List of Symbols and Abbreviations
- 95% CI
95% Conficence Intervals
- ACE
angiotensin‐converting enzyme
- CHARMS
checklist for critical appraisal and data extraction for systematic reviews of prediction modeling studies
- COVID‐19
Coronavirus disease 2019
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- GWAS
Genome‐wide association studies
- HLA‐B
Human Leukocyte Antigen B
- ICU
Intensive Care Unit
- OR
odds ratio
- PCR
polymerase chain reaction
- PICOTS
Population, Index predictive/prognostic factor, Predictive/prognostic comparators, Outcomes, Timing and Setting
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TMPRSS2
type 2 transmembrane serine proteases
- WHO
World Health Organization
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has been emerged as a global health emergency with consequences of magnitude both at health, social, and economy level. According to the World Health Organization (WHO), as for May 25, 2021, more than 167,000,000 confirmed COVID‐19 cases have been confirmed, including 3,472,068 deaths. 1
Several risk factors, both modifiable and nonmodifiable, could influence the susceptibility of acquiring severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. The severity of the symptoms among infected patients varies considerably from being asymptomatic to developing a critical illness with lethal complications, and some genetic and clinical characteristics of patients have been proposed as determinants of poor outcomes. 2
SARS‐CoV‐2 uses the angiotensin‐converting enzyme 2 (ACE2) receptor for cell entry, and thus it has been suggested that host genetic factors may play a role in susceptibility to COVID‐19. According to published evidence, people with ACE2 polymorphism who have type 2 transmembrane serine proteases (TMPRSS2) would be at a higher risk of SARS‐CoV‐2 infection. In addition, patients possessing HLA‐B*15:03 genotype may become immune to the infection. 3
Demographic factors may have an impact in the risk of COVID‐19 infection, disease severity, and mortality. Recently published systematic reviews found that men and patients with advanced age have a higher risk for COVID‐19 infection and poor prognosis. 2 , 4 Additionally, obesity, smoking, hypertension, diabetes, malignancy, coronary heart disease, hypertension, chronic liver disease, chronic obstructive pulmonary disease, and chronic kidney disease have been also correlated to severe COVID‐19 symptoms. 2 Acute respiratory distress syndrome, shock, and acute kidney injury were most likely to prevent recovery. 2
Blood group might also be associated with the susceptibility for SARS‐CoV‐2 infection and also with the risk of developing severe COVID‐19. Different mechanisms have been proposed that might explain the association between ABO blood groups and SARS‐CoV‐2 infection. It has been suggested that anti‐A and/or anti‐B antibodies serve as viral neutralizing antibodies by binding to A and/or B antigens expressed on the viral envelope, thereby preventing the interaction of the virus and ACE2 receptor, lastly limiting the entry of the virus into the lung epithelium and infection of target cells. 5 As for disease complications, the increased ACE1 activity in group A individuals could predispose to cardiovascular complications, accounting for severe COVID‐19. 5 In addition, variation of von Willebrand factor and Factor VIII levels by ABO type, with higher levels in group A individuals, could contribute to risk of thromboembolic disease and severe COVID‐19. 5 Another hypothesis is that ABO blood group influences the glycosyltransferase activity and the risk of venous thromboembolism, which is frequent in severe COVID‐19 cases. 6 Genome‐wide association studies (GWAS) also demonstrated that ABO locus variants correlate with increased plasma lipid and inflammatory markers. 5 Although the association between ABO group and COVID‐19 infection and severity is plausible in terms of biological mechanisms, its translation into clinical significance is uncertain. Analyzing this issue is of high interest, as ABO group of the patients may be an additional factor that could influence the preventive and therapeutic management. For instance, the identification of a blood group as a risk or prognostic factor may guide individual or collective decisions on vaccination (especially when prioritization is required due to resource constraints) or the use of preventive measures or treatments at an early stage, and it also may suggest novel treatment targets and enhance the design of new studies, introducing this characteristic as a covariate. Since the beginning of the pandemic, numerous studies have been published examining the association of ABO blood group with the risk of COVID‐19 infection or severity, and some reviews have attempted to synthesize this evidence. 7 , 8 , 9 , 10 , 11 However, they have important limitations and show contrasting and inconclusive results.
The main objectives of this systematic review were to analyze the association between ABO blood group and the risk of COVID‐19 infection (objective 1) and the association between ABO blood group and the risk of suffering complications and death related to COVID‐19 (objective 2).
2. METHODS
2.1. Review questions
This review analyzes the value of the ABO group as a predictive factor and as a prognostic factor. The review questions according to PICOTS system (Population, Index predictive/prognostic factor, Predictive/prognostic comparators, Outcomes, Timing and Setting) based on modified CHARMS 12 (checklist for critical appraisal and data extraction for systematic reviews of prediction modeling studies) for objective 1 and 2 are as follows:
PICOTS for objective 1 (COVID‐19 infection prediction): P: any adult population; I: ABO blood group; C: adjusted for age, sex, comorbidities, ethnicity or other factors when possible; O: COVID‐19 infection; T: anytime; S: any setting.
PICOTS for objective 2 (COVID‐19 severity): P: adults with COVID‐19 infection; I: ABO blood group; C: adjusted for age, sex, comorbidities, ethnicity, or other factors when possible; O: hospitalization, ICU admission, mechanical ventilation, mortality; T: ABO group anytime, outcome at least 2 weeks after positive test; S: any setting.
2.2. Types of studies
Case–control, cohort, and cross‐sectional studies were eligible for inclusion. Studies must provide data for at least one pre‐established outcome variable in order to be eligible for inclusion. Noncomparative studies and studies in which all the participants are people under 18 years were excluded.
