Abstract
In COVID‐19 patients, cytokine storm due to excessive immune responses can cause severe complications. In this study, we investigated the effect of curcumin nanomicelles on clinical outcome and cellular immune responses subtypes changes in COVID‐19 patients. A randomized, triple‐blinded, placebo‐controlled study was done. Forty COVID‐19 patients were included into two groups of nano‐curcumin and placebo. The nano‐curcumin group received 40 mg of nano‐curcumin capsule, four times per day for 2 weeks. Clinical signs and gene expression of TBX21, GATA3, RORC and FOXP3 genes and IFN‐γ, IL‐4, IL‐17 and TGF‐β cytokines serum levels were measured at time points of 0, 7 and 14 days. Serum levels of IFN‐γ (p = .52) and IL‐17 (p = .11) decreased, while IL‐4 (p = .12) and TGF‐β (p = .14) increased in the nano‐curcumin group compared with placebo on day 14. Moreover, gene expressions of TBX21 (p = .02) and FOXP3 (p = .005) genes were significantly decreased and increased between nano‐curcumin and placebo groups on day 7, respectively. It can be concluded that administration of nano‐curcumin in inflammatory phase of COVID‐19 can accelerate recovering of the acute inflammatory phase by modulating inflammatory immune responses. Therefore, it is suggested that this supplement in inflammatory diseases, including COVID‐19, can be effective in controlling the inflammatory responses.
Keywords: clinical trial, COVID‐19, curcumin, immunity, inflammation, signs and symptoms
1. INTRODUCTION
Acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the cause of coronavirus 2019 (COVID‐19), is associated with broad signs from mild flu symptoms to high risk of acute respiratory distress syndrome and mortality (Huang et al., 2020). Patients with COVID‐19 may face excessive immune responses followed by serious and life‐threatening pulmonary damage. In response to the virus, the immune cells may produce a large amount of inflammatory cytokines, which cause dangerous phenomenon of cytokine storms. In fact, cytokine release syndrome is one of the leading causes of severe lungs damage and death in COVID‐19 patients (Conti et al., 2020; Ghaebi, Osali, Valizadeh, Roshangar, & Ahmadi, 2020).
Curcumin, a traditional herb, is one of the derivatives of Curcuma longa (turmeric). Several in‐vivo and in‐vitro studies have shown that curcumin and its analogs significantly inhibit the production and secretion of pro‐inflammatory cytokines, such as IL‐1, IL‐6, IL‐8 and TNF‐α (Garg, Ahuja, Sankar, Kumar, & Moss, 2012; He et al., 2015). In this regard, animal studies have investigated the role of curcumin in inflammatory lung involvement, and their results showed that curcumin could actively reduce IL‐1b, IL‐6 and TNF‐α cytokines following the infectious lung injuries. In addition, curcumin can reduce productions of several cytokines and chemokines, such as MCP1, MIPI1, CXCL family, SDF1 and MMP family (Dai et al., 2018; Sordillo & Helson, 2015). The mechanism of immunomodulation of curcumin is widely investigated (Biswas, McClure, Jimenez, Megson, & Rahman, 2005). First, curcumin prevents activation of IKKb (Cohen, Veena, Srivatsan, & Wang, 2009). Second, curcumin increases expression of IkBa (Jobin et al., 1999). Third, curcumin activates AMPK (Han, Xu, Guo, & Huang, 2018) and inhibits nuclear translocation of NF‐kB and P65, which ultimately reduces inflammation (Xu & Liu, 2017). Another inflammatory mediator regulated by curcumin is cycloxygenase 2 (COX‐2) (Khan & Khan, 2018). Therefore, curcumin is probably able to shift immune responses through the above‐mentioned mechanisms. The balance of immune responses in infectious and non‐infectious diseases plays an important role in the prognosis of the diseases. The main cellular immune responses are including T helper1, T helper2, T Regulatory and T helper 17 lymphocytes responses, which shifting balances towards each of these responses can change the immune system condition (McMichael & Rowland‐Jones, 2001). Curcumin can also prevent the accumulation and migration of a wide range of immune cells, including peripheral mononuclear cells (PMNs) cells, CD4+ and CD8+ T lymphocytes and NK cells (Avasarala et al., 2013).
Curcumin also has antiviral effects on, such as, respiratory syndrome virus (RSV) (Yang, Li, Li, Wang, & Huang, 2017) different subtypes of IAV, H1N1, H2N2, H3N2 and H5N1 influenza (Kannan & Kolandaivel, 2017) as well as SARS‐CoV (Wen et al., 2007) through various molecular mechanisms.
On the other hand, despite curcumin's enormous beneficial properties, it has major limitations in administration due to its physicochemical properties. Among these limitations are the low aqueous solubility, low bioavailability and its low absorption from the gastrointestinal tract, which prevents its proper functions. Therefore, nano‐formulations of curcumin such as nanomicelles have been synthetized, which improve absorption, increase bioavailability and thus provide a suitable replacement for conventional form of curcumin (Gera et al., 2017; Hatamipour, Sahebkar, Alavizadeh, Dorri, & Jaafari, 2019).
Paying attention to the cytokine storm caused by SARAS‐COV‐2 virus, as well as the antiinflammatory efficacy of curcumin‐containing nanomicelles, in the present study, we studied the effect of curcumin‐containing nanomicelles on the T lymphocyte cells immune responses balance including T helper1, T helper 2, T regulatory and T helper 17 cells and its clinical outcome in the treatment of COVID‐19.
2. MATERIAL AND METHODS
2.1. Study design and patients' selection
The study protocol of the present was published previously (Hassaniazad et al., 2020). This is a randomized, triple‐blinded, placebo‐controlled study. A total of 40 COVID‐19 patients were included, who were referred to COVID‐19 wards of Shahid Mohammadi Hospital, Bandar Abbas, Iran. To have the most baseline homogeneity, the patients were divided randomly into the groups of nano‐curcumin and placebo using Excel software randomization method. For this purpose, a participant's variable equal to the number of participants in the two groups of placebo and nano‐curcumin was defined in Excel software. Then, using the randomization function, the participants list containing two groups of placebo and nano‐curcumin were randomly assigned, and the patients admitted to the study were allocated to the groups according to the randomly created list.
