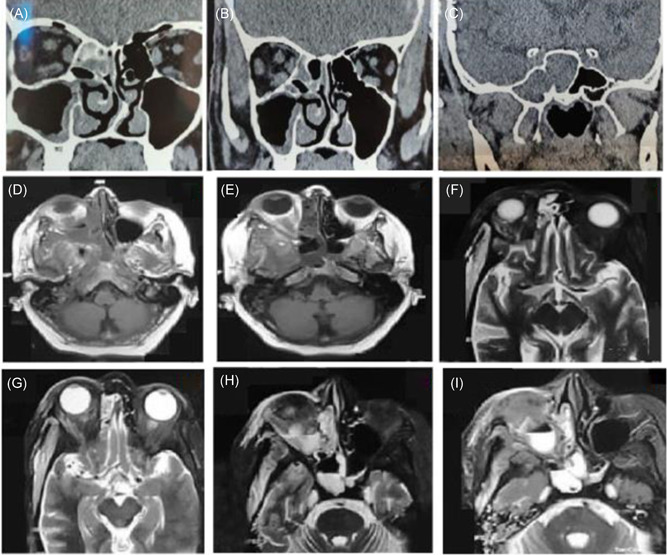
Post‐COVID mucormycosis radiographic imaging presenting: (A−C) Computed tomography (CT) scans of intracranial involvement in paranasal sinuses, ethmoid group. (D−I) Axial, magnetic resonance imaging of mucormycosis induced intra‐orbital extension in post‐COVID patients. Reproduced from (33), Copyright 2021, with permission Cambridge Press.COVID, coronavirus disease
Glucocorticoids are reported to cause immunosuppressive effects via transcriptional upregulation or repression of specific genes, especially nuclear factor κB (NF‐κB). 71 , 72 It also affects T‐lymphocyte activation, lower T‐lymphocyte proliferation, lymphokine migration, and induces delayed‐type hypersensitivity 73 and severe impact on post‐COVID‐19 illness. A study published in Mycopathologia analyzed the risk of uncontrolled diabetes mellitus in several cases. 45 In this study, COVID‐19‐positive patients with last‐stage kidney disease and diabetes mellitus identified with mucormycosis after >21 days. Additionally, hyphae with nodal rhizoids and brown‐colored sporangiospores in spherical sporangia in terminal sporangiophores were also observed in Lactophenol Cotton Blue (LCB) mount, which suggested the presence of R. microsporus. This case report confirmed the glucocorticoids induced hyperglycemic condition in COVID‐19 patients, which aggravated mortality by 87.5% due to diabetes mellitus, ARDS, and multiorgan dysfunction. However, another study of pulmonary mucormycosis by Zurl et al. showed that a patient (53 years, male) was hospitalized after treatment for secondary Acute Myeloid Leukemia (AML) and severe neutropenia. 40 The slight bilateral infiltrates in CT images and lower platelet along with lymphocyte counts in laboratory tests confirmed SARS‐CoV‐2 infection. After treatment with tocilizumab and high‐dose glucocorticoids, the patient slightly recovered; leukocyte cytospin test, viral PCRs, and fungal biomarkers were negative, and no new infiltrates were observed in chest X‐ray. Lung autopsy after death the patient confirmed the invasive pulmonary mucormycosis, however, unlike lung sample, PCR of throat swab sample showed SARS‐CoV‐2 coinfection in the patient. This study suggested a correlation between intensive chemotherapy and Myelodysplastic Syndromes (MDS), which leads to a prolonged neutropenic phase and can be considered as an additional risk factor for potential pulmonary fungal infection. The ARDS condition and corticosteroid treatment are also reported to trigger a fungal infection in COVID‐19 patients, and the patient showed positive tests against fungal biomarkers, such as galactomannan and 1,3‐ß‐d‐glucan. However, a routine biomarker for mucormycosis detection was lacking and made the situation complicated to diagnose coinfection rapidly and accurately.
