Abstract
Introduction
Endemic coronaviruses have been found in acute bronchiolitis, mainly as a coinfecting virus. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has been responsible for respiratory illness in hospitalized children. The characteristics of patients with bronchiolitis have not been extensively described.
Methods
Cross‐sectional study of patients with bronchiolitis and SARS‐CoV‐2 infection enrolled in a prospective multicenter cohort of children hospitalized with COVID‐19 in Spain from March 1, 2020 to February 28, 2021.
Results
Twelve of 666 children infected with SARS‐CoV‐2 who required hospital admission met the diagnostic criteria for bronchiolitis (1.8%). Median age was 1.9 months (range: 0.4–10.1). Six cases had household contact with a confirmed or probable COVID‐19 case. Main complaints were cough (11 patients), rhinorrhea (10), difficulty breathing (8), and fever (8). Eleven cases were classified as mild or moderate and one as severe. Laboratory tests performed in seven patients did not evidence anemia, lymphopenia, or high C‐reactive protein levels. Chest X‐rays were performed in six children, and one case showed remarkable findings. Coinfection with metapneumovirus was detected in the patient with the most severe course; Bordetella pertussis was detected in another patient. Seven patients required oxygen therapy. Albuterol was administered in four patients. One patient was admitted to the pediatric intensive care unit. Median length of admission was 4 days (range: 3–14). No patient died or showed any sequelae at discharge. Two patients developed recurrent bronchospasms.
Conclusion
SARS‐CoV‐2 infection does not seem to be a main trigger of severe bronchiolitis, and children with this condition should be managed according to clinical practice guidelines.
Keywords: bronchiolitis, coronavirus, infants, pandemic
1. INTRODUCTION
The coronaviruses 229E and OC43, discovered in the 1960s, and NL63 and HKU1, identified in the 2000s, have been described as the causative pathogen in upper respiratory tract infections, asthma, bronchiolitis, pneumonia, and croup, with more severe disease occurring in infants, the elderly, and immunocompromised individuals. Among hospitalized children, clinical manifestations and severity of illness were similar across the four human coronavirus (HCoV) types, and children under 2 years of age and those with chronic complex conditions were found to be at risk of increased disease severity. 1 As with endemic coronaviruses, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the disease that causes coronavirus disease 2019 (COVID‐19), is responsible for respiratory illness in hospitalized children. 2 Pneumonia has been the main clinical diagnosis in pediatric patients with COVID‐19. 3
Acute bronchiolitis (AB) is the most common cause of hospitalization among infants during the first 12 months of life. 4 In infants seeking care for AB, respiratory syncytial virus (RSV) is the most common infection. 5 Previously, human coronavirus (HCoVs) were an infrequent cause of single‐infection AB. 5 , 6 Although endemic coronavirus was found in 12% of children hospitalized with AB during the pre‐COVID‐19 era, it has been reported that 85% of endemic coronavirus AB cases had a coinfecting virus, mainly RSV. Coinfection has not been associated with increased disease severity. 7
To the best of our knowledge, there are scarce reports of infants with AB in whom SARS‐CoV‐2 was the only infection detected. 8 SARS‐CoV‐2 infection has been found to have a low impact on pediatric acute respiratory disease hospitalizations, and AB seems to be an infrequent diagnosis. 9 Several mechanisms have been proposed to explain the varying severity of SARS‐CoV‐2 infection between adult and pediatric patients. 10
We analyzed data from a prospective multicenter cohort of children hospitalized with COVID‐19 in Spain, one of the countries with the highest prevalence of COVID‐19 in Europe, 11 , 12 to describe the characteristics of patients with diagnosed AB secondary to SARS‐CoV‐2.
2. MATERIALS AND METHODS
2.1. Study design, setting, and population
We performed a cross‐sectional study of patients with AB and SARS‐CoV‐2 infection enrolled in the Prospective Epidemiological Study of COVID‐19 in Children of the Spanish Pediatric Association (EPICO‐AEP), from March 1, 2020 to February 28, 2021. EPICO‐AEP is a multicenter nationwide study aiming to describe COVID‐19 in Spanish children. 13 A representative sample of 10% of all hospitals in Spain recruited patients, including all major pediatric hospitals in each Spanish region. All affiliated pediatricians received an email invitation to participate in the project from the Spanish Pediatric Association. All centers showing interest were included in the project. No facilities were excluded. Children younger than 18 years of age infected with SARS‐CoV‐2 who received care at any of the 80 participating hospitals were included in this registry. For the present analysis, the inclusion criteria were (i) children under 2 years of age with AB admitted to the hospital and (ii) positive reverse‐transcriptase polymerase chain reaction (RT‐PCR) assay for SARS‐CoV‐2 performed using nasopharyngeal swab/aspirate.
