Abstract
Prognostic predictors are of paramount interest for prompt intervention and optimal utilization of the healthcare system in the ongoing context of the COVID‐19 pandemic. The platelet‐to‐lymphocyte count ratio (PLR), has emerged as a potential tool for risk stratification of critically ill patients with sepsis. The current systematic review explores the utility of PLR as a prognostic predictor of COVID‐19 patients. We screened the electronic databases until May 15, 2021 after enrolling in PROSPERO (CRD42021220269). Studies evaluating the association between PLR on admission and outcomes in terms of mortality and severity among COVID‐19 patients were included. We retrieved 32 studies, with a total of 2768 and 3262 COVID‐19 patients for mortality and disease severity outcomes. Deceased and critically ill patients had higher PLR levels on admission in comparison to survivors and non‐severe patients (mean differences [MD] = 66.10; 95% confidence interval [CI]: 47.75–84.44; p < 0.00001 and MD = 86.74; 95% CI: 67.7–105.7; p < 0.00001, respectively). A higher level of PLR on admission in COVID‐19 patients is associated with increased morbidity and mortality. However, the evidence is of low quality and further studies regarding the cut‐off value of PLR are the need of the hour.
Keywords: Coronavirus disease 2019, Platelet‐lymphocyte count ratio, Severe acute respiratory syndrome coronavirus‐2
Highlights
Impact of raised PLR on admission in COVID‐19 patients:
-
1.
↑ risk of mortality (MD = 66.10; 95%CI 47.75‐84.44; p < 0.00001).
-
2.
↑ risk of severity (MD = 86.74;95%CI 67.7‐105.7; p < 0.00001).
1. INTRODUCTION
Even after a year of emergence of the severe acute respiratory syndrome coronavirus‐2 (SARS CoV‐2), the coronavirus disease 2019 (COVID‐19) pandemic still has overwhelmed the medical infrastructure around the globe. Thus, early detection of severe cases is of paramount importance in the context of this pandemic as a method of triage and optimal allocation of resources.
The platelet‐to‐lymphocyte ratio (PLR) is an easily obtainable ratio from complete blood count (CBC) panels. Recently, it has been proposed as a better indicator of inflammation when compared to white blood cell count (WBC) alone. Increased PLR has been observed in patients with chronic inflammatory conditions like autoimmune diseases, rheumatic disorders, cancers, and diabetes. 1 , 2 , 3 , 4 , 5 Various studies have indicated a correlation between elevated PLR and mortality in acute pulmonary embolism, advanced cancers, and gynecologic malignancies. 3 , 4 , 6
Similarly, inflammation is central to the pathogenesis of COVID‐19 and the progress of inflammation or dysfunctional immune response has been associated with severe COVID‐19 disease. 7 , 8 It is therefore conceivable that patients with a pre‐existing chronic inflammatory state will be susceptible to severe COVID‐19 disease. In this meta‐analysis, we analyzed the studies which had reported PLR on admission and examined the outcome of COVID‐19 disease (severity and mortality) and the ability of PLR to predict progression to severe COVID‐19 disease.
PLR as a marker of pre‐existing pro‐inflammatory or chronic inflammatory state can be used as a predictor of COVID 19 disease progression. There have been several studies that have examined the relationship between admission PLR and its ability to predict mortality in COVID 19 disease. In this meta‐analysis, we aim to systematically analyze the current evidence for the utility of PLR on admission as a prognostic predictor of SARS CoV‐2 infection, as per the “Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA‐P) guidelines”.
2. METHODS
2.1. Protocol and registration
We prospectively registered the protocol of this systematic review in PROSPERO (ID: CRD42021220269). This study is without any divergence from the reported protocol.
2.2. Search strategy
Independently, SS, SK, and PK searched the major electronic databases (PubMed, Medline, and Embase), Google Scholar (https://scholar.google.com), preprint platforms MedRxiv (https://www.medrxiv.org), and Clinical trial database (https://ClinicalTrials.gov) from January 1, 2020 to May 15, 2021, with the following keywords: “COVID‐19” OR “SARS‐CoV‐2” AND “PLR” OR “Platelet‐to‐lymphocyte count ratio.”
2.3. Inclusion and exclusion criteria
Prospective and retrospective comparative cohort studies, case series with a control group, cross‐sectional studies, controlled clinical trials, case‐control studies, and randomized controlled trials (RCT), evaluating PLR on admission in COVID‐19 patients were looked for inclusion. We assessed mortality as the primary outcome and disease severity as the secondary outcome. The articles except in the English language, without full retrievable text or appropriate control group, were excluded (PRISMA flow diagram). 9 , 10
2.4. Study selection
SS, SK, and PK screened all the available abstracts independently after removing the duplications to exclude the irrelevant articles. Then the full‐texts of the eligible studies were screened to check the inclusion criteria. Any disagreements were resolved in consultation with a fourth researcher (AKS).