In the case of studies providing data for objective 1, COVID‐19‐positive patients were compared with people without COVID‐19. In order to separate the effect on COVID‐19 infection and severity, studies focusing exclusively on severe patients were excluded. Thus, studies in which the study period were limited to the first period (weeks) of the pandemic, when COVID‐19 tests were carried out only to suspected patients with severe symptoms, and those studies in which COVID‐19‐positive cases were identified exclusively from hospitalized patients were excluded. We did not consider comparisons between expected and observed blood group distributions, so studies with historical controls or comparisons of cases with population data (not matched with COVID‐19 diagnostic registries) were excluded.
For addressing objective 2, participants with COVID‐19 diagnosis with or without the outcomes of interest were compared. For this objective, studies could include patients of any severity.
Studies based on genetic analysis were included in the case blood group status were inferred from polymorphisms or the mutations identified.
2.3. Outcome variables
The following outcome variables were selected for responding to the first objective:
COVID‐19 infection determined by either polymerase chain reaction (PCR), antigen or antibody tests.
The second objective, which consisted on analyzing the association between blood group and COVID‐19 morbidity and mortality, was restricted to people with a confirmed COVID‐19 diagnosis and was analyzed through the following outcome variables:
Hospitalization
Admission to intensive care unit (ICU)
Need of mechanical ventilation
Mortality
2.4. Search strategy
A search was carried out in Medline in December 30, 2020, and in MedRxive and BioRxive in January 11, 2021. The searches were updated in May 4, 2021. The following terms were applied and combined: “COVID‐19,” “SARS‐CoV‐2,” “ABO,” “blood group.”
No language restrictions were applied.
2.5. Selection of studies
Two independent reviewers carried out the selection of articles. In a first phase, titles and abstracts of the identified references were screened based on pre‐established eligibility criteria. In a second phase, full texts of every article considered for inclusion in the first phase were reviewed, and a decision was made independently by each reviewer on their inclusion or exclusion. Discrepancies were resolved by discussion among all the review authors. Selection of studies was carried out using Rayyan‐Intelligent Systematic Review software. 13 Results of the screening process were reported using a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement (PRISMA) flow diagram. 14
2.6. Data extraction and management
Two reviewers independently extracted data from the included studies using a previously designed data extraction form. Extracted data by one reviewer were cross‐checked by the other reviewer. Discrepancies were resolved by discussion between these two authors.
When necessary, corresponding authors of the included studies were contacted in order to obtain additional information relevant for the analyses.
2.7. Data analyses
Adjusted and unadjusted data were analyzed separately. Outcome variables were expressed as the proportion of all selected patients with at least one event. The Mantel–Haenszel model was used to calculate the odds ratio (OR) and 95% confidence intervals (95% CI) for each outcome variable. A random effects model was used to combine results from the identified studies.
In the case of studies that provided only an estimate for a comparison but specific data for each compared arm are not available, results were presented in a narrative form and were not be included in the meta‐analyses.
I2 statistic was used to test for heterogeneity between included studies. We considered I2 > 65% as indicative of substantial heterogeneity. In case heterogeneity was found in any outcome variables, a sensitivity analysis was carried out limiting to studies with low risk of bias.
Funnel plots were carried out for outcomes in which data were obtained from 10 or more studies to explore the possibility of publication bias.
Review Manager software (RevMan version 5.3) was used for quantitative data synthesis and analyses.
2.8. Assessment of the quality of the evidence
Two reviewers independently assessed the risk of bias for each included study using the Newcastle‐Ottawa Scale, which consisted of the following domains: selection, comparability, exposure (only for case–control studies), and outcome (only for cohort and cross‐sectional studies). 15 The total score for each study range from 0 to 9 points. We defined studies with a score of 0–3 points were considered to be of high risk of bias, 4–6 of moderate risk of bias, and 7–9 points of low risk of bias. Discrepancies between authors when rating the studies were discussed until a consensus was reached.
Overall quality of the evidence was assessed following GRADE methodology. 16 The GRADE approach provides an initial rating of “low” quality to observational studies and addresses specific reasons to downgrading (study limitations, inconsistency of the results, directness of the evidence, imprecision, probability of publication bias) and upgrading (large magnitude of an effect, dose–response gradient, and the effect of plausible residual confounding) the initial punctuation. Study limitations were evaluated based on the risk of bias assessment of each study. Inconsistency was analyzed base on obtained I2 for each comparison. Imprecision was rated based on the confidence intervals of the overall estimates, assuming a minimal important difference threshold of 10% for mortality and 15% for the rest of the outcome variables. Publication bias assessment was based on funnel‐plots´ interpretation.
The protocol of the present review is available in Open Science Framework (osf.io/k2r35). The review was carried out in accordance with the PRISMA recommendations. 17
3. RESULTS
A total of 1433 references were identified through database searching. After removal of duplicates, 1405 references remained for screening. In the first phase, the titles and abstracts of these references were reviewed, and 1260 references were excluded. In the second phase, the full text of the 145 remaining references was reviewed, and of these, we excluded 77 references for not adhering to the protocol (n = 41 due to study design, n = 19 due to outcome, n = 14 due to publication type, n = 3 due to population). Therefore, 68 references were included in the review, which corresponded to 63 different studies (see Appendix S1). Of these, 30 studies were used for analyzing the first objective and 42 for analyzing the second objective. Fifty‐nine studies were included in quantitative synthesis (meta‐analyses). The PRISMA Flow Diagram is shown in Figure 1. Fourteen studies were published only as preprints, seven analyzing the first objective (Atergeleh et al., Belaouni et al., Chang et al., González et al., Singh et al., Stone et al., and Vasallo et al.), six analyzing the second objective (Dite et al., Ishaq et al., Mankelow et al., Mendy et al., Regina et al., and Zeng et al.), and one analyzing both (Martín et al.).
FIGURE 1.