2.2. Definitions
In accordance with the classical criteria by McConnochie et al., AB was defined as the first episode of acute wheezing in children aged less than 2 years, beginning as a viral upper respiratory infection (coryza, cough, or fever). 14 Patients with previous episodes of wheezing were excluded.
2.3. Ethics statement
This study was approved by the Ethics Committee of the coordinating hospital (Hospital Universitario 12 de Octubre, Madrid, number: 20/101) and by the ethics committees of all other participating centers, and informed consent was obtained from the parents or guardians of all children included.
2.4. Laboratory methods
Confirmed infection was defined as the detection of SARS‐CoV‐2 nucleic acid by RT‐PCR. The reported sensitivity of RT‐PCR for the envelope (E)‐gene and RNA‐dependent RNA polymerase (RdRp) gene assays is 5.2 and 3.8 copies per reaction at 95% detection probability, respectively. 15 Both genes needed to be amplified in RT‐PCR to report a positive result. RT‐PCR for SARS‐CoV‐2 was performed on the premises of each participating center.
2.5. Statistical analysis
Study data were standardized and prospectively collected by researchers from each center, entering the data into an encoded, confidential, unique online database using REDCap electronic data capture tools (Biomed Inform, 2009). 16 Data collected included clinical and sociodemographic variables. Continuous variables, ranges, interquartile ranges (IQR), and medians were presented in the case of nonnormally distributed variables and means and standard deviations when variables were normally distributed. Unless specified otherwise, the denominator for each percentage was the number of subjects within the population group, without considering missing observations. Data were analyzed using SPSS software 20.0 (IBM Corp.).
3. RESULTS
By the end of February 2021, 666 children infected with SARS‐CoV‐2 who required hospital admission had been included in the registry, and 306 of them had a respiratory illness (45.9%). The most common diagnoses of admitted patients are reflected in Figure 1. Thirteen patients were infants diagnosed as having AB. One patient was excluded as they did not meet the diagnostic criteria for AB. A total of 12 patients were included, representing 1.8% of admissions throughout the first year of the pandemic. Eight cases were admitted during the first wave of the pandemic, which lasted from March 2020 to May 2020 (Figure 2).
Figure 1.

SARS‐CoV‐2 hospital admissions due to a respiratory illness. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Figure 2.
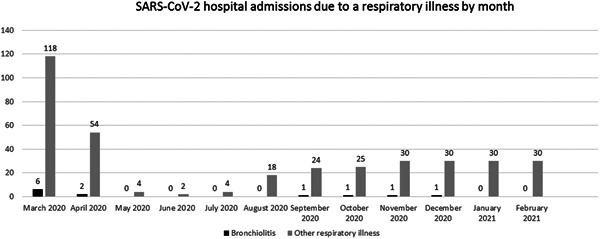
SARS‐CoV‐2 hospital admissions due to a respiratory illness by month. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
The epidemiological and clinical characteristics of these infants are summarized in Table 1. The median age at presentation of AB was 1.9 months (range: 0.4–10.1 months). Six out of 12 were below 8 weeks of age. Six cases had household contact with confirmed or probable COVID‐19 cases. None had underlying medical disorders. In all cases, RT‐PCR for SARS‐CoV‐2 using nasopharyngeal swabs or aspirates detected viral nucleic acid on admission in the first sample.
Table 1.
Epidemiological and clinical characteristics
| Case number | Age in months | Sex | Comorbidities | Close contact with a confirmed or probable case of COVID‐19 | Days of fever (from start to end) | Temperature at ED (°C) | Heart rate at ED | Respiratory rate at ED | Oxygen saturation at ED (%) | Bronchiolitis score at ED | Reason for admission |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.4 | Male | No | Yes (parents) | 2 | 38.5 | 158 | 70 | 93 | Moderate | Severity, age |
| 2 | 0.7 | Female | No | No | 1 | 38.2 | 115 | 52 | 94 | Moderate | Severity, age |
| 3 | 1.3 | Female | No | No | No | 36.1 | 150 | 49 | 99 | Mild | Age |
| 4 | 1.4 | Male | No | Yes (aunt) | 1 | 37.2 | 130 | 60 | 98 | Mild | Age |
| 5 | 1.5 | Female | No | Yes (father) | 2 | 37.7 | 140 | 34 | 100 | Mild | Age |
| 6 | 1.8 | Male | No | Yes (parents) | 3 | 37.9 | 169 | 65 | 95 | Severe | Severity |
| 7 | 2.0 | Male | No | No | 1 | 37.9 | 145 | 36 | 97 | Mild | Age |
| 8 | 3.1 | Female | No | Yes (uncle) | 6 | 36.8 | n.a. | 29 | 99 | Mild | Caregiver‐reported apnea |
| 9 | 4.7 | Male | No | No | No | 36.7 | 129 | n.a. | 99 | Moderate | Severity, feeding difficulties |
| 10 | 6.1 | Female | No | Yes (father) | 3 | 38.4 | 131 | 28 | 100 | Mild | Extreme family distress |
| 11 | 7.6 | Male | No | No | 4 | 36.5 | 135 | 45 | 93 | Moderate | Severity |
| 12 | 10.1 | Male | No | No | No | 37.6 | 160 | 50 | 94 | Moderate | Severity |
Abbreviations: ED, emergency department; n.a., not available.