2.5. Data extraction
SS and SK extracted the data regarding first author, year of publication, type of study, place, sample size, PLR on admission, disease severity, and mortality in COVID‐19 patients in a pre‐conceived data extraction sheet from all included studies individually. Dichotomous data were collected in terms of the number of incidents and the total number of patients in the respective group and means and SD were extracted for the continuous data. Studies with missing data have been described separately.
Due to lack of consensus regarding defining the severity of the disease among studies, any patient either requiring mechanical ventilation or with a ratio of the partial pressure of arterial blood oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg was considered as severe/critically ill and the rest of the patients are defined as mild/moderate ill patients.
2.6. Risk of bias assessment
SS and PK independently assessed the included studies for any potential bias. The difference of opinion was resolved by consulting with AKS. “The Risk Of Bias In Non‐randomized Studies—of Interventions (ROBINS‐I)” tool 11 was used for assessing the risk of bias in non‐randomized studies. It includes the following seven domains: “bias due to confounding,” “selection of participants, classification of interventions,” “deviations from intended interventions,” “missing data,” “measurement of outcomes,” and “selection of the reported result.” Every domain is graded as “Low,” “Moderate,” “Serious,” and “Critical.”
2.7. Quality of the evidence
Independently PK and SS used the “Grading of Recommendations Assessment, Development and Evaluation (GRADE)” tool, which has five downgrading factors (“study limitations, indirectness, imprecision, consistency of effect, and publication bias”) and three upgrading factors (“dose‐response relation, large magnitude of the effect, and plausible confounders or biases”) 12 , 13 for assessing the quality of evidence. Each outcome was graded in terms of either “High” or “Moderate” or “Low” or “Very low”. 14 , 15 , 16 , 17 , 18 , 19 The difference of opinion was resolved with the suggestion of AKS.
2.8. Data synthesis
SS and PK used Review Manager version 5 for conducting this frequentist meta‐analysis. The odds ratio (OR) for dichotomous data, and mean differences (MDs) for continuous data along with the 95% confidence intervals (CIs) respectively were assessed as per the Cochrane Handbook for Systematic Reviews of Interventions. 20 The I 2 statistic was used for evaluating the statistical heterogeneity, a value of >50% was accepted as significant heterogeneity. Publication bias was assessed with the help of a funnel plot.
3. RESULTS
3.1. Basic characteristics
Thirty‐three studies 21 ‐ 52 out of 979 distinguished publications were incorporated as per the aforementioned inclusion criteria (Figure 1 and Table 1). Twenty‐nine articles were peer‐reviewed, and three were preprints. 32 , 33 , 44 ALthough 20 articles evaluated PLR on admission to assess the severity of COVID‐19 patients, 14 articles addressed PLR on admission between survivors and non‐survivors. Among the included studies, six studies had a moderate degree of bias (Figure 2). The publication bias is represented qualitatively in the Funnel plot (Figure S1).
Figure 1.

PRISMA‐2009 flow diagram
Table 1.
Characteristics of included studies
| SN | Author, year | Type of study, center | Country | Total no. of patients | PLR cut off value | Outcome |
|---|---|---|---|---|---|---|
| 1. | Fois et al. (2020) 21 | Retrospective, SC | Italy | 119 | 240 | Nonsurvivors had a higher PLR in comparison to the surviving COVID‐19 patients (AUC: 0.57; 95% CI: 0.47–0.66). |
| 2. | Abrishami et al. (2020) 22 | Prospective, MC | Iran | 100 | NS | An elevated PLR has a positive corelation with mortality and it can be one of the cost‐effective prognostic markers of COVID‐19 |
| 3. | Pan et al. (2020) 23 | Retrospective, SC | China | 120 | NS | High PLR associated with risk of in hospital death in persons with COVID‐19 |
| 4. | Rokhni et al. (2020) 24 | Retrospective, SC | Iran | 233 | 200 | Nonsurvivors had a high level of PLR (14.84) in comparison to survivors (13.9) at admission. |
| 5. | Asgar et al. (2020) 25 | Retrospective, SC | Pakistan | 191 | 201.16 | Elevated PLR is positively corelated with morbidity and mortality of COVID‐19 patients (AUC: 0.703, PPV: 81.8%) |
| 6. | Wang et al. (2020) 26 | Retrospective, SC | China | 450 | NS | The mortality rate of COVID‐19 positively correlated with higher neutrophil‐to‐lymphocyte ratio, PLR |
| 7. | Wang et al. (2020) 27 | Retrospective, SC | China | 131 | NS | Mortality is associated with higher variation of PLR [187.33, IQR: 139.24–332.76] in compare to surviving patients [169.23, IQR: 115.23–222.96] |