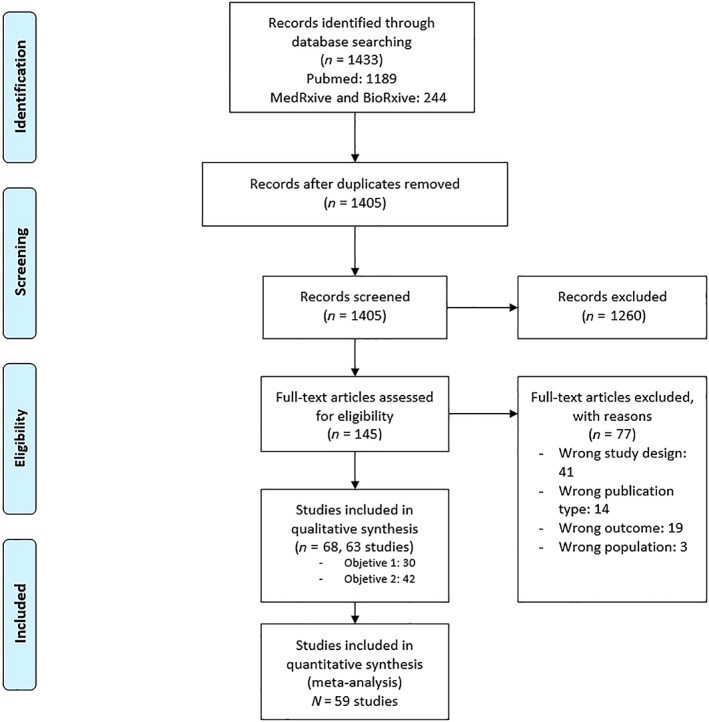
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement (PRISMA) flow diagram [Color figure can be viewed at wileyonlinelibrary.com]
Characteristics of the 63 included studies are shown in Table S1. Twenty‐six were cohort studies (41.3%), 19 (30.2%) case–control studies and the 18 remaining studies (28.6%) had a cross‐sectional design. A 61.9% of the included studies were multicenter (n = 39), 36.5% unicenter (n = 23), and such information was not available in one study. Of the included studies, 30 were carried out in Europe (47.6%), 18 in America (28.6%), and the remaining ones in Asia or Africa (15 studies, 23.8%). The number of the participants in each study was highly variable, ranging from 128 to 3,733,332 participants, totaling 6,470,438 participants. The available number of participants varied for the different analyses, reaching a maximum of 1,564,162 for infection, 36,154 for hospitalization, 32,690 for ICU admission, 10,210 for mechanical ventilation, and 39,542 for mortality. ABO group distribution for participants in studies analyzing infection was 38.3% group A, 12.1% group B, 4.2% group AB, and 45.4% group O.
Meta‐analysis of adjusted results was not possible due to insufficient data (a summary of adjusted results is shown in Table S2). All meta‐analyses are from unadjusted data.
For each of the outcomes, a sensitivity analysis was performed excluding studies published only as pre‐prints (Table S3).
3.1. COVID‐19 infection
A total of 30 of the included studies analyzed the susceptibility of COVID‐19 infection associated to ABO blood group, with 29 of them providing data for the meta‐analyses. Results are shown in Table 1. The O blood group was associated to a significantly lower susceptibility of COVID‐19 infection compared to the non‐O blood group (odds ratio [OR] 0.88 95% confidence interval [CI] 0.82–0.94; I2: 91%; 29 studies; 1,564,162 participants) (Figure 2). This result remained similar when comparing O blood group with group A (OR 0.88 95% CI 0.82–0.94; I2: 89%; 29 studies; 1,309,081 participants), B (OR 0.91 95% CI 0.83–0.99; I2: 86%; 29 studies; 899,262 participants), and AB (OR 0.83 95% CI 0.73–0.95; I2: 84%; 28 studies; 774,713 participants) independently. Results from the sensitivity analyses restricting to studies of low risk of bias showed no significant differences with O versus non‐O or with each of the rest of the blood groups independently (A, B, AB) (Table S4). However, heterogeneity persisted after the sensitivity analyses.
TABLE 1.