Patients in both groups received treatment in accordance with the latest version of the COVID‐19 treatment guideline, and inclusion into the study did not interfere with the main treatments (Table 1). An informed consent was obtained from all patients prior to the participation. The present study was approved by the Research Ethics Committee of Hormozgan University of Medical Sciences (HUMS.REC.1399.174) and Iranian Registry of Clinical Trials (IRCT code: IRCT20200611047735N1, https://www.irct.ir/trial/48843). The inclusion criteria of patients were as follows: 18–75 years of age, both genders, lack of participation in other clinical trials, diagnosis of COVID‐19 infection by a specialist and confirmation with COVID‐19 Real‐Time PCR test. The exclusion criteria also were as follows: pregnancy or lactation, allergy to turmeric or curcumin, smoking, patient connected to the ventilator, SaO2 less than 90% or PaO2 less than 8 kPa, having comorbidities (such as severe renal failure, glomerular filtration rate less than 30 ml/min, liver failure, congestive heart failure or chronic obstructive pulmonary disease), history of gallstones, history of gastritis or active gastrointestinal ulcer. The obtained demographic data, clinical and laboratory parameters are summarized in Tables 2 and 3.
TABLE 1.
Medications and supplements intake in two groups of placebo and nano‐curcumin
| Medications and supplements | Placebo n = 20 | Nano‐curcumin n = 20 | p value a |
|---|---|---|---|
| Vitamin D | 20 (100%) | 20 (100%) | >.9999 |
| Vitamin C | 20 (100%) | 20 (100%) | >.9999 |
| Zinc sulfate | 20 (100%) | 20 (100%) | >.9999 |
| L‐carnitine | 20 (100%) | 20 (100%) | >.9999 |
| Hydroxychloroquine | 9 (45%) | 9 (45%) | >.9999 |
| Kaletra® | 11 (55%) | 10 (50%) | >.9999 |
| Corticosteroids | 17 (85%) | 18 (90%) | >.9999 |
| Diphenhydramine | 13 (65%) | 10 (50%) | .5231 |
| Enoxaparin | 14 (70%) | 16 (80%) | .7164 |
| Heparin | 6 (30%) | 4 (20%) | .7164 |
| Pantoprazole | 20 (100%) | 20 (100%) | >.9999 |
Fishet's exact test was done to compare the frequencies between two groups.
TABLE 2.
Demographic and clinical characteristics of COVID‐19 patients in nano‐curcumin and placebo groups at the time of enrollment
| Placebo | Nano‐curcumin | p value | |
|---|---|---|---|
| Age, years | 48.3 ± 11 | 48.7 ± 10.8 | .90 a |
| Sex, N (%) | Male: 12 (60%) | Male: 10 (50%) | .29 b |
| Female: 8 (40%) | Female: 10 (50%) | ||
| Lung involvement (by CT scan finding) | Mild: 1 (5%) | Mild: 0 (0%) | .43 b |
| Moderate: 10 (50%) | Moderate: 8 (40%) | ||
| Severe: 9 (45%) | Severe: 12 (60%) | ||
| Length of stay, days | 5.2 ± 2.9 | 5.8 ± 3.1 | .53 a |
Note: Data presented as mean ± SD or percent.
Independent sample T‐test was used to evaluate the mean differences between groups.
Chi‐square test was used to evaluate the frequency differences between groups.
TABLE 3.
Laboratory and clinical findings in nano‐curcumin and placebo patients in three time points following intervention
| Day 0* | p value | Day 7* | p value | Day 14* | p value | ||
|---|---|---|---|---|---|---|---|
| Fever (°C) | Placebo | 36.7 ± 0.3 | .3688 a | 36.6 ± 0.2 | .2421 a | 36.5 ± 0.2 | .7857 a |
| Nano‐curcumin | 36.5 ± 0.3 | 36.5 ± 0.2 | 36.6 ± 0.2 | ||||
| Oxygen saturation level % | Placebo | 92 ± 5.5 | .6751 a | 96 ± 2 | .9998 a | 97.1 ± 1.2 | .9903 a |
| Nano‐curcumin | 93.5 ± 3.4 | 95.9 ± 2.4 | 97 ± 2.1 | ||||
| Myalgia, N (%) | Placebo | 11 (55%) | .3203 b | 0 | – | 0 | – |
| Nano‐curcumin | 15 (75%) | 0 | 0 | ||||
| Cough, N (%) | Placebo | 10 (50%) | .5231 b | 1 (5%) | – | 0 | – |
| Nano‐curcumin | 13 (65%) | 1 (5%) | 0 | ||||
| Headache, N (%) | Placebo | 8 (40%) |
.5273 b |
0 | – | 0 | – |
| Nano‐curcumin | 11 (55%) | 0 | 0 | ||||
| Sore throat, N (%) | Placebo | 9 (45%) |
.3203 b |
0 | – | 0 | – |
| Nano‐curcumin | 5 (25%) | 0 | 0 | ||||
| Chills, N (%) | Placebo | 9 (45%) | .3406 b | 0 | – | 0 | – |
| Nano‐curcumin | 13 (65%) | 0 | 0 | ||||
| Anosmia, N (%) | Placebo | 5 (25%) | .5006 b | 0 | – | 0 | – |
| Nano‐curcumin | 8 (40%) | 0 | 0 | ||||
| PT, seconds | Placebo | 13.9 ± 1 | .1096 a | 14 ± 1 | .6377 a | 13.9 ± 1 | .4082 a |
| Nano‐curcumin | 13.01 ± 1 | 13.5 ± 1.2 | 13.2 ± 1 | ||||
| PTT, seconds | Placebo | 33.9 ± 5 | .9998 a | 34.1 ± 10 | .7877 a | 33.8 ± 8 | .9994 a |
| Nano‐curcumin | 34 ± 6 | 31.9 ± 8 | 33.6 ± 8 | ||||
| International normalized ratio (INR) | Placebo | 1.12 ± 0.13 | .1752 a | 1.18 ± 0.23 | .1567 a | 1.16 ± 0.21 | .1948 a |
| Nano‐curcumin | 1.04 ± 0.12 | 1.06 ± 0.12 | 1.05 ± 0.08 | ||||
| Qualitative C‐reactive protein (CRP) | Placebo |
Zero: 2 (10%) +1: 3 (15%) +2: 5 (25%) +3: 10 (50%) |
.986 b |
Zero: 11 (55%) +1: 4 (20%) +2: 3 (15%) +3: 2 (10%) |
.931b |
Zero: 16 (80%) +1: 0 (0%) +2: 3 (15%) +3: 1 (5%) |
.171 a |
| Nano‐curcumin |
Zero: 2 (10%) +1: 3 (15%) +2: 6 (30%) +3: 9 (45%) |
Zero: 11 (55%) +1: 5 (25%) +2: 3 (15%) +3: 1 (5%) |
Zero: 16 (80%) +1: 3 (15%) +2: 1 (5%) +3: 0 (0%) |
Data presented as mean ± SD or percent.
Independent sample T‐test was used to evaluate the mean differences between groups.
Chi‐square test was used to evaluate the frequency differences between groups.