| 8. | Allahverdiyev et al. (2020) 28 | Retrospective, SC | Turkey | 455 | NS | The mortality rate of COVID‐19 positively correlated with higher PLR, NLR, RDW |
| 9. | Güneysu et al. (2020) 29 | Retrospective, SC | Turkey | 169 | 148.85 | NLR, PLR, and CRP values can be used as early predictors of mortality in Covid‐19 patients (AUC: 0.660; 95% CI: 0.577−0.743). |
| 10. | Jimeno et al. (2021) 30 | Retrospective, SC | Spain | 119 | NS | Deceased COVID‐19 patients had a higher PLR (200; IQR: 336.7–131.7) on admission in comparison to the survivors (193.3; IQR: 284.1–147) |
| 11. | Kalabin et al. (2021) 31 | Retrospective, SC | USA | 184 | NS | No significant difference in PLR was found among the deceased in comparison to the survivors. |
| 12. | Arachana et al. (2021) 32 | Cross Sectional, SC | India | 302 | 205 | The cut‐off of PLR > 205 for predicting the mortality ha a has 42% sensitivity and 49% specificity |
| 13. | Nasir et al. (2021) 33 | Retrospective, SC | Bangladesh | 99 | NS | No significant difference in PLR was found among the deceased in comparison to the survivors |
| 14. | Ashgar et al. (2020) 34 | Retrospective, SC | Pakistan | 100 | 153.65 | Elevated PLR on admission is associated with disease severity (AUC: 0.696, 95% CI: 0.576–0.816), and mortality (AUC: 0.671, 95% CI: 0.535–0.808). |
| 15. | Bastug et al. (2020) 35 | Retrospective, SC | Turkey | 191 | 175.78 | PLR is a prognostic predictor for patients with severe COVID‐19 (AUC: 0.715, 95% CI: 0.61–0.81). |
| 16. | Ai‐Ping Yang et al. (2020) 36 | Retrospective, SC | China | 93 | 180 | Elevated age, PLR, and NLR can be independently associated with advancing COVID‐19 severity (AUC: 0.784, 95% CI: 0.666–0.901) |
| 17. | Zeng et al. (2020) 37 | Retrospective, MC | China | 217 | NS | Higher PLR was associated with severe COVID‐19. |
| 18. | Xue et al. (2020) 38 | Retrospective, SC | China | 114 | NS | PLR > 229 has a positive predictive value of 69.8% for disease severity |
| 19. | Gong et al. (2020) 39 | Retrospective, MC | China | 381 | NS | PLR and RDW help to predict the severity of COVID‐19 patients. |
| 20. | Wang et al. (2020) 40 | Retrospective, SC | China | 61 | 200.8 | The PLR was significantly elevated in the severe group compared with the group with common symptoms (OR: 0.112; 95% CI: 0.032–0.387) |
| 21. | Kong et al. (2020) 41 | Prospective, SC | China | 40 | 191.7 | A higher PLR was associated with poor outcome. |
| 22. | Kazancioglu et al. (2020) 42 | Retrospective, SC | Turkey | 120 | 230 | The elevated PLR during follow‐up may be more useful compared to NLR to predict the disease severity. |
| 23. | Sha lin et al. (2020) 43 | Retrospective, SC | China | 68 | NS | High PLR, low monocyte counts, and low lymphocyte counts were independent correlates of severe illness in SARS‐COV‐2 infection. |
| 24. | Huang et al. (2020) 44 | Prospective, SC | China | 415 | 222.5 | Elevated NLR, and PLR are independent risk factor of severe COVID‐19 patient |
| 25. | Sun et al. (2020) 45 | Retrospective, SC | China | 116 | 226.67 | Severe COVID‐19 patients had a higher PLR at presentation |
| 26. | Weili Wang et al. (2020) 46 | Retrospective, SC | China | 123 | 189.11 | PLR is a potential predictor of poor clinical outcome in Covid‐19 patients (AUC: 0.788, 95% CI: 0.826–0.944) |
| 27. | Yan Zhao et al. (2020) 47 | Retrospective, SC | China | 285 | 274 | Initial PLR is found to be higher in SARS‐CoV‐2 virus‐infected group than in influenza A. |
| 28. | Yutian Zhou et al. (2020) 48 | Retrospective, SC | China | 304 | NS | Critically ill COVID‐19 patients had an elevated PLR. It is an important predictor for severity grading. |
| 29. | Waris et al. (2021) 49 | Retrospective, SC | Pakistan | 101 | NS | A significant association was observed in platelet‐lymphocyte ratio and disease severity |
| 30. | Erdogan et al. (2021) 50 | Retrospective, SC | Turkey | 304 | NS | PLR can be used as a significant biomarker for predicting prognosis of patients. |
| 31. | Ok et al. (2021) 51 | Retrospective, SC | Turkey | 139 | NS | PLR can help identify high‐risk cases with COVID‐19. |
| 32. | Zhu et al. (2021) 52 | Retrospective, SC | China | 111 | NS | PLR can play an important role in the severity of COVID‐19 and had a potential value for monitoring the process of severe cases. (OR: 1.004; 95% CI: 1.000–1.008; p = 0.06) |
| 33. | Seyit et al. (2020) 53 | Retrospective, SC | Turkey | 233 | 100.8 | Baseline PLR was significantly higher in COVID‐19 patients in comparison to COVID‐19 negative patient. AUC: 0.669 (0.590–0.747) |
Abbreviations: AUC, area under curve; IQR, interquartile range; MC, multi‐center; NLR, neutrophil‐to‐lymphocyte ratio; NS, not specified; PLR, platelet‐to‐lymphocyte ratio; RDW, red cell distribution width; SC, single‐center.