Results
| Infection | Hospitalization | Admission to ICU | Mechanical ventilation | Mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies (participants) | OR (95% CI) | I2 | Studies (participants) | OR (95% CI) | I2 | Studies (participants) | OR (95% CI) | I2 | Studies (participants) | OR (95% CI) | I2 | Studies (participants) | OR (95% CI) | I2 | |
| O versus non‐O | 29 (1,564,162) | 0.88 (0.82–0.94) | 91% | 11 (35,848) | 0.99 (0.94–1.05) | 0% | 20 (32,690) | 1.01 (0.93–1.09) | 0% | 12 (10,210) | 1.09 (0.96–1.24) | 0% | 26 (37,755) | 0.99 (0.92–1.06) | 4% |
| O versus A | 29 (1,309,081) | 0.88 (0.82–0.94) | 89% | 11 (30,562) | 1.01 (0.93–1.10) | 29% | 18 (25,475) | 1.01 (0.92–1.11) | 0% | 10 (6878) | 1.10 (0.84–1.45) | 49% | 25 (28,815) | 0.93 (0.86–1.00) | 0% |
| O versus B | 29 (899,262) | 0.91 (0.83–0.99) | 86% | 11 (20,960) | 1.02 (0.94–1.12) | 0% | 18 (20,039) | 0.98 (0.84–1.14) | 17% | 10 (6093) | 1.02 (0.80–1.30) | 27% | 25 (22,068) | 1.11 (1.01–1.23) | 0% |
| O versus AB | 28 (774,713) | 0.83 (0.73–0.95) | 84% | 11 (19,805) | 0.96 (0.72–1.28) | 64% | 18 (15,489) | 0.96 (0.79–1.16) | 0% | 10 (4580) | 1.07 (0.68–1.68) | 32% | 24 (17,457) | 0.99 (0.84–1.16) | 0% |
| A versus non‐A | 29 (1,560,198) | 1.08 (1.02–1.15) | 87% | 11 (36,154) | 0.99 (0.90–1.08) | 49% | 18 (32,209) | 1.00 (0.91–1.10) | 8% | 10 (9599) | 0.91 (0.67–1.24) | 65% | 27 (39,542) | 1.13 (1.03–1.23) | 17% |
| A versus B | 29 (789,217) | 1.04 (0.96–1.13) | 82% | 11 (19,363) | 0.93 (0.78–1.11) | 62% | 18 (16,720) | 0.98 (0.81–1.17) | 35% | 10 (5019) | 0.86 (0.57–1.29) | 61% | 25 (20,107) | 1.20 (1.08–1.33) | 0% |
| A versus AB | 28 (664,666) | 0.95 (0.85–1.05) | 75% | 11 (16,935) | 1.01 (0.88–1.15) | 0% | 18 (13,212) | 0.98 (0.80–1.19) | 0% | 10 (3506) | 0.86 (0.44–1.67) | 62% | 24 (15,483) | 1.08 (0.92–1.26) | 0% |
| B versus non‐B | 29 (1,564,162) | 1.01 (0.94–1.09) | 82% | 11 (36,168) | 1.08 (0.92–1.25) | 58% | 18 (20,779) | 0.97 (0.84–1.11) | 0% | 10 (9599) | 1.04 (0.81–1.32) | 33% | 25 (37,591) | 0.88 (0.80–0.96) | 0% |
| B versus AB | 28 (254,978) | 0.94 (0.86–1.04) | 63% | 11 (5606) | 1.07 (0.88–1.29) | 21% | 18 (6734) | 0.99 (0.80–1.22) | 0% | 13 (2721) | 1.04 (0.64–1.68) | 31% | 25 (8776) | 0.91 (0.77–1.09) | 0% |
| A/AB versus B/O | 29 (1,564,162) | 1.13 (1.06–1.21) | 90% | 11 (36,154) | 0.98 (0.89–1.07) | 51% | 18 (31,539) | 0.99 (0.91–1.08) | 0% | 11 (9731) | 0.93 (0.76–1.13) | 33% | 25 (37,551) | 1.10 (1.02–1.18) | 0% |
| B/AB versus A/O | 29 (1,743,669) | 1.27 (1.16–1.38) | 91% | 11 (36,154) | 1.04 (0.91–1.18) | 52% | 18 (32,209) | 1.03 (0.89–1.20) | 34% | 10 (9599) | 1.06 (0.78–1.43) | 58% | 25 (37,591) | 0.89 (0.82–0.97) | 0% |
Abbreviations: CI, confidence interval; ICU, intensive care unit; OR, odds ratio.
Bold values indicate there is a statistically significant difference between the groups
FIGURE 2.
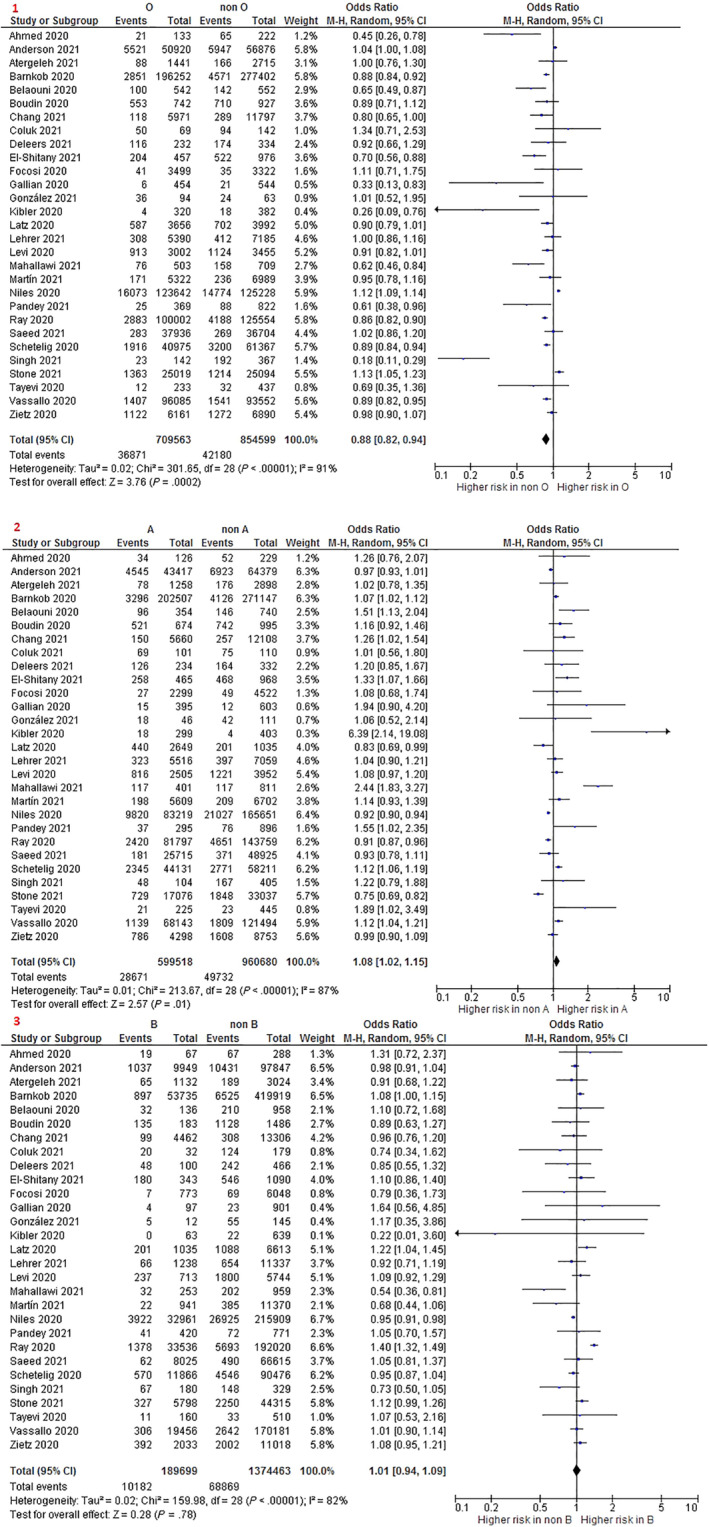
Forest plot for coronavirus disease 2019 (COVID‐19) infection. 1. O versus non‐O; 2. A versus non‐A; and 3. B versus non B [Color figure can be viewed at wileyonlinelibrary.com]
A significantly higher risk of COVID‐19 infection was found when comparing A blood group with non‐A (OR 1.08 95% CI 1.02–1.15; I2: 87%; 29 studies; 1,560,198 participants) (Figure 2). However, the differences were lost when restricting to studies with low risk of bias, and the heterogeneity persisted (Table S4). No significant differences were found in the risk of COVID‐19 infection between participants with A blood group and those with groups B and AB.