2.2. Study design
The sample size was calculated according to the Sakpal et al.'s study (Sakpal, 2010) and based on the comparing two proportions formula. To have a two arm designed clinical trial with 80% power and two‐sided 5% significance, and given the efficacy of nano‐curcumin in previous COVID‐19 clinical trial study (Saber‐Moghaddam et al., 2021), 20 sample size in each arm of the study was calculated.
The tested medication in this trial was an herbal supplement containing soft gels containing curcuminoids nanomicelles. This drug has been commercialized and registered as the Sinacurcumin® soft gel 40 mg by Exir Nano Sina Company, Tehran, Iran (IRC:1228225765).
The nano‐curcumin group participants received Sinacurcumin® soft gel 40 mg, four times per day (after breakfast, lunch, dinner and before bedtime) for 2 weeks. In the placebo group, capsules with the same appearance and characteristics (placebo capsules, Exir Nano Sina Company, Tehran, Iran) were prescribed, similar to the nano‐curcumin administration. In order to blind, the coded capsule containers method for nano‐curcumin and placebo capsules were used to have the triple‐blinded RCT for the participants, physicians and nurses collaborating in the study and data collectors. The capsule containers were coded in such a way that none of the participants, physicians and nurses collaborating in the study and data collectors knew about the coding. The capsules were then given in order of their codes to the patients entered the study. Assessing participant compliance was also done by methods of patient self‐reporting of medication use as well as counting of unused capsules in every visit by the physician and calculating the patient's compliance (Lafleur & Oderda, 2004). In case of deterioration of the patient and inconsistency with the inclusion criteria of the study, the patient was excluded from the study and received the necessary treatment according to the guidelines. Also, there were no co‐interventions for the participants in the present trial (Figure 1).
FIGURE 1.
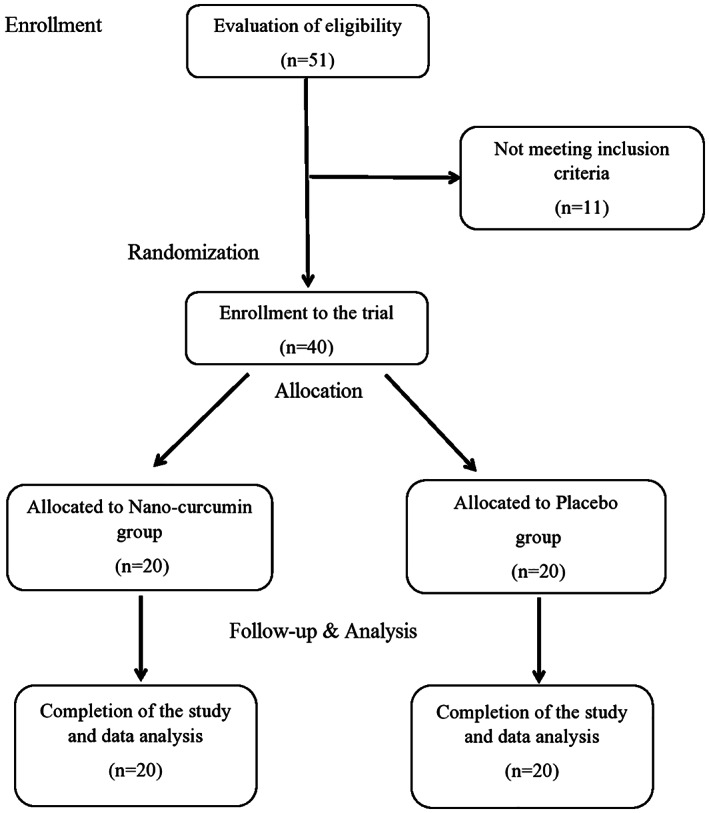
Flowchart of the designed trial on the COVID‐19 patients
Blood samples of all participants were collected three times of days 0, 7 and 14 after administration of the nano‐curcumin or placebo. Bloods were centrifuged at 3000 rpm, and serum was separated in different microtubes and stored at −80°C until cytokine assay. Complete clinical symptoms and laboratory findings including peripheral blood and serum parameters including lactate dehydrogenase (LDH), white blood cells (WBC) count, prothrombin time (PT), partial thromboplastin time (PTT), international normalized ratio (INR) and C‐reactive protein (CRP qualitative measurement kit, ENISON, Tehran, Iran) were measured and recorded in mentioned time points as seen in Table 3. To study the immune response balance changes, gene expressions containing T helper 1 cells transcription factor TBX21, T helper 2 cells transcription factor GATA‐3, regulatory T cells transcription factor FOXP3 and T helper 17 cells transcription factor ROR‐γT as well as serum level of IFN‐γ, IL‐4, IL‐17 and TGF‐β cytokines levels in both groups of nano‐curcumin and placebo were measured in three time points of 0, 7 and 14 days after treatments.
2.3. Analysis of TBX21, GATA‐3, FOXP3 and ROR‐γT mRNA expression by real‐time PCR
Total RNA was extracted from whole blood samples using the RiboExTM kit (Geneall, Seoul, South Korea), according to the manufacturer's instructions. Subsequently, complementary DNA (cDNA) was synthesized using SMOBIO Reverse Transcription Kit II (SMOBIO, SMOBiO Technology, Hsinchu, Taiwan). For gene expression study by real‐time PCR, a total volume of 25 μl, containing primers, cDNA, and the SYBR Green Master Mix, was used. The optimal conditions for amplification of each gene expression were calculated separately based on the annealing temperature of each primer pairs. The sequences of the primers are presented in Table 4. The GAPDH, as the housekeeping gene, was also used to normalize the transcript level of the target genes. Real‐time PCR was performed triplicates for every sample. Finally, mRNA expression levels were obtained using the 2−∆∆CT method, where ΔCt = target gene Ct − housekeeping gene Ct.
TABLE 4.
Quantitative real‐time PCR primers sequences and their annealing temperature
| Gene | Sequence (5′–3′) | Annealing temperature °C |
|---|---|---|
| TBX21 | F: ATTGCCGTGACTGCCTACCAGA | 62 |
| R: GGAATTGACAGTTGGGTCCAGG | ||
| GATA3 | F: ACCACAACCACACTCTGGAGGA | 63 |
| R: TCGGTTTCTGGTCTGGATGCCT | ||
| RORC | F: GAGGAAGTGACTGGCTACCAGA | 62 |
| R: GCACAATCTGGTCATTCTGGCAG | ||
| FOXP3 | F: GGCACAATGTCTCCTCCAGAGA | 62 |
| R: CAGATGAAGCCTTGGTCAGTGC | ||
| GAPDH | F: GGTGTGAACCATGAGAAGTAT | 62 |
| R: AGTCCTTCCACGATACCAA |
2.4. Measurement of serum cytokine level
To evaluate the COVID‐19 patients' serum level of IFN‐γ, IL‐4, IL‐17 and TGF‐β cytokines at days 0, 7 and 14 following treatments by nano‐curcumin or placebo, an enzyme‐linked immunosorbent assay (ELISA) method was used by Karmania Pars Gene ELISA kits (Karmania Pars Gene Co, Kerman, Iran) according to the manufacturer's instructions. The developed color was finally read using a plate reader (Biotek, Winooski, VT) at the wavelength of 450/620 nm, and the serum concentration of cytokines was measured according to the cytokine's standards of each cytokine kit.