Figure 2.
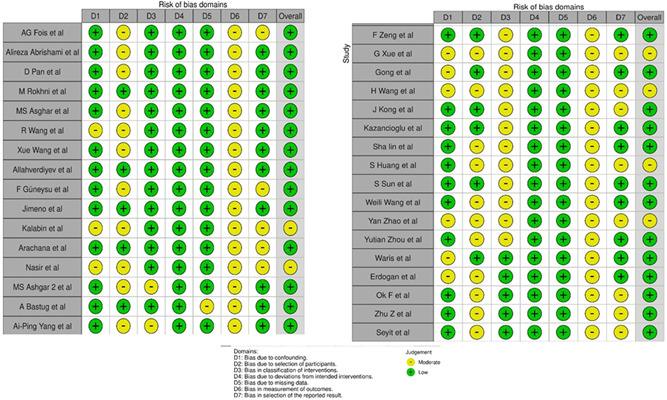
ROBINS‐I assessment for the included non‐randomized cohort studies
3.2. Meta‐analyses
3.2.1. Mortality
Fourteen articles with a total of 2768 patients were evaluated for mortality in COVID‐19. PLR on admission was significantly higher among the deceased in comparison to the survivors (MD = 66.10; 95% CI: 47.75–84.44; I 2 = 89%, p < 0.0001) (Figure 3).
Figure 3.
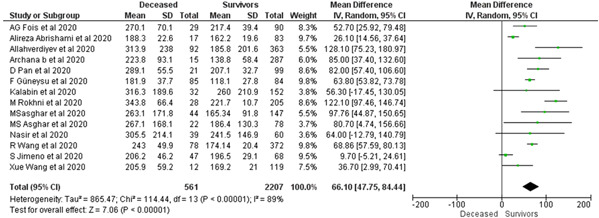
The impact of the baseline PLR on mortality in COVID‐19 patients. PLR, platelet‐to‐lymphocyte count ratio
3.2.2. Severity
Twenty studies with an aggregate of 3262 patients were evaluated for the severity of COVID‐19. Critically ill patients are associated with increased PLR on admission (MD = 86.74; 95% CI: 67.7–105.7; I 2 = 95%, p < 0.0001) (Figure 4A).
Figure 4.
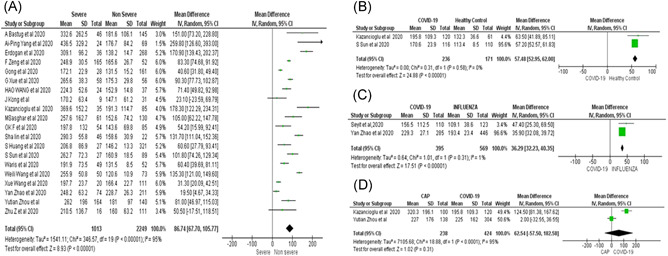
(A) The impact of baseline PLR on disease severity in COVID‐19 patients. (B) The impact of baseline PLR on disease severity in COVID‐19 patients in comparison to healthy controls. (C) The impact of baseline PLR on disease severity in COVID‐19 patients in comparison to patients with Influenza. (D) The impact of baseline PLR on disease severity in COVID‐19 patients in comparison to patients with community‐acquired pneumonia (CAP). PLR, platelet‐to‐lymphocyte count ratio
3.2.3. Subgroup analysis
In subgroup analyses, the baseline PLR was found to be significantly elevated in COVID‐19 patients in comparison to healthy controls (MD = 57.48; 95% CI: 52.95–62; I 2 = 0%) (Figure 4B), as well as similar patients with influenza (MD = 36.29; 95% CI: 32.23–40.35; I 2 = 1%) (Figure 4C). However, there was no significant difference in similarly ill patients with community‐acquired pneumonia (CAP) (MD = 62.54; 95% CI: −57.5–182.58; I 2 = 95%) (Figure 4D).
3.2.4. Significant heterogeneity is found among studies assessing mortality, severity, and subgroup analysis in patients with CAP
3.3. Quality of evidence
We found a low quality of evidence on the impact of raised PLR on COVID‐19 mortality and severity (Table 2).
Table 2.
GRADE evidence profile of COVID‐19 studies
| Outcome | No. of participants | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Quality of evidence (Grade) | Relative effect | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total no. | Intervention | Control | ||||||||
| Mortality | 2768 | 561 | 2207 | No | No | Yes | No | None | Low ⊕⊕⊝⊝ | MD = 66.1 (95% CI: 47.7–84.4) |
| Severity | 3262 | 1013 | 2249 | No | No | Yes | No | None | Low ⊕⊕⊝⊝ | MD = 86.74 (95% CI: 67.7–105.7) |
Abbreviations: CI, confidence interval; MD, mean difference.