A significant difference was not found between B blood group versus non‐B group (Figure 2) and between B versus AB group. Analyses yielded similar results when restricting to studies with low risk of bias (Table S4).
One study that could not be included in the meta‐analysis also showed a lower risk of infection in O group compared to A. 18
A subgroup analysis on risk of COVID‐19 infection discriminating between studies with patients identified as blood donors and those where blood determinations were probably part of clinical necessity is shown in Table S5. For blood donors, results were similar to the overall population. For studies where blood determinations respond to clinical necessity, results were similar to the overall population, except for the comparison B versus non‐B (group B showed a higher infection risk in this subgroup, while no difference was obtained for overall population).
3.2. Hospitalization
A total of 11 of the included studies analyzed the risk of COVID‐19‐positive participants of being hospitalized. Results are shown in Table 1.
No significant differences were found in any of the comparisons between ABO blood groups when analyzing the risk of hospitalization. Results for O versus non‐O, A versus non‐A, and B versus non‐B are shown in Figure S1.
3.3. Admission to Intensive Care Unit
A total of 20 studies provided data of the incidence of COVID‐19‐positive participants of being admitted to an ICU. Results are shown in Table 1. None of the blood groups showed a different risk of ICU admission compared to the others. Results for O versus non‐O, A versus non‐A, and B versus non‐B are shown in Figure S2.
3.4. Mechanical ventilation
Data regarding the requirement of mechanical ventilation were available in 13 of the included studies. Results are shown in Table 1.
There were found no significant differences in any of the comparisons in the requirement of mechanical ventilation. Results for O versus non‐O, A versus non‐A, and B versus non‐B are shown in Figure S3.
The study by Ellinghaus et al. did not provide data for the meta‐analysis but showed similar results. 19
3.5. Mortality
Data on the incidence of mortality among the COVID‐19‐positive participants were obtained from 27 of the included studies. Results are shown in Table 1.
Mortality risk was similar with O versus non‐O blood group (OR 0.99 95% CI 0.92–1.06; I2: 4%; 26 studies; 37,755 participants) (Figure 3), O versus A group (OR 0.93 95% CI 0.86–1.00; I2: 0%; 25 studies; 28,815 participants), and O versus AB group (OR 0.99 95% CI 0.84–1.16; I2: 0%; 24 studies; 17,457 participants). However, mortality was higher with O blood group compared to B blood group (OR 1.11 95% CI 1.01–1.23; I2: 0%; 25 studies; 22,068 participants).
FIGURE 3.
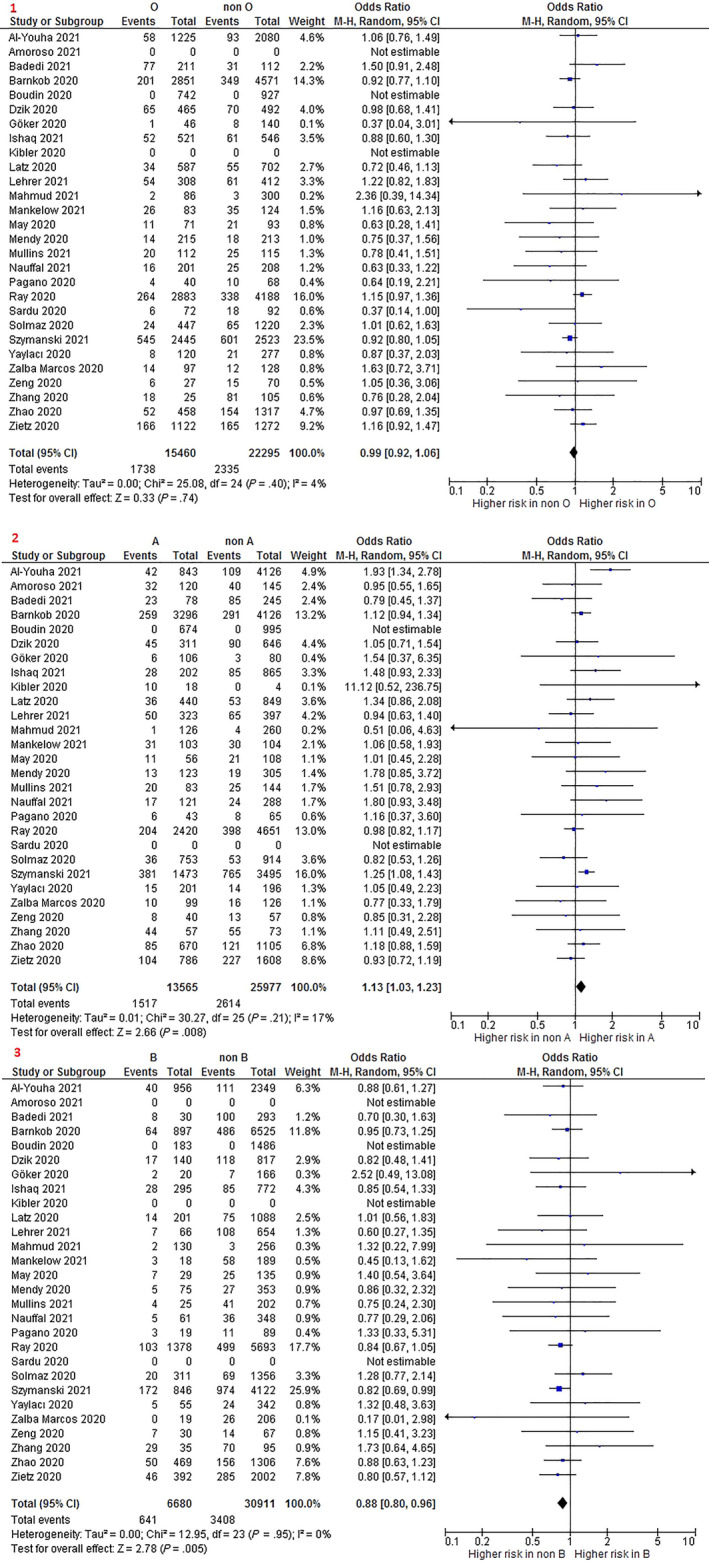
Forest plot for mortality. 1. O versus non‐O; 2. A versus non‐A; and 3. B versus non‐B [Color figure can be viewed at wileyonlinelibrary.com]
A blood group showed a higher risk of mortality when compared to non‐A group (OR 1.13 95% CI 1.03–1.23; I2: 17%; 27 studies; 39,542 participants) (Figure 3) and B group (OR 1.20 95% CI 1.08–1.33; I2: 0%; 25 studies; 20,107 participants). Significant differences were not found between participants with A versus AB blood group.
Participants with B blood group showed a lower mortality risk than participants with non‐B blood group (OR 0.88 95% CI 0.80–0.96; I2: 0%; 25 studies; 37,591 participants) (Figure 3). A similar mortality risk was found when comparing B versus AB blood groups.
The two studies that could not be included in the meta‐analysis showed an increased risk of mortality in A group 20 and a lower risk in B group, 21 respectively.
3.6. Risk of bias and quality of the evidence
Results of the risk of bias assessment of included studies are shown in Table S6. A 54% of the included studies had a moderate risk of bias, and the remaining studies (46%) were qualified as low risk of bias studies.