2.5. Statistical analysis
The statistical analysis was performed by GraphPad PRISM software, version 8. Results have been reported as percentage or mean ± SD. Kolmogorov–Smirnov test was used to check the normality distribution of the variables. Independent sample t test and its nonparametric Mann–Whitney U test (if applicable) were used to compare mean difference of variables between two groups. The analysis of variance (ANOVA) and Kruskal–Wallis tests (if applicable) as well as Bonferroni Post‐Hoc tests were also used to compare mean difference of variables. Fisher's exact and Chi square tests were also used to compare proportions between the groups. The p value lower than .05 was considered as significant.
3. RESULTS
3.1. Clinical manifestation and overall improvement
According to the inclusion criteria, 40 patients were eligible to enroll in the study, 20 patients in nano‐curcumin and 20 patients in placebo group completed the 2 weeks duration trial. As seen in Table 1, there were no significant differences between prescribed medications and supplements between placebo and nano‐curcumin groups. Also, the baseline‐characteristics of patients at the time of enrollment are summarized in Table 2. The demographics and clinical characteristics were not statistically different between nano‐curcumin and placebo groups at the time of enrollment.
3.2. Clinical outcome of nano‐curcumin or placebo administrations in COVID‐19 patients
All the subjects were evaluated in terms of clinical signs and symptoms including fever, oxygen saturation level, myalgia, cough, sore throat, headache, anosmia and laboratory indices such as cell blood count (CBC), lactate dehydrogenase (LDH) activity, PT, PTT, INR and CRP, at three time points of 0, 7 and 14 days in both nano‐curcumin and placebo groups.
As shown in Table 3, there were no statistically significant differences in the clinical manifestations between nano‐curcumin and placebo groups after the treatment at 0, 7 and 14 days. As seen in Table 3, despite the lack of significant differences, the CRP level in patients decreased in the second week after nano‐curcumin administration compared with placebo group, so that 5% of patients in nano‐curcumin group had the CRP level more than +2, compared with 20% of patients in placebo group.
The laboratory findings are also shown in Figure 2. One of the remarkable findings, despite the lack of statistically significant difference between the placebo and nano‐curcumin groups, was the increase in the mean percentage of lymphocytes in the nano‐curcumin group compared with the placebo. The mean percentage of lymphocytes in nano‐curcumin group on days 7 and 14 was 31.6% ± 13.3 and 32.6% ± 12.8, respectively, while this frequency was observed in the placebo group as 23.5% ± 13.3 and 29.7 ± 11.8 on days 7 and 14, respectively. This trend was also observed for other laboratory indices such as neutrophils percent, neutrophil to lymphocyte ratio (NLR) and LDH activity (Figure 2). It should be mentioned that no specific adverse reactions related to curcumin were observed in nano‐curcumin receiving patients.
FIGURE 2.
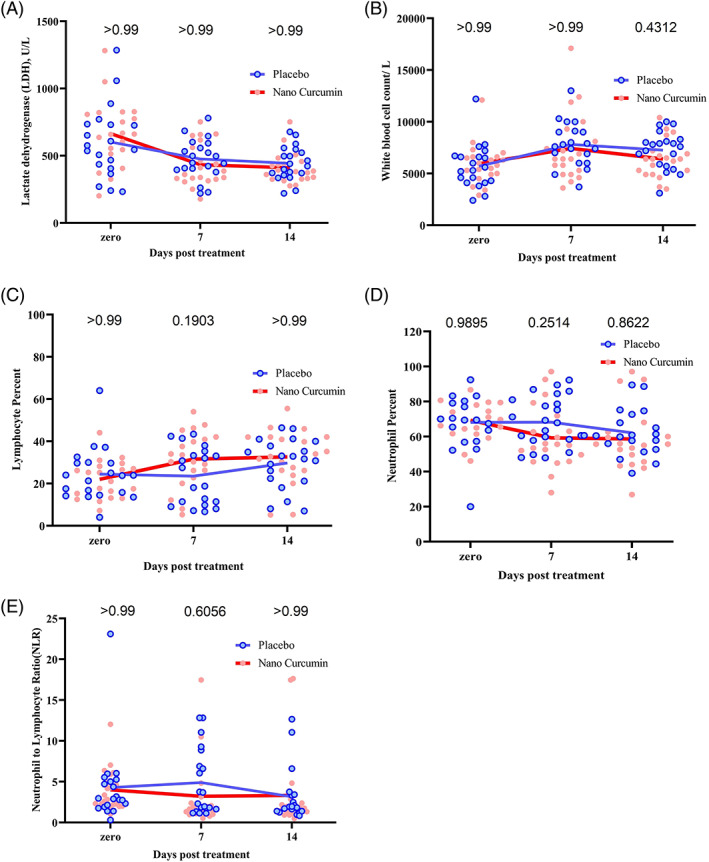
Laboratory indices changes in nano‐curcumin and placebo groups on days 0, 7, and 14 after treatment. (a) Mean serum LDH activity in placebo and nano‐curcumin groups, (b) white blood cells count in placebo and nano‐curcumin groups, (c) lymphocyte frequency in placebo and nano‐curcumin groups, (d) lymphocyte frequency in placebo and nano‐curcumin groups, and (e) neutrophil to lymphocyte ratio (NLR) in placebo and nano‐curcumin groups. The continuous line in the graphs represents the mean value of variables. The point in the graphs represents one of the participants in the study. The two‐way ANOVA and Bonferroni's multiple comparison Post‐Hoc test was used to evaluate the mean differences between placebo and nano‐curcumin groups in the time points
3.3. mRNA expression study of TBX21, GATA3, RORC and FOXP3 genes
TBX21, GATA3, RORC and FOXP3 mRNA expression levels in COVID‐19 patients were assessed and compared between nano‐curcumin and placebo groups in time points of days 0, 7 and 14 following treatment. As shown in Figure 3a, TBX21 gene expression decreased at days 7 and 14 time points in the nano‐curcumin group compared with the placebo group. This decrease at day 7 showed a significant difference between two groups (p value: .0252). RORC gene expression also downregulated in nano‐curcumin group compared with placebo group at days 7 and 14 time points. However, this reduction in RORC gene expression between the two groups was not statistically significant in none of the time points (p value >.05). Moreover, as shown in Figure 3d, upregulation of FOXP3 and GATA3 genes was seen in the nano‐curcumin group compared with the placebo group. The difference in FOXP3 gene expression on day 7 between placebo and nano‐curcumin groups was also statistically significant (p value: .005).