3.4. Publication bias
The publication bias was assessed for the studies on COVID‐19 mortality. As per the Funnel plot, qualitatively a publication bias is likely in view of some smaller studies with large effects (Figure S1).
4. DISCUSSION
We have identified low‐quality evidence with variability that PLR value on admission has the potential ability of discrimination in COVID‐19 patients predicting the mortality and severity.
The PLR, a nonspecific inflammatory marker, implies concurrent interaction between platelet count and lymphocyte count, reflects aggregation, as well as inflammatory pathways. It has been found to be elevated in response to many acute as well as chronic proinflammatory conditions 54 , 55 , 56 and associated with a poor prognosis in patients with COPD 57 and carcinomas. 58 , 59 , 60 A recent study has found a correlation between raised PLR and poor prognosis of sepsis‐induced acute kidney injury, and mortality (OR: 1.02, 95% CI: 1.003–1.039). 61
Another recent systematic review 62 also echoed that an elevated PLR is associated with severe illness in COVID‐19 patients than in those with mild disease (SMD: 0.68; 95% CI: 0.43–0.93; I 2 = 58%).
Although it has been widely acknowledged that both lymphopenias, as well as, thrombocytopenia are associated with poor outcomes in SARS‐COV‐2 infection, 63 , 64 , 65 the exact mechanism of elevated PLR is still not clear. Platelets play a crucial role in the inflammatory response particularly at the endothelium injury 66 and can be activated even in response to proinflammatory cytokine or infectious factors without any vascular damage. 67 The interaction between circulatory leukocytes and proinflammatory cytokine activity of platelets leads to the release of cytokines. Direct viral invasion of the hematopoietic cells or bone marrow stromal cells, 68 injury of pulmonary endothelial cells leading to activation, and aggregation of platelets resulting into thrombus may lead to alteration of platelets and megakaryocytes. 69 , 70
A recent study found after an initial elevation subsequent decline of platelet count in critically ill COVID‐19 patients. The activated platelets not only augments lymphocyte adhesion to the endothelium, orients the lymphocytes towards endothelial veins of various inflammatory sites but also release the platelet factor‐4 to hinder the agglutinin‐A, thereby impeding lymphocyte generation. 71
On the contrary, the abundancy of ACE 2 receptors in lymphocytes makes vulnerable to SARS‐COV‐2 invasion, 72 acute tissue sequestration similar to previous outbreaks of the severe acute respiratory syndrome, 73 increased utilization by the elevated interleukin‐6, 74 or SARS‐COV‐2 mediated direct stimulation of NLRP3 inflammasome resulting in pyroptosis 75 in lymphocytes may predispose significant lymphocytopenia. Probably, a more severe lymphocytopenia than thrombocytopenia leading results in an elevated PLR.
The change in PLR during the hospital course from baseline seems to be linearly correlated with disease severity and period of hospital stay in COVID‐19 patients. More difference is associated with prolongation of hospitalization along with severe pneumonia. A cut off of 126.7 for difference in PLR had 100% sensitivity and 81.5% specificity (p = 0.014). 71 Similarly, Kazancioglu et al. 42 also reported a decline of PLR in the non‐severe group in contrast to a sharp rise of PLR in critically ill COVID‐19 patients from admission till the finishing of treatment.
Although Mousavi et al. 76 have reported a strong correlation between elevated PLR (>233) and mortality in Covid‐19 patients (p = 0.034), Zhao et al. 47 reported an elevated PLR of 274 (AUC: 0.69) has a specificity: 79% and sensitivity: 57%. Similarly, another study with 233 hospitalized COVID‐19 patients also reported raised PLR > 102.8 (AUC: 0.669) with sensitivity: 70% and specificity: 50%. 53
Irrespective of different cut‐off values of PLR at admission, it cannot be ignored that elevated PLR is associated with increased morbidity and mortality in SARS‐COV2 infection.
4.1. Strengths and limitations
Our study is one of the extensive and comprehensive systematic reviews of the effectiveness of PLR on admission in patients with COVID‐19 for predicting the mortality and severity, and may be considered at the moment as important evidence for decision‐making.
The majority of the included studies are retrospective in nature and from Asian countries. Although in the current scenario, the prognostic role of PLR in COVID‐19 is promising, our findings are heterogeneous, medium in effect, and of low‐quality evidence. We also acknowledged that the cut‐off value of PLR and the point of evaluation is yet to be standardized, and information in this regard is still evolving.
5. CONCLUSION
PLR is a potential predictive biomarker for stratifying risk and aiding prompt decisions about an escalation of management, and further large‐scale prospective studies in this context are the need of the hour.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Soumya Sarkar: Conceptualization, search strategy, study selection, data extraction, data synthesis, risk of bias assessment, and drafted the manuscript. Sundara Kannan: Study selection and data extraction. Puneet Khanna: Conceptualization, search strategy, study selection, risk of bias assessment, quality of the evidence assessment, and editing. Akhil Kant Singh: Study selection, data extraction, risk of bias assessment, quality of the evidence assessment, and editing.