Funnel‐plots for each of the outcomes are shown in Figures S4–S7. A possible publication bias was identified in COVID‐19 infection results for comparisons O versus non‐O (Figure S4.1) and A versus non‐A (Figure S4.2) and also in admission to ICU for the comparison O versus non‐O (Figure S6.1). Results of the overall assessment of the quality of the evidence are shown in Table 2. The quality of the evidence for COVID‐19 infection results was rated as “very low” for all the comparisons. Results for hospitalization, admission to ICU, and mortality were qualified as “low” or “very low.”
TABLE 2.
Quality of the evidence assessment
| Infection | Hospitalization | Admission to intensive care unit (ICU) | Mechanical ventilation | Mortality | |
|---|---|---|---|---|---|
| O versus non‐O | Very low a , b , c , d | Low | Very low d | Very low b | Low |
| A versus non‐A | Very low b , d | Low | Low | Very low b | Very low b |
| B versus non‐B | Very low b | Very low b | Very low b | Very low b | Very low b |
Downgraded for study limitations (risk of bias).
Downgraded due to inconsistency of the results.
Downgraded due to imprecision.
Downgraded due to publication bias.
4. DISCUSSION
The findings of this systematic review and meta‐analysis about the association of blood groups and COVID‐19 suggest a higher risk of infection in A group compared to non‐A, but not with each group independently, and a lower risk of infection in O group compared to non‐O groups and to each individual group. We also found a higher risk of infection in patients without anti‐A or anti‐B antibodies. No significant association was found with any blood groups when analyzing the risk of hospitalization, ICU admission, or mechanical ventilation among patients with COVID‐19. Regarding mortality, group B showed a lower risk compared to non‐B groups, to A group and to O group, and A group showed a higher risk when comparing with non‐A groups and with B but not with O or AB. Participants without anti‐A antibodies (A/AB) also showed a higher risk of death, while those without anti‐B antibodies (B/AB) showed a lower risk.
Some other reviews have previously assessed the risk of COVID‐19 infection and mortality. In general, they coincide in showing an increased risk of infection in A group (vs. non‐A) and a decreased risk in O group (vs. non‐O). 7 , 9 , 11 , 22 , 23 However, our analysis comparing the risk of each group with the other groups individually may add some relevant information. O group showed a lower risk of infection compared to non‐O groups but also with A, B, and AB. Group A, however, only showed a higher risk when comparing with non‐A. As non‐A group is mostly composed of participants with O group (represents 74% of non‐A), it may be reflecting the protective effect of O group instead of a higher risk in A. The higher risk in those lacking anti‐A and anti‐B antibodies also supports this hypothesis. Regarding mortality, Bhattacharjee et al. failed to find a relationship between people with and without anti‐A antibodies and COVID‐19 severe outcomes or death, 8 Pourali et al. found no association of any of the ABO group with mortality, 23 Liu et al. showed an increased risk of death in A group compared to non‐A, 7 while Wu et al. found a lower risk in group B. 22
Mechanisms underlying the relationship between some blood groups and risk of COVID‐19 infection or mortality are still unclear, but numerous hypotheses have raised. The lower susceptibility of people with O blood group to get infected by viruses had already been reported by Cheng et al. in 2005 for SARS‐CoV, 24 and another study suggested that adhesion of S protein and ACE2 can be inhibited by anti‐A natural antibody for SARS‐CoV. 25 ACE2 has been also suggested to be the receptor for SARS‐CoV‐2, so the anti‐A and anti‐B natural antibodies produced in individuals with blood group O could potentially block viral adhesion to cells, which could explain the lower risk of infection in O group. Deleers et al. found that ABO antibody levels were significantly lower in COVID‐19 patients compared to controls. These findings could indicate that patients with low levels of ABO antibodies are at a higher risk of being infected and could therefore support causality.