4.4.3. COVID‐19 and mucormycosis coinfection associated with conjunctival mucosa
Not restricted to pulmonary, nasal, or oral mucormycosis, conjunctival mucosa (rhino‐orbito‐cerebral) derived fungal infection is another coinfection in COVID‐19 patients. COVID‐19 has a propensity to induce extensive pulmonary disease along with alveolo‐interstitial pathology, which is itself a risk factor for invasive fungal infection in sinuses and lungs. 74
Major rhino‐orbito‐cerebral mucormycosis‐associated cases have been found in COVID‐19 patients with a history of ketoacidosis diabetes mellitus with initial symptoms of acute sinusitis, nasal discharge, headache, and fever, which later spread to the orbital system. The first case in this regard was published in Ophthalmic plastic and reconstructive surgery, where, a COVID‐19 patient (60 years, male) with insulin‐dependent diabetes, demonstrated asymmetric retrobulbar fat stranding and extensive opacification of right maxillary, ethmoid, and frontal sinuses, and was suspected for acute invasive fungal rhinosinusitis with mild proptosis, erythema, and edema of the eyelids and conjunctival chemosis. 75 Treatment with retrobulbar injections of liposomal amphotericin B had to be replaced with posaconazole due to acute kidney injury, while dexamethasone had to be stopped due to hyperglycemic condition. This led to the death of the patient and the case suggested finding other alternatives such as tocilizumab (IL‐6 inhibitor) for SARS‐CoV‐2 infection. Similarly, in another study, a COVID‐19 patient (33 years, female) reported progressive left lid swelling and maxillary hypoesthesia, which further added proptosis with hyperemic conjunctiva and an opaque cornea. 52 Swelling of soft tissue residing in the left inferior turbinate and thickening of maxillary, ethmoid, and sphenoid mucosa on the ipsilateral side were observed in the patient. She also exhibited proptosis‐associated soft tissue swelling in the left side of preorbital and midfacial structures in CT imaging and clinicians suspected rhino‐orbital mucormycosis. However, after treatment with imipenem/linezolid and amphotericin B, the patient faced refractory metabolic acidosis along with pulmonary insult and disseminated intravascular coagulopathy induced acute kidney injury, which led to death with unresponsive septic shock. Another case in Lilavati Hospital and Research Center, Mumbai, India showed bilateral lid edema with right eye prominence after managing diabetes in a hospitalized COVID‐19 patient (60 years, male). 74 The MRI imaging illustrated, soft tissue swelling in right preseptal, malar, premaxillary and retrobulbar regions, mucosal thickening in frontal, maxillary, and ethmoidal sinus (Figure 5). The ophthalmic evaluation identified proptotic right eye with conjunctival edema in the periorbital region with necrosis in soft tissue along with medial half of upper and lower lids, which were signs of keratitis. Along with radio‐diagnostic imaging and clinical picture, an invasive nasal biopsy from middle turbinate in Sabourauds Dextrose Agar culture also identified broad aseptate filamentous fungal hyphae, likely to mucormycosis. Similarly, opacification of paranasal sinuses and their extension to the posterior orbital space were observed through CT imaging, whereas the MRI showed disease extension into the anterior cranial fossa in an Iran‐based case report. 76 Another case in this study also exhibited unilateral opacifications of the left orbit and paranasal sinuses along with endoscopically observed blackish necrotic tissues in paranasal sinuses in the SARS‐CoV‐2 and mucormycosis coinfected patient.
Figure 5.
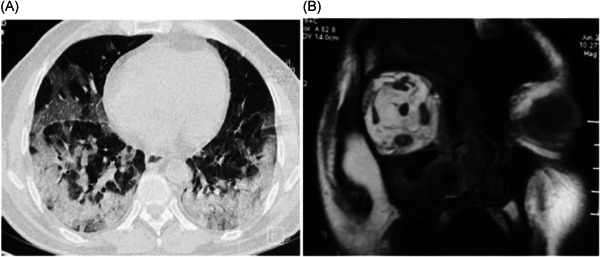
(A) Computed tomography (CT) representation of peripheral ground‐glass opacities in both lungs of mucormycosis in SARS‐CoV‐2 coinfection. (B) Magnetic resonance imaging (MRI) imaging of coronal section (T1) presenting irregular hypersensitivity of retrobulbar space in COVID‐19 patient, which suggested the presence of fungal infection. Reproduced from (74), Copyright 2020, with permission Cureus
Furthermore, sino‐orbital, 77 rhinocerebral, 78 rhino‐orbital cerebral, 79 and gastrointestinal 51 mucormycosis with SARS‐CoV‐2 coinfection are other major concerns in the present pandemic situation. Angioinvasion and subsequent thrombosis‐induced tissue necrosis are hallmarks of cerebral involvement with mucormycosis and present as black necrotic eschars. 79 However, in patients with rhinocerebral mucormycosis and SARS‐CoV‐2 coinfection, the typical finding on cranial MRI imaging showed cavernous sinus enhancement with an intracranial abscess in infratemporal fossa and fungal extension into the sinus. 78 For additional sensitivity, a chest X‐ray was indicated because it revealed atelectasis and pneumonia in the left lobe of the lungs, whereas a CT scan showed peripheral bilateral lung infiltrate along with chronic sinusitis. Another aggravation of mucormycosis in the gastrointestinal tract was also reported in a COVID‐19‐positive patient (86 years, male) and CT imaging confirmed SARS‐CoV‐2 infection with GGO with consolidative abnormalities. 51 However, large gastric ulcers at greater and lesser curvature with dirty debris and deep hemorrhagic base in the absence of active bleeding were observed via esophagogastroduodenoscopy (EGD). These studies showed the severity of mucormycosis infection and their rapid progression in COVID‐19 patients, especially with diabetes comorbid conditions. Therefore, health‐care professionals and authorized clinicians should act promptly in the direction of adopting a multidisciplinary approach in diagnostic and therapeutic interventions for the reversal of an underlying condition.