FIGURE 3.
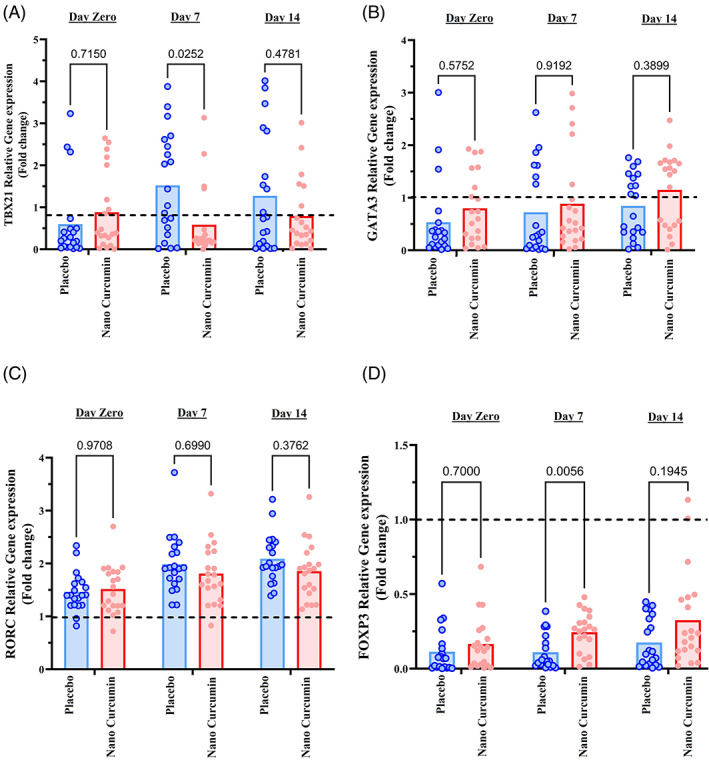
Gene expression study of TBX21, GATA3, RORC, and FOXP3 genes in COVID‐19 patients in nano‐curcumin and placebo groups at time points of days 0, 7, and 14 subsequent of treatment. (a) Gene expression study of TBX21, (b) gene expression study of GATA3, (c) gene expression study of RORC, and (d) gene expression study of FOXP3. The bar charts in the graphs represent the mean value of variables. The point in the graphs represents one of the participants in the study. The two‐way ANOVA and Bonferroni's multiple comparison Post‐Hoc test was used to evaluate the mean differences between placebo and nano‐curcumin groups in the time points
3.4. Serum level of IL‐17, IL‐4, IFN‐γ and TGF‐β cytokines
Based on the results shown in Figure 4a, serum IFN‐γ cytokine levels were not significantly different between nano‐curcumin and placebo groups at days of 0, 7 and 14 time points (p value>.05). However, IFN‐γ serum level was higher in the placebo group (195.5 ± 69.3 pg/ml) on the time point of day 7 compared with the nano‐curcumin group (182 ± 60.7 pg/ml). Moreover, despite the lack of statistical significance differences, IL‐4 serum cytokine increased at times points of 7 and 14 days in the nano‐curcumin group compared with the placebo group (p value>.05) (Figure 4b).
FIGURE 4.
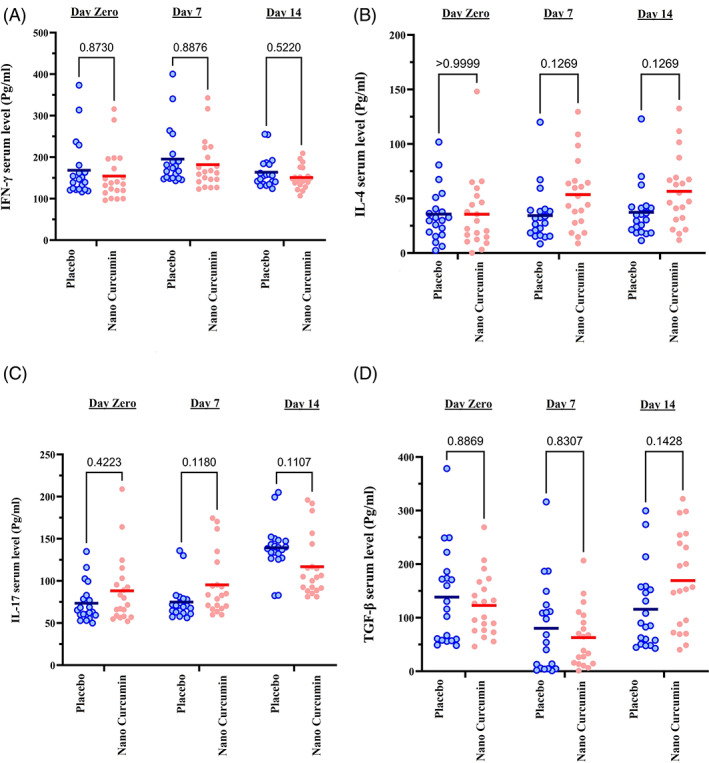
COVID‐19 patients' serum level measurements of IL‐4, IFN‐γ, IL‐17, and TGF‐β cytokines in the nano‐curcumin and placebo groups at the three time points of 0, 7, and 14 days of treatments using ELISA technique. (a) IFN‐g serum level, (b) IL‐4 serum level, (c) IL‐17 serum level, and (d) TGF‐β serum level. The lines in the charts represent the mean value of variables. The point in the graphs represents one of the participants in the study. The two‐way ANOVA and Bonferroni's multiple comparison Post‐Hoc test was used to evaluate the mean differences between placebo and nano‐curcumin groups in the time points
Also, as seen in Figure 4c, the serum level of IL‐17 in the nano‐curcumin group increased at day 7 time point (95.3 ± 37.2 pg/ml), but decreased at day 14 time point (116.8 ± 36.2 pg/ml) compared with the placebo group (74.8 ± 21.3 g/ml and 139.3 ± 28.4 g/ml at days 7 and 14 time points, respectively; p value >.05).
Serum levels of the cytokine TGF‐β also decreased at time point of day 7 in the curcumin group (62.9 ± 55.1 pg/ml) but increased at time point of day 14 (169.3 ± 90.3 pg/ml) compared with the placebo group (80.2 ± 84.1 pg/ml and 115.8 ± 76.2 pg/ml at days 7 and 14 time points, respectively) (p > .05) (Figure 4d).
