Abstract
Due to current advances and growing experience in the management of coronavirus Disease 2019 (COVID‐19), the outcome of COVID‐19 patients with severe/critical illness would be expected to be better in the second wave compared with the first wave. As our hospitalization criteria changed in the second wave, we aimed to investigate whether a favorable outcome occurred in hospitalized COVID‐19 patients with only severe/critical illness. Among 642 laboratory‐confirmed hospitalized COVID‐19 patients in the first wave and 1121 in the second wave, those who met World Health Organization (WHO) definitions for severe or critical illness on admission or during follow‐up were surveyed. Data on demographics, comorbidities, C‐reactive protein (CRP) levels on admission, and outcomes were obtained from an electronic hospital database. Univariate analysis was performed to compare the characteristics of patients in the first and second waves. There were 228 (35.5%) patients with severe/critical illness in the first wave and 681 (60.7%) in the second wave. Both groups were similar in terms of age, gender, and comorbidities, other than chronic kidney disease. Median serum CRP levels were significantly higher in patients in the second wave compared with those in the first wave [109 mg/L (interquartile range [IQR]: 65–157) vs. 87 mg/L (IQR: 39–140); p < 0.001]. However, intensive care unit admission and mortality rates were similar among the waves. Even though a lower mortality rate in the second wave has been reported in previous studies, including all hospitalized COVID‐19 patients, we found similar demographics and outcomes among hospitalized COVID‐19 patients with severe/critical illness in the first and second wave.
Keywords: COVID‐19, demographic characteristics, mortality, the first versus second wave, Turkey
Highlights
Even though a lower mortality rate in the second wave has been reported in previous studies, including all hospitalized COVID‐19 patients, we found similar AQ4demographics and outcomes among hospitalized COVID‐19 patients with severe/critical illness in the first and second wave.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) was first identified in Wuhan, China, over a year ago and still remains a major health problem worldwide. According to the WHO data, over four million people have died due to COVID‐19. Many countries including Turkey have experienced the second wave of the pandemic. 1 In Turkey, the first case of COVID‐19 was announced on March 11, 2020, with the first related death occurring on March 17. The first wave of the pandemic reached a peak between April and May 2020, then the number of daily cases declined gradually and stabilized during the summer. The second wave of the pandemic occurred between October and November 2020. 1 , 2
Istanbul is the most populated city in Turkey with a population of nearly 15 million and is also one of the top cities of Turkey, with the highest number of COVID‐19 cases. Our hospital has been caring for COVID‐19 patients since the beginning of the pandemic and is one of the 14 tertiary education and research hospitals on the European side in Istanbul. We have served 113 036 outpatients and 5043 hospitalized COVID‐19 patients during the first 10 months of the pandemic. In our hospital, we have used immunsuppressive therapy including corticosteroids and anti‐interleukin agents, for COVID‐19 patients with a severe illness at the beginning of the first wave, however; we used these treatments more often and started them earlier in the second wave. In addition, we were more experienced in dealing with COVID‐19 and its complications in the second wave. Aside from all this, new variants of coronavirus would cause differences in the clinical characteristics and outcomes of the affected patients between the two waves. A previous study from Spain included all hospitalized COVID‐19 patients and reported a lower mortality rate in the second wave compared with the first wave. 3 However, this finding may be because of demographic differences of hospitalized patients between the first and second waves. Hospitalization of less severe cases may cause a lower mortality rate in the second wave. For instance, we had to hospitalize all suspected or confirmed COVID‐19 patients who were >50 years old or had any comorbidities in the first wave. However, we hospitalized only patients with severe or critical illness in the second wave. Even though our hospitalization criteria changed in the second wave, there were no differences in the definitions of disease severity. Therefore, we aimed to compare the demographics and outcomes of hospitalized COVID‐19 patients with only severe or critical illness between the first and second wave and find out whether the mortality rate of COVID‐19 patients with severe/critical illness also differs between the waves.