The most frequent complaints were cough (11 patients), rhinorrhea, 10 and work of breathing. 8 Eight presented fever on admission and one developed fever during admission. Median temperature in the emergency department (ED) was 37.6°C (range: 36.1–38.5°C); none presented fever >39°C during the course of the disease. The median time of fever before hospital admission was 2 days (range: 0–6 days) and none of them presented fever lasting more than 6 days (median: 2 days; range: 1–6 days). Gastrointestinal symptoms were infrequent (one case of diarrhea and one with vomiting), and none presented skin lesions.
At admission, median percutaneous oxygen saturation in room air was 97% (range: 93%–100%) and respiratory rate was 49 rpm (range: 28–70 rpm). According to the AB scoring system used in each center, six cases were classified as mild, five as moderate, and one as severe. The reason for admission in patients with mild AB was age <8 weeks in four cases, apnea reported by caregivers in one case, and extreme family distress in the other case.
The results of diagnostic tests and the clinical course of these patients are included in Table 2. Laboratory tests were ordered in seven patients. None evidenced anemia, lymphopenia, or thrombocytopenia. Median values of C‐reactive protein at admission were 1.6 mg/L (range: 0.4–6.1 mg/L). A search for coinfection was performed in nine patients. PCR assay for multiple respiratory viruses (including RSV, influenza, adenovirus, rhinovirus, enterovirus, parainfluenza, human metapneumovirus [hMPV], bocavirus, and HCoVs other than SARS‐CoV‐2) was performed in six patients and one patient was positive for hMPV. As a single RSV antigen test performed in other two cases presented a negative result, RSV coinfection was ruled out in a total of eight patients. Additionally, PCR assays for Mycoplasma pneumoniae, Chlamydia pneumoniae, and Bordetella pertussis were performed in five patients and one patient was positive for Bordetella pertussis. Chest X‐rays (CXR) were performed in six children, five of which were normal at admission; one case had perihilar infiltrates. One patient who had a normal CXR developed a consolidation on the third day of admission.
Table 2.
Diagnostic tests and clinical course
| Case number | Hemoglobin (g/dl) | Leukocytes (cells/µl) | Lymphocytes (cells/µl) | Platelets (cells/µl) | C‐reactive protein (mg/L) | Coinfection | Chest X‐ray | Days of oxygen therapy | Albuterol | Other treatments | Days of admission |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 12830 | 3690 | 389000 | 1.3 | Not performed | Normal | 6 | No | No | 8 |
| 2 | – | – | – | – | – | Negative | Not performed | 3 | No | No | 4 |
| 3 | 14 | 12900 | 8980 | 704000 | 5.3 | Bordetella | Normal | 4 | No | Azithromycin | 4 |
| 4 | 10.6 | 5200 | 1100 | 303000 | 6.1 | Negative | Not performed | 0 | No | No | 4 |
| 5 | – | – | – | – | – | Not performed | Not performed | 0 | No | No | 3 |
| 6 | 12.1 | 11250 | 6200 | 331000 | 2.2 | Metapneumovirus | Normal at admission; consolidation at day 3 | 12 | Yes | Epinephrine, hypertonic saline, remdesivir, corticosteroids, antibiotics, azithromycin, hydroxychloroquine | 14 |
| 7 | – | – | – | – | – | Not performed | Perihilar infiltrates | 0 | No | No | 3 |
| 8 | 10.4 | 9280 | 5790 | 234000 | 0.5 | Negative | Normal | 0 | No | No | 4 |
| 9 | 13.6 | 18730 | 13800 | 478000 | 0.4 | Negative | Not performed | 2 | Yes | Corticosteroids | 4 |
| 10 | 12.6 | 12290 | 3800 | 346000 | 1.6 | Negative | Normal | 0 | Yes | Azithromycin | 3 |
| 11 | – | – | – | – | – | Negative | Not performed | 4 | No | No | 4 |
| 12 | – | – | – | – | – | Negative | Not performed | 3 | Yes | No | 3 |
Seven out of 12 infants required oxygen therapy, 6 of them only by nasal cannula. The patient coinfected with hMPV was first on high‐flow nasal cannula (HFNC), followed by continuous positive airway pressure (CPAP), and finally required mechanical ventilation (MV) for 6 days. Length of oxygen therapy ranged from 2 to 12 days. None of the five remaining patients received any respiratory support.