It has also been proposed that COVID‐19 mortality may be related to the thrombotic risk that manifests in this disease. 26 The known relationship of blood groups to von Willebrand factor levels 27 (with individuals with group O having the lowest levels) may have an influence on mortality through thrombotic risk. However, thromboprophylaxis has become widespread after the first wave, so this effect may have been diluted.
In this case, the lower mortality in group B could be partly explained by demographic differences. Group B is more often of Asian origin and is very rare in Europe and America, where most of the included studies come from. This could be accompanied by a less aging population with fewer comorbidities. In relation to this, it should be noted that despite biological plausibility, association should not be confused with causality, and that factors such as ethnicity/race may play an important role in COVID‐19‐related outcomes. It is known that there are ethnicities/races with a skewed ABO frequency, and at the same time, they often share characteristics (such as socio‐demographic status, lifestyle, or overweight) that may influence the likelihood of infection or poorer health outcomes from COVID‐19. Despite this, as can be seen in Table S2, very few studies have taken these confounding factors into account in their analyses (Niles et al. and Saeed et al. for infection, Mendy et al. for hospitalization and Apea et al. and Nauffal et al. for mortality), pointing to the need for quality studies that take these aspects into account.
Blood transfusions also could be a modulator of outcome, through donor blood or due to the different profile of transfused patients (it is to be expected that blood donors and transfusion recipients will have different characteristics). Studies in which blood group determinations were probably part of clinical necessity, blood group determinations are likely associated with more blood transfusions. In the subgroup analysis discriminating these studies and those with blood donors, the risk of COVID‐19 infection differ when comparing A versus non‐A and B versus non‐B. Muñiz‐Diaz et al. also found different results in this outcome between patients transfused during hospitalization and blood donors, and Pagano et al. found that a higher proportion of type A patients were transfused compared to patients with other blood groups. Unfortunately, only these two studies provided information about transfused patients with COVID‐19, opening up a possible field for further research. Most of the previous published reviews were performed early in the pandemic and included very few studies. In our review, we have been able to collect a considerably higher number of studies following rigorous selection criteria, conducted not only at the beginning of the pandemic, which included more types of patients. This approach reduced the potential bias in estimating the association of ABO blood group and susceptibility of COVID‐19 infection and complications and also allowed having a significantly larger volume of data than in previous reviews, thus contributing to generate more solid evidence. Moreover, previous reviews have other limitations that we have tried to address. For instance, studies with historical controls or using population‐based comparators not matched with COVID‐19 diagnostic registries were excluded in order to seek greater precision. In addition, to clearly distinguish the effect of ABO in susceptibility of COVID‐19 infection and morbidity/mortality (predictive vs. prognostic factor), studies with different inclusion criteria were sought to address any or both questions, and analyses were conducted separately.
Our review has also some limitations. Especially for the outcome of COVID‐19 infection, high heterogeneity was found between the studies, so the overall estimates should be interpreted with caution. This heterogeneity may be due to the different populations, study designs, follow‐up periods, and assessment of the outcome or quality of the studies. When restricting the analyses showing high heterogeneity to studies with low risk of bias heterogeneity persisted, and the protective effect of the O group became nonstatistically significant. However, this may be due to the sample size reduction. Furthermore, meta‐analysis include unadjusted results, due to lack of adjusted data, so the presence of some confounding factors such as age, sex, or race, that could affect the results, cannot be ruled out. Little or no information is available on other factors that may affect the results, such as vaccination status, access to health care resources, or existence of different dominant mutations (only 4 studies provided information of genetics). Yet, available adjusted results are consistent with unadjusted effect estimates, especially for the protective effect of 0 group in COVID‐19 infection.
This systematic review includes preprint articles that have not yet been peer‐reviewed, and this could compromise the certainty of their findings. However, pandemic emergency circumstance has caused many articles to be advanced in this way, and thus we considered this strategy as the most appropriate in order to generate updated evidence, and risk of bias of these studies was also assessed. Moreover, sensitivity analyses excluding studies published only as preprints showed similar results to those of the main analyses.
Our review brings together all available information on the association between blood type and COVID‐19 infection and severity. Searches were carried out both in PubMed and repositories, maximizing the sensibility of the process. In addition, a rigorous methodology was followed to analyze and synthesize data, also assessing the risk of bias and the quality of the evidence. Screening of the references, data extraction, and quality assessment was carried out independently by two review authors, which ensures the quality of the process.
Recognizing people's susceptibility according to blood group can help understand the pandemic dynamics in different areas and also anticipate the possible outcomes, thus limiting the adverse impact of the infection. Generated evidence can also be considered as the basis for future research to increase knowledge about SARS‐COV‐2 transmission and pathogenesis and to search for new therapeutic targets or protective mechanisms.