5. CONCLUDING REMARKS
In this systematic review, we studied ~30 case reports and observational studies in which patients showed mucormycosis in COVID‐19 patients, among them, 70% of patients died due to the lack of timely diagnosis. These studies also demonstrated that COVID‐19‐associated mucormycosis is majorly linked to immunity deterioration, extensive use of steroids, and broad‐spectrum antibiotics, used during treatment of critically ill SARS‐CoV‐2 patients. The curve of multipathogen coinfection in COVID‐19 patients also increased in the initial month of the year 2021 and has compromised reliable clinical diagnosis and treatment. Therefore, clinicians should be aware of the possibility of secondary infections, especially in patients with pre‐existing risk factors, such as diabetes mellitus, neutropenia, and immunocompromised conditions (HIV, tumor, or organ transplant). In this study, we analyzed case reports and observational studies that have reported pulmonary, orbital, cerebral, and gastrointestinal mucormycosis involvement in SARS‐CoV‐2 coinfection and concluded that ~80% of cases had a history of hyperglycemic conditions. However, post‐COVID mucormycosis showed a lower risk of fungal coinfection‐associated mortality. We consider that it is important to identify the potential association among biomarkers and pathways of relevant coinfection of the current scenario. As PCR is a major diagnostic method for SARS‐CoV‐2 detection and histopathology is mainly considered for mucormycosis, radio diagnostic methods are the most reliable technique for coinfection detection. Still, no standard protocols have been developed or differentiating features classified to date that can demonstrate the severity of SARS‐CoV‐2 and mucormycosis individually. Our purpose in this article was to assist clinicians in the management of fungal coinfection in COVID‐19 patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The work is financially supported by the Extramural Research grant (File No. EMR/2016/007564) and Young Scientist Scheme (YSS/2015/000023) by the Science and Engineering Research Board (SERB), Government of India; Technology Development Program (TDP) (TDP/BDTD/33/2019), the Department of Science and Technology (DST), Government of India; Biotechnology Industry Research Assistance Council (BIRAC) (File No. BT/IIPME0211/02/16), Government of India and Department of Biotechnology (DBT), Government of India, which are highly acknowledged.
Soni S, Pudake RN, Jain U, Chauhan N. A systematic review on SARS‐CoV‐2‐associated fungal coinfections. J Med Virol. 2021;94:99‐109. 10.1002/jmv.27358
Contributor Information
Utkarsh Jain, Email: ujain@amity.edu.