The patient coinfected with hMPV who required MV was treated with albuterol, epinephrine, hypertonic saline, remdesivir, corticosteroids, antibiotics, azithromycin, and hydroxychloroquine. Excluding this patient, albuterol was administered in three patients for 2–3 days, and none received epinephrine or hypertonic saline. Intravenous corticoids were given in one case and azithromycin was administered in two cases (one of them had a confirmed coinfection with Bordetella pertussis). No patients received any other antiviral treatment.
One patient was admitted to the pediatric intensive care unit (PICU). The median length of admission was 4 days (range: 3–14 days). No patient died or showed any sequelae at discharge.
As of April 2021, the follow‐up period ranged from 5 to 13 months depending on the date of discharge. Two patients have had further episodes of bronchospasm. None has required further hospitalization.
4. DISCUSSION
Although SARS‐CoV‐2 infection may be associated with AB in the absence of a viral coinfection, SARS‐CoV‐2 AB has not been a frequent cause of admission during the first year of the COVID‐19 pandemic in Spain, one of the European countries most severely affected by the disease. 11 , 12 These findings are similar to those described during the pre‐COVID‐19 era in which HCoVs were an infrequent cause of single‐infection AB. 5 , 6 When admitted, patients with SARS‐CoV‐2 AB did not have a more severe disease course.
In our series of patients with AB, hospital admission was mostly motivated by patient age below 8 weeks. Young age is a known risk factor for severe disease in AB caused by other viruses. 15 , 16 Therefore, local protocols usually recommend hospital admission for young infants with AB regardless of severity. Moreover, in the early stages of the pandemic, a more conservative approach regarding age was to be expected by attending pediatricians in the ED.
According to clinical practice guidelines, clinicians should diagnose AB and assess disease severity based on history and physical examination; radiographic or laboratory studies should not be obtained routinely. 17 , 18 An investigation by Parikh et al. showed that blood testing was ordered in 29% and CXR in 52% of patients admitted due to AB. 19 In our series, laboratory studies were ordered in 7/12 patients, likely due to concerns surrounding this emerging pathogen, though no remarkable results were found. On the other hand, a CXR was ordered in 6/12 cases despite a nonsevere course in most cases, and remarkable findings were found only in the patient coinfected with hMPV who required MV.
Nowadays, pharmacological treatments should not be administered to infants and children with a diagnosis of AB. 17 , 18 Nevertheless, it continues to be commonplace for hospitalized patients to receive bronchodilators (58%), antibiotics (33%), or steroids (16%) in the United States. 19 Similar figures of overuse of nonrecommended drugs have been reported in Spain. 20 In our series, bronchodilators were given to 4/12 patients. This low rate may reflect a cautious approach taken when considering nebulized treatments in the COVID‐19 era due to the risk associated with these therapy approaches, but also due to the current strong evidence base advising against them.
The nonsevere course of most cases of SARS‐CoV‐2 AB is reflected by the degree of supplemental oxygen needed during admission. Five patients did not require any oxygen at all, and in another six cases an ordinary nasal cannula was sufficient. Only the patient coinfected with hMVP required HFNC, CPAP, and MV. PICU admission was indicated in this unique patient, which is consistent with other series in which approximately 6%–17% of hospitalized children with AB required intensive care. 21 , 22 Although the patient with the worst clinical evolution had a viral coinfection, most of the infants tested showed no presence of coinfection. Therefore, our study cannot contribute data on the behavior of this emerging virus in coinfection with other pathogens.