In conclusion, available information suggests that there may be an association between ABO blood group and COVID‐19 infection and mortality. O group would be associated to a reduced risk of COVID‐19 infection (OR 0.88) but would not influence the prognosis of the disease. With less confidence, group A would be a risk factor for COVID‐19 infection (OR 1.08) and mortality (OR 1.13). Group B would not modify the risk of COVID‐19 infection but would present a lower risk of mortality (OR 0.88). These findings should be interpreted with caution considering the high heterogeneity found between the studies when analyzing the risk of infection and a low or very low quality of the evidence.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. Carlos Jericó has received funding from Vifor Pharma España SL®, Zambon®, and Bial® for lectures, consultancies, or travel grants. Jose Antonio García‐Erce has received funding from Terumo®, Hemonetics®, Inmucor®, Abbott®, Roche®, and Macopharma® as Director, Travel and Congress grants for Banco de Sangre y Tejidos de Navarra's workers.
Gutiérrez‐Valencia M, Leache L, Librero J, Jericó C, Enguita Germán M, García‐Erce JA. ABO blood group and risk of COVID‐19 infection and complications: A systematic review and meta‐analysis. Transfusion. 2022;62:493–505. 10.1111/trf.16748
Marta Gutiérrez‐Valencia and Leire Leache contributed equally to this study.
Funding information Macopharma®; Roche®; Abbott®; Inmucor®; Hemonetics®; Terumo®; Bial®; Zambon®; Vifor Pharma España SL®
REFERENCES
- 1. WHO World Health Organization (WHO) Coronavirus (COVID‐19) Dashboard.
- 2. Li X, Zhong X, Wang Y, Zeng X, Luo T, Liu Q. Clinical determinants of the severity of COVID‐19: a systematic review and meta‐analysis. PLoS One. 2021;16:e0250602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. SeyedAlinaghi S, Mehrtak M, MohsseniPour M, MirzaPour P, Barzegary A, Habibi P, et al. Genetic susceptibility of COVID ‐ 19: a systematic review of current evidence. Eur J Med Res. 2021;26:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pijls BG, Jolani S, Atherley A, Derckx RT, Dijkstra JIR, Franssen GHL, et al. Demographic risk factors for COVID‐19 infection, severity, ICU admission and death: a meta‐analysis of 59 studies. BMJ Open. 2021;11:e044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goel R, Bloch EM, Pirenne F, Al‐Riyami AZ, Crowe E, Dau L, et al. ABO blood group and COVID‐19: a review on behalf of the ISBT COVID‐19 working group. Vox Sang. 2021;116:849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yildirim Z, Sahin OS, Yazar S, Bozok CV. Genetic and epigenetic factors associated with increased severity of Covid‐19. Cell Biol Int. 2021;45:1158–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu N, Zhang T, Ma L, Zhang H, Wang H, Wei W, et al. The impact of ABO blood group on COVID‐19 infection risk and mortality: a systematic review and meta‐analysis. Blood Rev. 2020;48:100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhattacharjee S, Banerjee M, Pal R. ABO blood groups and severe outcomes in COVID‐19: a meta‐analysis. Postgrad Med J. 2020. 10.1136/postgradmedj-2020-139248. [DOI] [PubMed] [Google Scholar]
- 9. Golinelli D, Boetto E, Maietti E, Fantini MP. The association between ABO blood group and SARS‐CoV‐2 infection: a meta‐analysis. PLoS One. 2020;15:e0239508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kabrah SM, Kabrah AM, Flemban AF, Abuzerr S. Systematic review and meta‐analysis of the susceptibility of ABO blood group to COVID‐19 infection. Transfus Apher Sci. 2021;60:103169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franchini M, Cruciani M, Mengoli C, Marano G, Candura F, Lopez N, et al. ABO blood group and COVID‐19: an updated systematic literature review and meta‐analysis. Blood Transfus. 2021;19:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta‐analysis of prognostic factor studies. BMJ. 2019;364:k4597. [DOI] [PubMed] [Google Scholar]
- 13.Rayyan‐Intelligent Systematic Review [Internet]. Available from: https://www.rayyan.ai/
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses.
- 16. Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html
- 17. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kolin DA, Kulm S, Christos PJ, Elemento O. Clinical, regional, and genetic characteristics of Covid‐19 patients from UK Biobank. PLoS One. 2020;15:e0241264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Severe Covid‐19 GWAS Group . Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383:1522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muñiz‐Diaz E, Llopis J, Parra R, Roig I, Ferrer G, Grifols J, et al. Relationship between the ABO blood group and COVID‐19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021;19:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Apea VJ, Wan YI, Dhairyawan R, Puthucheary ZA, Pearse RM, Orkin CM, et al. Ethnicity and outcomes in patients hospitalised with COVID‐19 infection in East London: an observational cohort study. BMJ Open. 2021;11:e042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu BB, Gu DZ, Yu JN, Yang J, Wang‐Qin S. Association between ABO blood groups and COVID‐19 infection, severity and demise: a systematic review and meta‐analysis. Infect Genet Evol. 2020;84:104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pourali F, Afshari M, Alizadeh‐Navaei R, Javidnia J, Moosazadeh M, Hessami A. Relationship between blood group and risk of infection and death in COVID‐19: a live meta‐analysis. New Microbes New Infect. 2020;37:100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng Y, Cheng G, Chui CH, Lau FY, Chan PKS, Ng MHL, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293:1450–1. [DOI] [PubMed] [Google Scholar]
- 25. Guillon P, Clément M, Sébille V, Rivain JG, Chou CF, Ruvoën‐Clouet N, et al. Inhibition of the interaction between the SARS‐CoV spike protein and its cellular receptor by anti‐histo‐blood group antibodies. Glycobiology. 2008;18:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Franchini M, Capra F, Targher G, Montagnana M, Lippi G. Relationship between ABO blood group and von Willebrand factor levels: from biology to clinical implications. Thromb J. 2007;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.