4. DISCUSSION
COVID‐19 outbreak, which has been a pandemic since 2020, has caused many deaths worldwide by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Many efforts have been made to achieve an effective therapeutic strategy for it, but no definitive approach has been reached to date. It has been shown that the excessive immune response and the development of cytokine storms are the key factor for the development of severe forms of the COVID‐19 disease and broad lung damage and mortality in COVID‐19 patients (Rodriguez‐Morales et al., 2020).
In the present study, we investigated the therapeutic role of curcumin‐containing nanomicelles as modulator of inflammatory immune responses in COVID‐19 patients. For this purpose, in the present clinical trial in two groups of placebo and nano‐curcumin, gene expression and cytokines serum levels related to cellular immune responses subgroups including Th1, Th2, Th17 and T regulatory were studied.
Despite curcumin's enormous beneficial properties, it has major limitations in administration due to its physicochemical properties. Among these limitations are the low aqueous solubility, low bioavailability and its low absorption from the gastrointestinal tract, which prevents its proper functioning. Therefore, biodegradable nanomicelles containing curcumin, which was used in our trial, have been synthetized to overcome these obstacles (Dei Cas & Ghidoni, 2019; Gera et al., 2017).
The positive effects of curcumin on controlling inflammation have been reported (Banez et al., 2020; Farhood et al., 2019; Kahkhaie et al., 2019; Shimizu et al., 2019). Saadati et al. (2019) studied the effect of curcumin on the non‐alcoholic fatty liver disease (NAFLD) patients and reported that curcumin administration reduced fibrosis, nuclear factor‐kappa B activity, liver enzymes and TNF‐α serum level (Saadati et al., 2019). In another study by Adibian et al., curcumin reduced the serum lipid profile as well as CRP and adiponectin of Type‐2 diabetes patients (Adibian et al., 2019). These results confirm the role of modulating the immune responses of curcumin in diseases of inflammatory origin.
Based on the prominent immunomodulatory role of curcumin, from the onset of the COVID‐19 pandemic, studies have been conducted on the therapeutic role of curcumin in COVID‐19 patients. Saber‐Moghaddam et al. (2021) reported that nano‐curcumin administration to COVID‐19 patients significantly improved clinical indices including length of hospital stay and blood oxygen level compared with placebo group due to antiinflammatory effects of curcumin on the recovery of COVID‐19 patients, confirming the results of our study (Saber‐Moghaddam et al., 2021). However, they did not perform molecular studies and focused on the clinical outcome of nano‐curcumin administration in COVID‐19 patients.
A study also was done by Valizadeh et al. on the effect of nano‐curcumin on the mRNA expression and cytokine secretion of IL‐1β, IL‐6, TNF‐α and IL‐18 on COVID‐19 patients, and the results showed that nano‐curcumin significantly reduced secretion of IL‐6 and IL‐1β and gene expression of these cytokines, which was consistent with our results, indicating the antiinflammatory effect of nano‐curcumin (Valizadeh et al., 2020). Tahmasebi et al. studied the role of curcumin on the frequency of Th17 cells in patients with COVID‐19, and they showed that nano‐curcumin significantly reduced the frequency of Th17 cells. This study is also consistent with our obtained results about reduction of interleukin‐17 serum level and its gene expression, which indicates the antiinflammatory role of curcumin. Roy et al. also stated possible role of curcumin in improving the severe side effects of COVID‐19 by the anticoagulant effects of curcumin on platelets, calcium and cyclooxygenase pathways. In COVID‐19 patients, coagulation disorders such as disseminated intravascular coagulopathy (DIC (can pose a significant risk to the COVID‐19 patient, so anticoagulant properties of curcumin may have a role in preventing coagulopathy complications (Roy et al., 2020). One of the strengths of our trial compared to the mentioned studies was the investigation of the immunomodulatory role of nano‐curcumin in patients with COVID‐19 by studying its effect on main subtypes of cellular immune response (Th1, Th2, Th17 and Treg in terms of gene expression and serum cytokines levels), which has not been performed specifically in other studies on COVID‐19.
The effect of nano‐curcumin on the improvement of laboratory indices, such as lymphocyte percentage, neutrophils percentage, LDH level and neutrophil to lymphocyte (NLR) ratio from the time point of day 7 after treatment, can be considered as one of our main findings. Based on the previous reports, the NLR, decreased lymphocytes and increased neutrophils count are diagnostic and prognostic factors for the assessment of infections severity (Berhane et al., 2019; Liu et al., 2017; Wang et al., 2020; Zhang et al., 2020). In this regard, Liu et al. reported that aged ≥ 50 and NLR ≥ 3.13 were the key indices for prediction of severe condition of COVID‐19 patients (Liu et al., 2020).
Another major part of our study was related to the effect of nano‐curcumin on the immune responses balance in COVID‐19 patients by studying of cellular immune responses' subgroups transcription factors gene expression. According to our results, nano‐curcumin significantly downregulated TBX1 gene of Th1 responses and upregulated FOXP3 gene of regulatory T cell population in COVID‐19 patients. Th1 responses intensify inflammatory responses centered on CD8 T lymphocytes, macrophages, due to IFN‐γ and IL‐12 cytokine secretions (Bruder, Srikiatkhachorn, & Enelow, 2006; Farhood et al., 2019; Farrar & Schreiber, 1993). In COVID‐19 patients, one of the main causes of lung tissue destruction is cytokine storm related to severe cellular immune responses to virus‐infected lung cells, so one way to control the exacerbation of COVID‐19 is to modulate cellular immune responses (Rodriguez‐Morales et al., 2020). According to obtained results, curcumin nanomicelles modulated cellular immune responses by reducing TBX1 gene expression associated with Th1 response. Furthermore, the expression of RORC gene related to Th17 responses and the expression of GATA3 gene related to Th2 response showed that nano‐curcumin can not only modulate inflammatory immune responses through Th1 and T regulatory responses, but also modulates the immune responses through Th17 and Th2 responses. Therefore, according to previous reports and our current findings, it seems that curcumin can modulate T cell‐mediated immune responses in COVID‐19 patients.
According to our results, inflammatory cytokines including IL‐17 and IFN‐γ were decreased, but TGF‐β and IL‐4 cytokines were elevated in the serum of nano‐curcumin‐receiving COVID‐19 patient. In support of our findings, Djalali et al. studied the effect of nano‐curcumin on Th1/Th17 balance and serum level of IFN‐γ and IL‐17 in migraine patients, and they stated that using nano‐curcumin in these patients significantly reduced the gene expression of IL‐17 compared with control (Djalali et al., 2020). Based on our findings, nano‐curcumin downregulated the expression of RORC gene of Th17 response in COVID‐19 patients. This finding indicates an immunomodulation in neutrophil‐rich inflammatory responses and inflammation following Th17‐related responses.