According to recent studies, hMPV is one of the five most common pathogens in severe AB, accounting for 6% of the cases in the United States and in half of them acting as a single infection. 22 It is difficult to determine whether the hMPV itself or the combination of the two infections was responsible for the severity in the single case requiring MV in the series. On the one hand, endemic coronavirus coinfection, mainly with RSV, has not been associated with increased disease severity. 7 On the other hand, hMPV, either alone or in coinfection with RSV, is a known trigger of severe AB. 22 , 23 Therefore, although the exact role of SARS‐CoV‐2 infection is impossible to discern in this case, it may not have played a key role in the complicated course.
Apart from the fact that SARS‐CoV‐2 does not seem to be a major trigger of AB, lockdown orders, physical distancing, mask‐wearing, and other nonpharmaceutical interventions do not only impact COVID‐19 but also the dynamics of various other infectious diseases. 24 , 25 Since the beginning of the pandemic, AB has been almost entirely absent due to the nonexistence of the 2020 winter RSV infection peak, first in the southern hemisphere 26 , 27 and then in the northern hemisphere. 28 , 29 Supporting this trend, the eight patients who were screened for RSV in our series were negative. Due to the nonexistence of RSV infection peaks, PICU admissions due to AB during the COVID‐19 pandemic have been reduced dramatically. 30
As with other well‐known viruses causing AB, neonatal apnea has been described as the presenting symptom of SARS‐CoV‐2 infection in a 16‐day‐old newborn who required PICU admission and nasal CPAP. 31 Similarly, another case report describes life‐threatening bronchospasm in an 11‐month‐old boy infected with SARS‐CoV‐2 with a past history consisting of four episodes of lower respiratory tract infection. 32 These alternative respiratory manifestations caused by SARS‐CoV‐2 infection in infants have not been addressed in this article.
Other limitations should be pointed out. First, as microbiologic tests were initially indicated only in patients who required admission, the frequency of SARS‐CoV‐2 AB cases not requiring hospitalization cannot be established. Second, the presence of other coinfecting viruses have not been ruled out in all patients so in some cases we cannot exclude the possibility that other viruses produced the AB and SARS‐CoV‐2 was a coinfection. Third, although the EPICO‐AEP project has gathered data from one of the largest cohorts of pediatric hospitalized patients with COVID‐19 assembled to date, the cohort of patients with AB remains small, and more data from other countries should be obtained to conduct meta‐analyses on this issue. Finally, since the first year of the pandemic did not coincide with the RSV season, the behavior of this emerging virus in coinfection with this well‐known trigger of AB is uncertain.
In conclusion, single SARS‐CoV‐2 infection does not seem to be a main trigger of severe AB in children, and individuals with this condition should be managed according to clinical practice guidelines for AB.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Jose Antonio Alonso‐Cadenas: Investigation (supporting); writing original draft (supporting). Elena Cobos‐Carrascosa: Data curation (supporting); investigation (supporting); project administration (supporting); writing review & editing (supporting). Inmaculada Bodegas: Investigation (supporting); writing review & editing (supporting). Manuel Oltra‐Benavent: Investigation (supporting); writing review & editing (supporting). Ane Plazaola: Investigation (supporting); writing review & editing (supporting). Cristina Epalza: Investigation (supporting); writing review & editing (supporting). Cinta Moraleda: Data curation (equal); investigation (equal); project administration (equal); writing review & editing (supporting). Alfredo Tagarro: Data curation (equal); investigation (equal); project administration (equal); writing review & editing (supporting).