Supporting information
Supplementary information.
Sarkar S, Kannan S, Khanna P, Singh AK. Role of platelet‐to‐lymphocyte count ratio (PLR), as a prognostic indicator in COVID‐19: A systematic review and meta‐analysis. J Med Virol. 2021;94:211‐221. 10.1002/jmv.27297
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26:372‐376. [DOI] [PubMed] [Google Scholar]
- 2. Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The platelet‐to‐lymphocyte ratio as an inflammatory marker in rheumatic diseases. Ann Lab Med. 2019;39:345‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li B, Zhou P, Liu Y, et al. Platelet‐to‐lymphocyte ratio in advanced cancer: review and meta‐analysis. Clin Chim Acta. 2018;483:48‐56. [DOI] [PubMed] [Google Scholar]
- 4. Jiang S, Liu J, Chen X, et al. Platelet‐lymphocyte ratio as a potential prognostic factor in gynecologic cancers: a meta‐analysis. Arch Gynecol Obstet. 2019;300:829‐839. [DOI] [PubMed] [Google Scholar]
- 5. Mertoglu C, Gunay M. Neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio as useful predictive markers of prediabetes and diabetes mellitus. Diabetes Metab Syndr. 2017;11(suppl 1):S127‐131. [DOI] [PubMed] [Google Scholar]
- 6. Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in acute pulmonary embolism: a systematic review and meta‐analysis. Int Angiol J Int Union Angiol. 2018;37:4‐11. [DOI] [PubMed] [Google Scholar]
- 7. García LF. Immune response, inflammation, and the clinical spectrum of COVID‐19. Front Immunol. 2020;16(11):1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA G. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383‐394. [DOI] [PubMed] [Google Scholar]
- 13. Norris SL, Meerpohl JJ, Akl EA, et al. The skills and experience of GRADE methodologists can be assessed with a simple tool. J Clin Epidemiol. 2016;79:150‐158. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407‐415. [DOI] [PubMed] [Google Scholar]
- 15. Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277‐1282. [DOI] [PubMed] [Google Scholar]
- 16. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. J Clin Epidemiol. 2011;64:1283‐1293. [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. J Clin Epidemiol. 2011;64:1294‐1302. [DOI] [PubMed] [Google Scholar]
- 18. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. J Clin Epidemiol. 2011;64:1303‐1310. [DOI] [PubMed] [Google Scholar]
- 19. Guyatt GH, Oxman AD, Sultan S, et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311‐1316. [DOI] [PubMed] [Google Scholar]
- 20. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in‐hospital mortality in COVID‐19 patients. Molecules. 2020;25(23):5725. 10.3390/molecules25235725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abrishami A, Eslami V, Baharvand Z, et al. Epicardial adipose tissue, inflammatory biomarkers and COVID‐19: Is there a possible relationship? Int Immunopharmacol. 2021;90:107174. 10.1016/j.intimp.2020.107174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan D, Cheng D, Cao Y, et al. A predicting nomogram for mortality in patients with COVID‐19. Front Public Health. 2020;8:461. 10.3389/fpubh.2020.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rokni M, Ahmadikia K, Asghari S, Mashaei S, Hassanali F. Comparison of clinical, para‐clinical and laboratory findings in survived and deceased patients with COVID‐19: diagnostic role of inflammatory indications in determining the severity of illness. BMC Infect Dis. 2020;20(1):869. 10.1186/s12879-020-05540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Asghar MS, Khan NA, Haider Kazmi SJ, et al. Hematological parameters predicting severity and mortality in COVID‐19 patients of Pakistan: a retrospective comparative analysis. J Community Hosp Intern Med Perspect. 2020;10(6):514‐520. 10.1080/20009666.2020.1816276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang R, He M, Yin W, et al. The Prognostic Nutritional Index is associated with mortality of COVID‐19 patients in Wuhan, China. J Clin Lab Anal. 2020;34:e23566. 10.1002/jcla.23566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang X, Li X, Shang Y, et al. Ratios of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte predict all‐cause mortality in inpatients with coronavirus disease 2019 (COVID‐19): a retrospective cohort study in a single medical centre. Epidemiol Infect. 2020;148:e211. 10.1017/S0950268820002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allahverdiyev S, Quisi A, Harbalioglu H, et al. The neutrophil to lymphocyte ratio and in‐hospital all‐cause mortality in patients with COVID‐19. Eur J Ther. 2020;26(3):251‐256. [Google Scholar]
- 29. Güneysu F, Guner NG, Erdem AF, Durmus E, Durgun Y, Yurumez Y. Can COVID‐19 mortality be predicted in the emergency room? J Coll Physicians Surg Pak. 2020;30(9):928‐932. 10.29271/jcpsp.2020.09.928 [DOI] [PubMed] [Google Scholar]
- 30. Jimeno S, Ventura PS, Castellano JM, et al. Prognostic implications of neutrophil‐lymphocyte ratio in COVID‐19. Eur J Clin Invest. 2021;51(1):e13404. 10.1111/eci.13404 [DOI] [PubMed] [Google Scholar]