Nidhi Chauhan, Email: nchauhan1@amity.edu.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Mallapaty S. What's the risk of dying from a fast‐spreading COVID‐19 variant? Nature. 2021;590(7845):191‐192. [DOI] [PubMed] [Google Scholar]
- 2. Thiagarajan K. Why is India having a covid‐19 surge? BMJ. 2021;373:n1124. [DOI] [PubMed] [Google Scholar]
- 3. Pemán J, Ruiz‐Gaitán A, García‐Vidal C, et al. Fungal co‐infection in COVID‐19 patients: should we be concerned? Rev Iberoam Micol. 2020;37(2):41‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allegra A, Di Gioacchino M, Tonacci A, Musolino C, Gangemi S. Immunopathology of SARS‐CoV‐2 infection: immune cells and mediators, prognostic factors, and immune‐therapeutic implications. Int J Mol Sci. 2020;21(13):4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chauhan N, Soni S, Jain U. Optimizing testing regimes for the detection of COVID‐19 in children and older adults. Expert Rev Mol Diagn. 2021:1‐18. 10.1080/14737159.2021.1962708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khurana S, Singh P, Sharad N, et al. Profile of co‐infections & secondary infections in COVID‐19 patients at a dedicated COVID‐19 facility of a tertiary care Indian hospital: implication on antimicrobial resistance. Indian J Med Microbiol. 2021;39(2):147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wee LE, Ko KKK, Ho WQ, Kwek GTC, Tan TT, Wijaya L. Community‐acquired viral respiratory infections amongst hospitalized inpatients during a COVID‐19 outbreak in Singapore: co‐infection and clinical outcomes. J Clin Virol. 2020;128:104436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwon‐Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (Zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012;54(suppl_1):S8‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morace G, Borghi E. Invasive mold infections: virulence and pathogenesis of mucorales. Int J Microbiol. 2012;2012:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahalaxmi I, Jayaramayya K, Venkatesan D, et al. Mucormycosis: an opportunistic pathogen during COVID‐19. Environ Res. 2021;201:111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antoniadi K, Iosifidis E, Vasileiou E, et al. Invasive mucormycosis in children with malignancies: report from the infection working group of the hellenic society of pediatric hematology‐oncology. J Pediatr Hematol Oncol. 2021;43(5):176‐179. [DOI] [PubMed] [Google Scholar]
- 12. Wand O, Unterman A, Izhakian S, Fridel L, Kramer MR. Mucormycosis in lung transplant recipients: a systematic review of the literature and a case series. Clin Transplant. 2020;34(2):e13774. [DOI] [PubMed] [Google Scholar]
- 13. Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID‐19: a systematic review of cases reported worldwide and in India. Diabetes Metab Syndr Clin Res Rev. 2021;15(4):102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lansbury L, Lim B, Baskaran V, Lim WS. Co‐infections in people with COVID‐19: a systematic review and meta‐analysis. J Infect. 2020;81(2):266‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chauhan N, Soni S, Gupta A, Aslam M, Jain U. Interpretative immune targets and contemporary position for vaccine development against SARS‐CoV‐2: a systematic review. J Med Virol. 2020;93(4):1967‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID‐19: a retrospective cohort study in a UK secondary‐care setting. Clin Microbiol Infect. 2020;26(10):1395‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Diabetes Federation. 2020. https://idf.org/our-network/regions-members/south-east-asia/members/94-india.html. Accessed May 21, 2021.
- 18. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962‐970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacIntyre CR, Chughtai AA, Barnes M, et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis. 2018;18(1):637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63(8):766‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vellingiri B, Jayaramayya K, Iyer M, et al. COVID‐19: a promising cure for the global panic. Sci Total Environ. 2020;725:138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu X, Ge Y, Wu T, et al. Co‐infection with respiratory pathogens among COVID‐2019 cases. Virus Res. 2020;285:198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song G, Liang G, Liucor W. Fungal co‐infections associated with global COVID‐19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;87:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahoo JP, Mishra AP, Pradhan P, Samal KC. Misfortune never comes alone ‐ the new “Black Fungus” accompanying COVID‐19 wave. Biot Res Today. 2021;3(5 SE‐Articles):318‐320. [Google Scholar]