ACKNOWLEDGMENTS
Project PI20/00095, from the Instituto de Salud Carlos III (Ministry of Economy, Industry and Competitiveness) and cofounded by the European Regional Development Fund. Elena Cobos is supported by a specific Research Project of the Spanish Society of Paediatrics (Asociación Española de Pediatría). EPICO‐AEP Working Group: Francisco José Sanz, María Isabel Iglesias‐Bouzas, Jose Antonio Alonso Cadenas, Blanca Herrero (Hospital Infantil Universitario Niño Jesús), Teresa del Rosal, Ana Méndez‐Echevarría, Talía Sainz, Clara Udaondo, Fernando Baquero, Cristina Calvo, Carlos Grasa, Paula R Molino, María José Mellado, María Ceano, Victor Galán, Marta Melgosa, Paula Garcia Sanchez, Sonsoles San Roman (Hospital Universitario La Paz), Alicia Hernanz Lobo, Mar Santos, Marisa Navarro, Elena Rincón, David Aguilera, Begoña Santiago, Jorge Huerta, Eduardo Bardón, Jorge Lorente (Hospital Universitario Gregorio Marañón), Pablo Rojo, Daniel Blázquez, Luis Prieto, Elisa Fernández‐Cooke, David Torres‐Fernández, Ángela Manzanares, Jaime Carrasco, Cristina Epalza, Jesús Contreras, Sara Domínguez, Sara Villanueva, Arantxa Gonzalez (Hospital Universitario 12 de Octubre), Cinta Moraleda, Alfredo Tagarro, Elena Cobos, Álvaro Ballesteros, Sara Domínguez‐Rodriguez (Instituto de Investigación 12 de Octubre), Gemma Pons, Silvia Simó, Miguel Lanaspa, Victoria Fumadó, Rosa María Pino, (Hospital Sant Joan de Déu), María Espiau, Jacques G. Rivière, Pere Soler‐Palacín, Antonio Soriano Arandes, Natalia Mendoza (Hospital Universitari Vall d'Hebron), Mercedes Herranz, María Urretavizcaya Martínez (Complejo Hospitalario de Navarra), Fernando Cabañas, Fátima Ara, Marta Baragaño, Rosa Maria Hernández Palomo, Inmaculada Bodegas (Hospital Universitario Quirón Salud Madrid), Rut del Valle, Ana González‐de‐Zárate, Mónica Pacheco, María Luisa Herreros, Julia Yebra, Beatriz Pérez‐Seoane, María Fernández, Teresa Raga, María de la Serna, Ane Plazaola, Juan Miguel Mesa, Rosa Batista, Ana Barrios, Ignacio Navarro, Jana Rizo, Teresa Reinoso, Alfonso Cañete (Hospital Universitario Infanta Sofía), María Dolores Martín, Elena Sáez, Olga Nerea Coya, Fernando Cava (BR Salud), Enrique Otheo, Juan Carlos Galán, José Luis Vázquez, Carmen Vázquez, Victor Quintero (Hospital Universitario Ramón y Cajal), Lola Falcón, Olaf Neth, Peter Olbrich, Walter Goicoechea, Cati Márquez, Marisol Camacho, Inés Marín Cruz (Hospital Universitario Virgen del Rocío), Laura Martín (Hospital Universitario Regional de Málaga), Lucía Figueroa (Hospital de Villalba), María Llorente (Hospital Universitario del Sureste), María Penin, Claudia García, María García, Teresa Alvaredo (Hospital Universitario Príncipe de Asturias), Mª Inmaculada Olmedo, Agustín López, María Jose Pérez (Hospital Universitario Puerta de Hierro), Elvira Cobo (Hospital Fundación Alcorcón), Mariann Tovizi (Hospital del Tajo), Pilar Galán (Hospital Fundación Fuenlabrada), Beatriz Soto, Sara Guillén (Hospital de Getafe), Adriana Navas (Hospital Universitario Infanta Leonor) M. Luz García (Hospital de Leganés), Sara Pérez (Hospital de Torrejón), Amanda Bermejo, Pablo Mendoza (Hospital de Móstoles), Gema Sabrido (Hospital Rey Juan Carlos), María José Hernández (Hospital Central de la Defensa), Ana Belén Jiménez (Fundación Jiménez Díaz), Arantxa Berzosa, José Tomás Ramos, Marta Illán (Hospital Clínico San Carlos), Ana López, Nerea Gallego (Hospital Universitari Son Espases), Beatriz Ruiz (Hospital Universitario Reina Sofía), Santiago Alfayate, Ana Menasalvas, Eloísa Cervantes (Hospital Clínico Universitario Virgen de la Arrixaca), María Méndez (Institut d'Investigació en Ciències de la Salut Germans Trias i Pujol), Ángela Hurtado (Instituto Hispalense de Pediatría), Cristina