- 31. Kalabin A, Mani VRK, Valdivieso SC, Donaldson B. Role of neutrophil‐to‐lymphocyte, lymphocyte‐to‐monocyte and platelet‐to‐lymphocyte ratios as predictors of disease severity in COVID‐19 patients. Infez Med. 2021;29(1):46‐53. [PubMed] [Google Scholar]
- 32. Archana B, Shyamsunder S, Das R. Validity of markers and indexes of systemic inflammation in predicting mortality in COVID 19 infection: a hospital based cross sectional study. medRxiv. 2021. 10.1101/2021.03.30.21254635 [DOI] [Google Scholar]
- 33. Nasir M, Perveen RA, Nazneen R, Zahan T, Ahmad SN, Chowdhury AS. Paradox of Predictors in Critically ill COVID‐19 Patients: Outcome of a COVID‐dedicated Intensive Care Unit. medRxiv. 2021. 10.1101/2021.04.23.21256009 [DOI] [Google Scholar]
- 34. Asghar MS, Haider Kazmi SJ, Ahmed Khan N, et al. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID‐19 patients in Pakistan: a single‐center retrospective study in a tertiary care hospital of Karachi. Cureus. 2020;12(6):e8712. 10.7759/cureus.8712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bastug A, Bodur H, Erdogan S, et al. Clinical and laboratory features of COVID‐19: predictors of severe prognosis. Int Immunopharmacol. 2020;88:106950. 10.1016/j.intimp.2020.106950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol. 2020;84:106504. 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zeng F, Deng G, Cui Y, et al. A predictive model for the severity of COVID‐19 in elderly patients. Aging. 2020;12(21):20982‐20996. 10.18632/aging.103980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xue G, Gan X, Wu Z, et al. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID‐19. Int Immunopharmacol. 2020;89(Pt A):107065. 10.1016/j.intimp.2020.107065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong J, Ou J, Qiu X, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID‐19): a multicenter study using the risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71(15):833‐840. 10.1093/cid/ciaa443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H, Xing Y, Yao X, et al. Retrospective study of clinical features of COVID‐19 in inpatients and their association with disease severity. Med Sci Monit. 2020;26:e927674. 10.12659/MSM.927674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kong J, Wang T, Di Z, et al. Analysis of hematological indexes of COVID‐19 patients from fever clinics in Suzhou, China. Int J Lab Hematol. 2020;42(5):e204‐e206. 10.1111/ijlh.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kazancioglu S, Bastug A, Ozbay BO, Kemirtlek N, Bodur H. The role of haematological parameters in patients with COVID‐19 and influenza virus infection. Epidemiol Infect. 2020;148:e272. 10.1017/S095026882000271X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin S, Mao W, Zou Q, Lu S, Zheng S. Associations between hematological parameters and disease severity in patients with SARS‐CoV‐2 infection. J Clin Lab Anal. 2021;35(1):e23604. 10.1002/jcla.23604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang S, Liu M, Li X, Shang Z, Zhang T, Lu H. Significance of neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio for predicting clinical outcomes in COVID‐19. medRxiv. 2020. 10.1101/2020.05.04.20090431 [DOI] [Google Scholar]
- 45. Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID‐19 in Wenzhou, China. Clin Chim Acta. 2020;507:174‐180. 10.1016/j.cca.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang W, Zhao Z, Liu X, et al. Clinical features and potential risk factors for discerning the critical cases and predicting the outcome of patients with COVID‐19. J Clin Lab Anal. 2020;34(10):e23547. 10.1002/jcla.23547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao Y, Yu C, Ni W, Shen H, Qiu M, Zhao Y. Peripheral blood inflammatory markers in predicting prognosis in patients with COVID‐19. Some differences with influenza A. J Clin Lab Anal. 2021;35(1):e23657. 10.1002/jcla.23657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou Y, Guo S, He Y, et al. COVID‐19 is distinct from SARS‐CoV‐2‐negative community‐acquired pneumonia. Front Cell Infect Microbiol. 2020;10:322. 10.3389/fcimb.2020.00322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Waris A, Din M, Khalid A, et al. Evaluation of hematological parameters as an indicator of disease severity in Covid‐19 patients: Pakistan's experience. J Clin Lab Anal. 2021;35:e23809. 10.1002/jcla.23809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Erdogan A, Can FE, Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR and LCR ratio in COVID‐19 patients. J Med Virol. 2021. 93(9):5555–5559. 10.1002/jmv.27097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ok F, Erdogan O, Durmus E, Carkci S, Canik A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID‐19 patients. J Med Virol. 2021;93(2):786‐793. 10.1002/jmv.26300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu Z, Cai T, Fan L, et al. Clinical value of immune‐inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332‐339. 10.1016/j.ijid.2020.