However, the limitations of the present study were the small sample size as well as the short treatment time. The short duration of treatment time was due to short duration of the disease and limitations of patients' follow‐up after discharging from the hospital.
In conclusion, the results of the present clinical trial study suggest that the use of nanomicelles containing curcumin in COVID‐19 patients can improve peripheral blood inflammatory indices 7 days after intervention. Also, using nano‐curcumin can modulate the immune responses by decreasing Th1 and Th17 responses and increasing T regulatory responses and further reducing the IL‐17 and IFN‐γ and increasing the suppressive cytokines TGF‐β and IL‐4. Therefore, it can be concluded that nano‐curcumin can accelerate the process of recovery from the acute inflammatory phase of COVID‐19 disease.
CONFLICT OF INTEREST
Prof. Mahmoud Reza Jaafari, one of the co‐authors, founded Exir Nano Sina Company, a Nano pharmaceutical company. The other co‐authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank the staff of COVID‐19 ward and the Laboratory Department of Shahid Mohammadi Hospital, Bandar Abbas, Iran, for their cooperation in this study.
Hassaniazad, M. , Eftekhar, E. , Inchehsablagh, B. R. , Kamali, H. , Tousi, A. , Jaafari, M. R. , Rafat, M. , Fathalipour, M. , Nikoofal‐Sahlabadi, S. , Gouklani, H. , Alizade, H. , & Nikpoor, A. R. (2021). A triple‐blind, placebo‐controlled, randomized clinical trial to evaluate the effect of curcumin‐containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID‐19 patients. Phytotherapy Research, 35(11), 6417–6427. 10.1002/ptr.7294
Funding informationThe financial supports of Hormozgan University of Medical Sciences, Bandar Abbas, Iran (grant no. 990126) is acknowledged. The Exir Nano Sina company had no role in funding or dedication of SinaCurcumin and placebo capsules, data collection, analysis, and interpretation of data.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Adibian, M. , Hodaei, H. , Nikpayam, O. , Sohrab, G. , Hekmatdoost, A. , & Hedayati, M. (2019). The effects of curcumin supplementation on high‐sensitivity C‐reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double‐blind, placebo‐controlled trial. Phytotherapy Research, 33(5), 1374–1383. [DOI] [PubMed] [Google Scholar]
- Avasarala, S. , Zhang, F. , Liu, G. , Wang, R. , London, S. D. , & London, L. (2013). Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral‐induced acute respiratory distress syndrome. PLoS One, 8(2), e57285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banez, M. J. , Geluz, M. I. , Chandra, A. , Hamdan, T. , Biswas, O. S. , Bryan, N. S. , … Ernst, R. (2020). A systemic review on the antioxidant and anti‐inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutrition Research, 78, 11–26. [DOI] [PubMed] [Google Scholar]
- Berhane, M. , Melku, M. , Amsalu, A. , Enawgaw, B. , Getaneh, Z. , & Asrie, F. (2019). The role of neutrophil to lymphocyte count ratio in the differential diagnosis of pulmonary tuberculosis and bacterial community‐acquired pneumonia: A cross‐sectional study at Ayder and Mekelle Hospitals, Ethiopia. Clinical Laboratory, 65(4), 527–533. [DOI] [PubMed] [Google Scholar]
- Biswas, S. K. , McClure, D. , Jimenez, L. A. , Megson, I. L. , & Rahman, I. (2005). Curcumin induces glutathione biosynthesis and inhibits NF‐κB activation and interleukin‐8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxidants & Redox Signaling, 7(1–2), 32–41. [DOI] [PubMed] [Google Scholar]
- Bruder, D. , Srikiatkhachorn, A. , & Enelow, R. I. (2006). Cellular immunity and lung injury in respiratory virus infection. Viral Immunology, 19(2), 147–155. [DOI] [PubMed] [Google Scholar]
- Cohen, A. N. , Veena, M. S. , Srivatsan, E. S. , & Wang, M. B. (2009). Suppression of interleukin 6 and 8 production in head and neck cancer cells with curcumin via inhibition of Iκβ kinase. Archives of Otolaryngology–Head & Neck Surgery, 135(2), 190–197. [DOI] [PubMed] [Google Scholar]
- Conti, P. , Ronconi, G. , Caraffa, A. L. , Gallenga, C. E. , Ross, R. , Frydas, I. , & Kritas, S. K. (2020). Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): Anti‐inflammatory strategies. Journal of Biological Regulators and Homeostatic Agents, 34(2), 1. [DOI] [PubMed] [Google Scholar]
- Dai, J. , Gu, L. , Su, Y. , Wang, Q. , Zhao, Y. , Chen, X. , … Li, K. (2018). Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF‐κB pathways. International Immunopharmacology, 54, 177–187. [DOI] [PubMed] [Google Scholar]
- Dei Cas, M. , & Ghidoni, R. (2019). Dietary curcumin: Correlation between bioavailability and health potential. Nutrients, 11(9), 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djalali, M. , Abdolahi, M. , Hosseini, R. , Miraghajani, M. , Mohammadi, H. , & Djalali, M. (2020). The effects of nano‐curcumin supplementation on Th1/Th17 balance in migraine patients: A randomized controlled clinical trial. Complementary Therapies in Clinical Practice, 41, 101256. [DOI] [PubMed] [Google Scholar]
- Farhood, B. , Mortezaee, K. , Goradel, N. H. , Khanlarkhani, N. , Salehi, E. , Nashtaei, M. S. , … Sahebkar, A. (2019). Curcumin as an anti‐inflammatory agent: Implications to radiotherapy and chemotherapy. Journal of Cellular Physiology, 234(5), 5728–5740. [DOI] [PubMed] [Google Scholar]
- Farrar, M. A. , & Schreiber, R. D. (1993). The molecular cell biology of interferon‐gamma and its receptor. Annual Review of Immunology, 11(1), 571–611. [DOI] [PubMed] [Google Scholar]
- Garg, S. K. , Ahuja, V. , Sankar, M. J. , Kumar, A. , & Moss, A. C. (2012). Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews, 10, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera, M. , Sharma, N. , Ghosh, M. , Huynh, D. L. , Lee, S. J. , Min, T. , … Jeong, D. K. (2017). Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget, 8(39), 66680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaebi, M. , Osali, A. , Valizadeh, H. , Roshangar, L. , & Ahmadi, M. (2020). Vaccine development and therapeutic design for 2019‐nCoV/SARS‐CoV‐2: Challenges and chances. Journal of Cellular Physiology, 235(12), 9098–9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S. , Xu, J. , Guo, X. , & Huang, M. (2018). Curcumin ameliorates severe influenza pneumonia via attenuating lung injury and regulating macrophage cytokines production. Clinical and Experimental Pharmacology and Physiology, 45(1), 84–93. [DOI] [PubMed] [Google Scholar]