García, Inés Amich, Yolanda Ruiz (Hospital San Pedro), Manuel Oltra, Álvaro Villarroya‐Villalba, Adela Cañete, Bienvenida Argiles (Hospital Universitari i Politècnic La Fe), Angustias Ocaña (Hospital La Moraleja), Isabel Romero, María Fernanda Guzmán (Hospitales Madrid), M.J. Pascual (Hospital Nisa), María Sánchez‐Códez (Hospital Universitario Puerta del Mar), Elena Montesinos (Consorci Hospital General Universitari de València), Julia Jensen, María Rodríguez (Hospital Universitario Infanta Cristina), Gloria Caro (Hospital Universitario Infanta Elena), Neus Rius, Alba Gómez (Hospital Universitari Sant Joan de Reus), Rafael Bretón (Hospital Clínico Universitario de Valencia), Margarita Rodríguez, Julio Romero, Juan Francisco Pascual Gazquez (Hospital Universitario Virgen de las Nieves), Ana Campos (Hospital Universitario Sanitas La Zarzuela), Mercedes García (Hospital de Mérida), Rosa María Velasco (Complejo Hospitalario de Toledo), Zulema Lobato (Althaia, Xarxa Assistencial Universitària de Manresa), Fernando Centeno, Elena Pérez, Alfredo Cano (Hospital Universitario Río Hortega), Paula Vidal (Hospital Clínico Universitario Lozano Blesa), Corsino Rey, Ana Vivanco, Maruchi Alonso (Hospital Universitario Central de Asturias), Pedro Alcalá, Javier González de Dios, Laura Ureña Horno (Hospital General Universitario de Alicante), Eduard Solé, Laura Minguell (Hospital Universitari Arnau de Vilanova), Itziar Astigarraga, Olatz Villate, Susana García Obregón (Hospital Universitario de Cruces), Mª Ángeles Vázquez, Miguel Sánchez, Leticia Martínez Campos (Hospital Universitario Torrecárdenas), Elena Díaz (Hospital Virgen de la Luz), Eduardo Consuegra, Susana Riesco, Almudena González, Maika Mendoza (Hospital Universitario de Salamanca), María Cabanillas (Complejo Asistencial Universitario de Palencia), Yeray Novoa‐Medina, Elena Colino‐Gil, Ana Reyes‐Domínguez, Luis Peña‐Quintana (Hospital Universitario Materno Infantil de las Palmas), Elisa Garrote, Maite Goicoechea, Ainhoa Gondra Sangroniz (Hospital Universitario de Basurto), Irene Centelles (Hospital General Universitari de Castelló), Santiago Lapeña, Sara Gutiérrez, Soraya Gutiérrez (Complejo Asistencial Universitario de León), Amparo Cavalle (PIUS Hospital de Valls), José María Olmos (Hospital Mare de Déu dels Lliris), Alejandro Cobo, Sara Díaz, Cristina Martinez Faci, Macarena Gonzalez Cruz, Beatriz Castro (Hospital Universitario de Canarias), Beatriz Jiménez, Cristina Alvarez Alvarez (Hospital Universitario Marqués de Valdecilla), Raúl González (Hospital Sant Joan d'Alacant), Miguel Lafuente, Matilde Bustillo (Hospital Infantil de Zaragoza), Natividad Pons, Julia Morata (Hospital Lluís Alcanyis), Elsa Segura (Hospital Universitario Son Llatzer de Palma de Mallorca), María Bernardino (Universidad Europea de Madrid), Marta Pareja León (Complejo Hospitalario Universitario de Albacete), Ana Domingo Ruiz (Hospital de Manacor), Eider Oñate, Nagore Garcia de Andoin Baran (Hospital Universitario Donostia), Nerea Domínguez‐Pinilla (Hospital Virgen de la Salud), María Teresa Coll Sibina (Hospital General de Granollers), María Jesús García García (Hospital Universitario de Cáceres), Marta Osuna (Hospital HM Montepríncipe), Raquel Portugal (Hospital Universitario de Burgos), Leonor García Maset (Hospital de Sagunto), Belén Sevilla (Hospital Universitario San Cecilio Granada), Noelia Berciano Jiménez (Hospital Virgen de la Macarena, Sevilla).
Andina‐Martinez D, Alonso‐Cadenas JA, Cobos‐Carrascosa E, et al. SARS‐CoV‐2 acute bronchiolitis in hospitalized children: neither frequent nor more severe. Pediatric Pulmonology. 2022;57:57‐65. 10.1002/ppul.25731
Cinta Moraleda and Alfredo Tagarro contributed equally to this study.