- 27. Zhang Y, Li WX, Huang KW, Cao ZX, Hao JY. Hospital acquired pneumonia occurring after acute stage of the serious SARS and its treating strategies. Nosocom Infect China. 2003;11(13):1081‐1087. [Google Scholar]
- 28. Yin CH, Wang C, Tang Z, Zhang SW, Wang BS. Clinical analysis of 146 patients with critical severe acute respiratory syndrome in Beijing areas. Clin J Emerg Med. 2004;1(13):12‐14. [Google Scholar]
- 29. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gangneux J‐P, Bougnoux M‐E, Dannaoui E, Cornet M, Zahar JR. Invasive fungal diseases during COVID‐19: we should be prepared. J Mycol Med. 2020;30(2):100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Silva LN, de Mello TP, de Souza Ramos L, Branquinha MH, Roudbary M, dos Santos ALS. Fungal infections in COVID‐19‐positive patients: a lack of optimal treatment options. Curr Top Med Chem. 2020;20(22):1951‐1957. [DOI] [PubMed] [Google Scholar]
- 33. Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dyer O. Covid‐19: India sees record deaths as “black fungus” spreads fear. BMJ. 2021;373:n1238. [DOI] [PubMed] [Google Scholar]
- 35. Sahoo JP, Panda B, Mishra AP, Samal KC. The unseen “fungal infections”–an extra thrust aggravating COVID second wave in India. Biot Res Today. 2021;3(5 SE‐Articles):354‐356. [Google Scholar]
- 36. Thomas L, Tay SY, Howard D, Falhammar H. Mucormycosis in a 40‐year‐old woman with diabetic ketoacidosis. Can Med Assoc J. 2020;192(16):E431‐E433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18(10):845‐854. [DOI] [PubMed] [Google Scholar]
- 38. Chauhan N, Soni S, Gupta A, Jain U. New and developing diagnostic platforms for COVID‐19: a systematic review. Expert Rev Mol Diagn. 2020;20(9):971‐983. [DOI] [PubMed] [Google Scholar]
- 39. Kontoyiannis DP. A potential explanation of a positive serum β‐Glucan assay in mucormycosis. Open Forum Infect Dis. 2016;3(4):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zurl C, Hoenigl M, Schulz E, et al. Autopsy proven pulmonary mucormycosis due to rhizopus microsporus in a critically Ill COVID‐19 patient with underlying hematological malignancy. J Fungi. 2021;7(2):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Skiada A, Lass‐Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):S93‐S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lackner M, Caramalho R, Lass‐Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol. 2014;9(5):683‐695. [DOI] [PubMed] [Google Scholar]
- 43. Patel R. MALDI‐TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61(1):100‐111. [DOI] [PubMed] [Google Scholar]
- 44. Revannavar SM, P SS, Samaga L, V KV. COVID‐19 triggering mucormycosis in a susceptible patient: a new phenomenon in the developing world? BMJ Case Rep. 2021;14(4):e241663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Garg D, Muthu V, Sehgal IS, et al. Coronavirus disease (Covid‐19) associated mucormycosis (CAM): case report and systematic review of literature. Mycopathologia. 2021;186:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abelenda‐Alonso G, Rombauts A, Gudiol C, et al. Influenza and bacterial coinfection in adults with community‐acquired pneumonia admitted to conventional wards: risk factors, clinical features, and outcomes. Open Forum Infect Dis. 2020;7(3):066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burrell A, Huckson S, Pilcher DV. ICU Admissions for sepsis or pneumonia in Australia and New Zealand in 2017. N Engl J Med. 2018;378(22):2138‐2139. [DOI] [PubMed] [Google Scholar]
- 48. González Ballester D, González‐García R, Moreno García C, Ruiz‐Laza L, Monje Gil F. Mucormycosis of the head and neck: report of five cases with different presentations. J Cranio‐Maxillofacial Surg. 2012;40(7):584‐591. [DOI] [PubMed] [Google Scholar]
- 49. Garcia‐Vidal C, Sanjuan G, Moreno‐García E, et al. Incidence of co‐infections and superinfections in hospitalized patients with COVID‐19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alves RC, Ferreira JS, Alves AS, et al. Systemic and gastrohepatic mucormycosis in dogs. J Comp Pathol. 2020;175:90‐94. [DOI] [PubMed] [Google Scholar]
- 51. Monte Junior E, Santos M, Ribeiro IB, et al. Rare and fatal gastrointestinal mucormycosis (zygomycosis) in a COVID‐19 patient: a case report. Clin Endosc. 2020;53(6):746‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Waizel‐Haiat S, Guerrero‐Paz JA, Sanchez‐Hurtado L, Calleja‐Alarcon S, Romero‐Gutierrez L. A case of fatal rhino‐orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID‐19. Cureus. 2021;13(2):e13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Luo Y, Xie Y, Zhang W, et al. Combination of lymphocyte number and function in evaluating host immunity. Aging. 2019;11(24):12685‐12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID‐19) in a patient co‐infected by HIV with a low CD4+ T‐cell count. Int J Infect Dis. 2020;96:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pramanik P, Pramanik P. Coincidence of uncontrolled diabetes mellitus and COVID‐19 Is a serious threat to Mucormycosis: a systematic review. World Wide J Multidiscip Res Dev. 2021;7(7):6‐13. [Google Scholar]