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seyit M, Avci E, Nar R, et al. Neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio and platelet to lymphocyte ratio to predict the severity of COVID‐19. Am J Emerg Med. 2021;40:110‐114. 10.1016/j.ajem.2020.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu L, Shao Z, Yu H, Zhang W, Wang H, Mei Z. Is the platelet to lymphocyte ratio a promising biomarker to distinguish acute appendicitis? Evidence from a systematic review with meta‐analysis. PLOS One. 2020;15(5):e0233470. 10.1371/journal.pone.0233470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Erre GL, Paliogiannis P, Castagna F, et al. Meta‐analysis of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratio in rheumatoid arthritis. Eur J Clin Invest. 2019;49(1):e13037. 10.1111/eci.13037 [DOI] [PubMed] [Google Scholar]
- 56. Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in acute pulmonary embolism: a systematic review and meta‐analysis. Int Angiol. 2018;37(1):4‐11. 10.23736/S0392-9590.17.03848-2 [DOI] [PubMed] [Google Scholar]
- 57. El‐Gazzar AG, Kamel MH, Elbahnasy OKM, El‐Naggar ME. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med. 2020;14(1):111‐116. 10.1080/17476348.2019.1675517 [DOI] [PubMed] [Google Scholar]
- 58. Kemal Y, Yucel I, Ekiz K, et al. Elevated serum neutrophil to lymphocyte and platelet to lymphocyte ratios could be useful in lung cancer diagnosis. Asian Pac J Cancer Prev. 2014;15:2651‐2654. [DOI] [PubMed] [Google Scholar]
- 59. Li B, Zhou P, Liu Y, et al. Platelet‐to‐lymphocyte ratio in advanced cancer: review and meta‐analysis. Clin Chim Acta. 2018;483:48‐56. 10.1016/j.cca.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 60. Tian C, Song W, Tian X, Sun Y. Prognostic significance of platelet‐to‐lymphocyte ratio in patients with ovarian cancer: a meta‐analysis. Eur J Clin Invest. 2018;48(5):e12917. 10.1111/eci.12917 [DOI] [PubMed] [Google Scholar]
- 61. Chen Y, Feng F, Li M, et al. Relationship between platelet/lymphocyte ratio and prognosis of patients with septic acute kidney injury: a pilot study. J Chin Med Assoc. 2020;83(11):1004‐1007. 10.1097/JCMA.0000000000000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simadibrata DM, Pandhita BAW, Ananta ME, Tango T. Platelet‐to‐lymphocyte ratio, a novel biomarker to predict the severity of COVID‐19 patients: a systematic review and meta‐analysis. J Intensive Care Soc. 2020;5:371. 10.1177/1751143720969587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58:1021‐1028. [DOI] [PubMed] [Google Scholar]
- 64. Huang I, Pranata R. Lymphopenia in severe coronavirus disease‐2019 (COVID‐19): systematic review and Meta‐analysis. J Intensive Care. 2020;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019(COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hamilos M, Petousis S, Parthenakis F. Interaction between platelets and endothelium: from pathophysiology to new therapeutic options. Cardiovasc Diagn Ther. 2018;8(5):568‐580. 10.21037/cdt.2018.07.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759‐2767. 10.1182/blood-2013-11-462432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eickmann M, Gravemann U, Handke W. Inactivation of three emerging viruses severe acute respiratory syndrome coronavirus, Crimean‐Congo haemorrhagic fever virus and Nipah virus—in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox Sang. 2020;115(3):146‐151. 10.1111/vox.12888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pilaczyńska‐Cemel M, Gołda R, Dąbrowska A, Przybylski G. Analysis of the level of selected parameters of inflammation,circulating immune complexes,and related indicators (neutrophil/lymphocyte, platelet/lymphocyte, CRP/CIC) in patients with obstructive diseases. Cent Eur J Immunol. 2019;44(3):292‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shinya K, Gao Y, Cilloniz C, et al. Integrated clinical, pathologic, virologic, and transcriptomic analysis of H5N1 influenza virus‐induced viral pneumonia in the rhesus macaque. J Virol. 2012;86(11):6055‐6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Qu R, Ling Y, Zhang YH, et al. Platelet‐to‐lymphocyte ratio is associated with prognosis in patients with coronavirus disease‐19. J Med Virol. 2020;92(9):1533‐1541. 10.1002/jmv.25767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li T, Qiu Z, Zhang L, et al. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189(4):648‐651. 10.1086/381535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS‐CoV‐2 infection‐a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9(1):727‐732. 10.1080/22221751.2020.1746199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang M. Cell Pyroptosis, a Potential Pathogenic Mechanism of 2019‐nCoV Infection (January 29, 2020). Available at SSRN: https://ssrn.com/abstract=3527420 or 10.2139/ssrn.3527420 [DOI]
- 76. Mousavi SA, Rad S, Rostami T, et al. Hematologic predictors of mortality in hospitalized patients with COVID‐19: a comparative study. Hematology. 2020;25(1):383‐388. 10.1080/16078454.2020.1833435 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