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Varghese L, Zachariah P, Vargas C, et al. Epidemiology and clinical features of human coronaviruses in the pediatric population. J Pediatric Infect Dis Soc. 2018;7(2):151‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang B, Liu S, Zhang J, et al. Children hospitalized for coronavirus disease 2019 (COVID‐19): a multicenter retrospective descriptive study. J Infect. 2020;81(2):e74‐e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003; 143(5 Suppl):S127‐S132. [DOI] [PubMed] [Google Scholar]
- 5. Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J. 2013;32(9):950‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvador García C, Moreno Docón A, Piñero JA, Alfayate Miguelez S, Iborra, Bendicho MA. [Aetiology of bronchiolitis in hospitalised children in South‐East Spain]. An Pediatr. 2012; 77(6):386‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansbach JM, Hasegawa K, Piedra PA, Sullivan AF, Camargo CA. Severe coronavirus bronchiolitis in the pre‐COVID‐19 era. Pediatrics. 2020;146(3):e20201267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimaud E, Challiol M, Guilbaud C, et al. Delayed acute bronchiolitis in infants hospitalized for COVID‐19. Pediatr Pulmonol. 2020;55(9):2211‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Melé M, Henares D, Pino R, et al. Low impact of SARS‐CoV‐2 infection among paediatric acute respiratory disease hospitalizations. J Infect. 2021;82(3):414‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimmermann P, Curtis N. Why is COVID‐19 less severe in children? A review of the proposed mechanisms underlying the age‐related difference in severity of SARS‐CoV‐2 infections. Arch Dis Child. 2020: archdischild‐2020‐320338. [DOI] [PubMed] [Google Scholar]
- 11. Instituto de Salud Carlos III COVID‐19 in Spain update, as of 1 March 2021. 2021. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_322_COVID-19.pdf
- 12. 2021. European Center for Disease Prevention and Control. COVID‐19 situation update worldwide, as of 1 March 2021. 2021. https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
- 13. Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID‐19) in children in Madrid, Spain. JAMA Pediatr. 2021;175(3):316‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McConnochie KM. Bronchiolitis. What's in the name? Am J Dis Child. 1983;137(1):11‐13. [PubMed] [Google Scholar]
- 15. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014; 134(5):e1474‐e1502. [DOI] [PubMed] [Google Scholar]
- 18. O'Brien S, Borland ML, Cotterell E, et al. Australasian bronchiolitis guideline. J Paediatr Child Health. 2019; 55(1):42‐53. [DOI] [PubMed] [Google Scholar]
- 19. Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133(1):e1‐e7. [DOI] [PubMed] [Google Scholar]
- 20. Ochoa Sangrador C, González de Dios J, Research Group of the aBREVIADo Project . Overuse of bronchodilators and steroids in bronchiolitis of different severity: bronchiolitis‐study of variability, appropriateness, and adequacy. Allergol Immunopathol. 2014;42(4):307‐315. [DOI] [PubMed] [Google Scholar]
- 21. Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000‐2009. Pediatrics. 2013;132(1):28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasegawa K, Goto T, Hirayama A, et al. Respiratory virus epidemiology among US infants with severe bronchiolitis: analysis of 2 multicenter, multiyear cohort studies. Pediatr Infect Dis J. 2019;38(8):e180‐e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNamara PS, Flanagan BF, Smyth RL, Hart CA. Impact of human metapneumovirus and respiratory syncytial virus co‐infection in severe bronchiolitis. Pediatr Pulmonol. 2007. 42(8):740‐743. 10.1002/ppul.20649 [DOI] [PubMed] [Google Scholar]
- 24. Wilder JL, Parsons CR, Growdon AS, Toomey SL, Mansbach JM. Pediatric hospitalizations during the COVID‐19 pandemic. Pediatrics. 2020;146(6):e2020005983. [DOI] [PubMed] [Google Scholar]
- 25. Angoulvant F, Ouldali N, Yang DD, et al. Coronavirus disease 2019 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and nonviral infections‐a time series analysis. Clin Infect Dis. 2021; 72(2):319‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Friedrich F, Ongaratto R, Scotta MC, et al. Early impact of social distancing in response to coronavirus disease 2019 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin Infect Dis. 2021; 72(12):2071‐2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeoh DK, Foley DA, Minney‐Smith CA, et al. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021; 72(12):2199‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Brusselen D, De Troeyer K, Ter Haar E, et al. Bronchiolitis in COVID‐19 times: a nearly absent disease? Eur J Pediatr. 2021;180(6):1969‐1973. 10.1007/s00431-021-03968-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guedj R, Lorrot M, Lecarpentier T, Leger P‐L, Corvol H, Carbajal R. Infant bronchiolitis dramatically reduced during the second French COVID‐19 outbreak. Acta Paediatr. 2021; 110(4):1297‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rambaud J, Dauger S, Morin L, et al. Bronchiolitis admissions to intensive care during COVID. Pediatrics. 2021; 147(4):e2021050103. [DOI] [PubMed] [Google Scholar]
- 31. González Brabin A, Iglesias‐Bouzas MI, Nieto‐Moro M, Martínez de Azagra‐Garde A, García‐Salido A. [Neonatal apnea as initial manifestation of SARS‐CoV‐2 infection]. An Pediatr. 2020;93(3):215‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. André MC, Pätzug K, Bielicki J, Gualco G, Busi I, Hammer J. Can SARS‐CoV‐2 cause life‐threatening bronchiolitis in infants? Pediatr Pulmonol. 2020;55(11):2842‐2843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