- 57. Singh A, Ahmad N, Varadarajan A, et al. Lactoferrin, a potential iron‐chelator as an adjunct treatment for mucormycosis–a comprehensive review. Int J Biol Macromol. 2021;187:988‐998. [DOI] [PubMed] [Google Scholar]
- 58. Erener S. Diabetes, infection risk and COVID‐19. Mol Metab. 2020;39:101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pal R, Banerjee M. Are people with uncontrolled diabetes mellitus at high risk of reinfections with COVID‐19? Prim Care Diabetes. 2021;15(1):18‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Brandão SCS, Ramos J, Dompieri LT, et al. Is Toll‐like receptor 4 involved in the severity of COVID‐19 pathology in patients with cardiometabolic comorbidities? Cytokine Growth Factor Rev. 2021;58:102‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Leal SM, Roy S, Vareechon C, et al. Targeting iron acquisition blocks infection with the fungal pathogens Aspergillus fumigatus and Fusarium oxysporum . PLoS Pathog. 2013;9(7):e1003436. Feldmesser Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morales‐Franco B, Nava‐Villalba M, Medina‐Guerrero EO, et al. Host‐pathogen molecular factors contribute to the pathogenesis of rhizopus spp. in diabetes mellitus. Curr Trop Med Reports. 2021;8(1):6‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. John TM, Jacob CN, Kontoyiannis DP. When uncontrolled diabetes mellitus and severe COVID‐19 converge: the perfect storm for mucormycosis. J Fungi. 2021;7(4):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID‐19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136:111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Muhoberac BB. What can cellular redox, iron, and reactive oxygen species suggest about the mechanisms and potential therapy of COVID‐19? Front Cell Infect Microbiol. 2020;10:569709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Szarpak L, Chirico F, Pruc M, Szarpak L, Dzieciatkowski T, Rafique Z. Mucormycosis—a serious threat in the COVID‐19 pandemic? J Infect. 2021;83(2):237‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kanwar A, Jordan A, Olewiler S, Wehberg K, Cortes M, Jackson BR. A Fatal case of rhizopus azygosporus pneumonia following COVID‐19. J Fungi. 2021;7(3):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Khan N, Gutierrez CG, Martinez DV, Proud KC. A case report of COVID‐19 associated pulmonary mucormycosis. Arch Clin Cases. 2020;07(03):46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kumar Chennamchetty V, Adimulapu S, Patel Kola B, De Padua M, C A, Raghavendra Rao MV. Post‐COVID pulmonary mucormycosis—a case report. IP Indian J Immunol Respir Med. 2021;6(1):62‐66. [Google Scholar]
- 70. Paul O, Tao JQ, West E, et al. Clinical characteristics and outcomes of 16 cases with COVID19 and mucormycosis: experience from a tertiary care center in India and review of literature. Res Sq. 2021. 10.21203/rs.3.rs-533347/v1 [DOI] [Google Scholar]
- 71. Ehrchen JM, Roth J, Barczyk‐Kahlert K. More than suppression: glucocorticoid action on monocytes and macrophages. Front Immunol. 2019;10:2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Escoter‐Torres L, Caratti G, Mechtidou A, Tuckermann J, Uhlenhaut NH, Vettorazzi S. Fighting the fire: mechanisms of inflammatory gene regulation by the glucocorticoid receptor. Front Immunol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus. 2020;12(9):10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute invasive rhino‐orbital mucormycosis in a patient with COVID‐19‐associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37(2):e40‐e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino‐orbital mucormycosis during steroid therapy in COVID‐19 patients: a case report. Eur J Ophthalmol. 2021:112067212110094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Maini A, Tomar G, Khanna D, Kini Y, Mehta H, Bhagyasree V. Sino‐orbital mucormycosis in a COVID‐19 patient: a case report. Int J Surg Case Rep. 2021;82:105957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Alekseyev K, Didenko L, Chaudhry B. Rhinocerebral mucormycosis and COVID‐19 pneumonia. J Med Cases. 2021;12(3):85‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. Secondary infection with rhino‐orbital cerebral mucormycosis associated with COVID‐19. Orbit. 2021:0:1‐5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