- Hassaniazad, M. , Inchehsablagh, B. R. , Kamali, H. , Tousi, A. , Eftekhar, E. , Jaafari, M. R. , … Alizade, H. (2020). The clinical effect of Nano micelles containing curcumin as a therapeutic supplement in patients with COVID‐19 and the immune responses balance changes following treatment: A structured summary of a study protocol for a randomised controlled trial. Trials, 21(1), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatamipour, M. , Sahebkar, A. , Alavizadeh, S. H. , Dorri, M. , & Jaafari, M. R. (2019). Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids. Iranian Journal of Basic Medical Sciences, 22(3), 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y. , Yue, Y. , Zheng, X. , Zhang, K. , Chen, S. , & Du, Z. (2015). Curcumin, inflammation, and chronic diseases: How are they linked? Molecules, 20(5), 9183–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Wang, Y. , Li, X. , Ren, L. , Zhao, J. , Hu, Y. , … Gu, X. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin, C. , Bradham, C. A. , Russo, M. P. , Juma, B. , Narula, A. S. , Brenner, D. A. , & Sartor, R. B. (1999). Curcumin blocks cytokine‐mediated NF‐κB activation and proinflammatory gene expression by inhibiting inhibitory factor I‐κB kinase activity. The Journal of Immunology, 163(6), 3474–3483. [PubMed] [Google Scholar]
- Kahkhaie, K. R. , Mirhosseini, A. , Aliabadi, A. , Mohammadi, A. , Mousavi, M. J. , Haftcheshmeh, S. M. , … Sahebkar, A. (2019). Curcumin: A modulator of inflammatory signaling pathways in the immune system. Inflammopharmacology, 27(5), 885–900. [DOI] [PubMed] [Google Scholar]
- Kannan, S. , & Kolandaivel, P. (2017). Antiviral potential of natural compounds against influenza virus hemagglutinin. Computational Biology and Chemistry, 71, 207–218. [DOI] [PubMed] [Google Scholar]
- Khan, M. A. , & Khan, M. J. (2018). Nano‐gold displayed anti‐inflammatory property via NF‐kB pathways by suppressing COX‐2 activity. Artificial Cells, Nanomedicine, and Biotechnology, 46(Suppl 1), 1149–1158. [DOI] [PubMed] [Google Scholar]
- Lafleur, J. , & Oderda, G. M. (2004). Methods to measure patient compliance with medication regimens. Journal of Pain & Palliative Care Pharmacotherapy, 18(3), 81–87. [PubMed] [Google Scholar]
- Liu, J. , Liu, Y. , Xiang, P. , Pu, L. , Xiong, H. , Li, C. , … Song, R. (2020). Neutrophil‐to‐lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. Journal of Translational Medicine, 18, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, W. J. , Zhao, M. , Liu, K. , Xu, K. , Wong, G. , Tan, W. , & Gao, G. F. (2017). T‐cell immunity of SARS‐CoV: Implications for vaccine development against MERS‐CoV. Antiviral Research, 137, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael, A. J. , & Rowland‐Jones, S. L. (2001). Cellular immune responses to HIV. Nature, 410(6831), 980–987. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Morales, A. J. , Cardona‐Ospina, J. A. , Gutiérrez‐Ocampo, E. , Villamizar‐Peña, R. , Holguin‐Rivera, Y. , Escalera‐Antezana, J. P. , … Henao‐Martinez, A. F. (2020). Clinical, laboratory and imaging features of COVID‐19: A systematic review and meta‐analysis. Travel Medicine and Infectious Disease, 34, 101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, A. , Sarkar, B. , Celik, C. , Ghosh, A. , Basu, U. , Jana, M. , … Ildiz, N. (2020). Can concomitant use of zinc and curcumin with other immunity‐boosting nutraceuticals be the arsenal against COVID‐19? Phytotherapy Research, 34(10), 2425–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadati, S. , Sadeghi, A. , Mansour, A. , Yari, Z. , Poustchi, H. , Hedayati, M. , … Hekmatdoost, A. (2019). Curcumin and inflammation in non‐alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterology, 19(1), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saber‐Moghaddam, N. , Salari, S. , Hejazi, S. , Amini, M. , Taherzadeh, Z. , Eslami, S. , … Elyasi, S. (2021). Oral nano‐curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease‐19 patients: An open label nonrandomized clinical trial. Phytotherapy Research, 35(5), 2616–2623. [DOI] [PubMed] [Google Scholar]
- Sakpal, T. (2010). Sample size estimation in clinical trial. Perspectives in Clinical Research, 1(2), 67–67. [PMC free article] [PubMed] [Google Scholar]
- Shimizu, K. , Funamoto, M. , Sunagawa, Y. , Shimizu, S. , Katanasaka, Y. , Miyazaki, Y. , … Morimoto, T. (2019). Anti‐inflammatory action of curcumin and its use in the treatment of lifestyle‐related diseases. European Cardiology Review, 14(2), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo, P. P. , & Helson, L. (2015). Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo, 29(1), 1–4. [PubMed] [Google Scholar]
- Valizadeh, H. , Abdolmohammadi‐Vahid, S. , Danshina, S. , Gencer, M. Z. , Ammari, A. , Sadeghi, A. , … Ghaebi, M. (2020). Nano‐curcumin therapy, a promising method in modulating inflammatory cytokines in COVID‐19 patients. International Immunopharmacology, 89, 107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , … Xiong, Y. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama, 323(11), 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, C.‐C. , Kuo, Y.‐H. , Jan, J.‐T. , Liang, P.‐H. , Wang, S.‐Y. , Liu, H.‐G. , … Lee, S.‐S. (2007). Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. Journal of Medicinal Chemistry, 50(17), 4087–4095. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , & Liu, L. (2017). Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF‐κB signaling pathway. Influenza and Other Respiratory Viruses, 11(5), 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. X. , Li, C. M. , Li, Y. F. , Wang, J. , & Huang, C. Z. (2017). Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale, 9(41), 16086–16092. [DOI] [PubMed] [Google Scholar]
- Zhang, B. , Zhou, X. , Zhu, C. , Song, Y. , Feng, F. , Qiu, Y. , … Zhu, B. (2020). Immune phenotyping based on the neutrophil‐to‐lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID‐19. Frontiers in Molecular Biosciences, 7, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