2. METHODS
2.1. Study design and participants
This single‐center retrospective observational study was approved by the Ethics Committee of Istanbul Gaziosmanpasa Training and Research Hospital (ethics approval number 203/12.2020). A total of 1649 adult patients were hospitalized in the first wave (between April 1 and May 31), and a total of 1790 adult patients were hospitalized in the second wave (between October 1 and November 30). Six hundred and forty‐two of these patients in the first wave and 1121 in the second wave had a positive nasopharyngeal real‐time polymerase chain reaction result for SARS‐CoV‐2. A total of 909 patients [228 (35.5%) in the first wave and 681 (60.7%) in the second wave] who met WHO definitions for severe or critical illness on admission or during follow‐up were included. 4 Demographics, comorbidities, C‐reactive protein (CRP) levels on admission, duration of hospitalization, and outcomes (intensive care unit [ICU] admission, mechanical ventilation, and death) were obtained from hospital‐based electronic health records. All patients were followed until death in the hospital or 30 days after discharge.
2.2. Definitions
The severity of COVID‐19 pneumonia was defined according to the WHO criteria. 4 Severe illness was defined as individuals having the following: (a) partial pressure of arterial oxygen (mmHg)/fraction of inspired oxygen (%) ≤300 mmHg; (b) respiratory rate > 30 breaths/min; or (c) oxygen saturation at rest <90% on room air. Critical illness was defined as individuals having: (a) acute respiratory distress syndrome; (b) sepsis; or (c) septic shock.
2.3. Treatment
During the COVID‐19 outbreak in Turkey, all outpatients and hospitalized patients with a suspicion of COVID‐19 or those with confirmed COVID‐19 received medical treatment free in public and private hospitals.
We followed the treatment guidelines by the Turkish Ministry of Health which have been updated several times during the pandemic. 5 In the first wave, our treatment regimen for hospitalized COVID‐19 patients included hydroxychloroquine (200 mg every 12 h, orally, 5–10 days), azithromycin (500 mg every 24 h, orally, for 5 days), favipiravir (first day 1600 mg, and then 600 mg every 12 h, orally, for 5–7 days) and lopinavir‐ritonavir (500 mg twice daily, orally, for 10–14 days). Patients with non‐severe illness received a combination of hydroxychloroquine and azithromycin. Patients with severe illness were treated with favipiravir or lopinavir‐ritonavir. Oseltamivir (75 mg every 12 h, orally, for 5–10 days) was also initiated during the influenza season. In addition, for patients with severe illness who had progressive disease, we used a combination of intravenous methylprednisolone (40–80 mg every 24 h, for 5–10 days) and tocilizumab (8 mg/kg single dose or in two divided doses). Progressive disease was defined as an increase in oxygen requirement or absence of clinical improvement despite favipiravir or lopinavir‐ritonavir treatment. All hospitalized patients were treated with a prophylactic dose of enoxaparin.
In the second wave, our standard treatment regimen for hospitalized COVID‐19 patients with severe illness included favipiravir (first day 1600 mg, and then 600 mg every 12 h, orally, for 10 days), methylprednisolone (80–1000 mg every 24 h, for 10–16 days), and therapeutic dose of enoxaparin. Tocilizumab (8 mg/kg single dose) and anakinra (400–1200 mg/day) were also given to severe patients who did not respond after 3 days of corticosteroid therapy.
2.4. Statistics analysis
The data were analyzed using IBM SPSS Statistics 25 program and checked for normal distribution with the Shapiro–Wilk test. Categorical variables were presented as counts and percentages. Continuous variables were presented as mean and standard deviation (SD) and compared by independent sample t‐test if normally distributed, otherwise, the median and interquartile range (IQR) were used to present the data and the Mann–Whitney U test was used for comparison. A χ 2 test was used to compare categorical data. p Values <0.05 indicate that the difference was statistically significant.
3. RESULTS
3.1. Comparison of characteristics of patients with severe/critical illness in the first versus second wave
Among 642 laboratory‐confirmed hospitalized COVID‐19 patients in the first wave and 1121 in the second wave, we included 228 patients (35.5%) in the first wave and 681 patients (60.7%) in the second wave who met the WHO definition of disease for analyses. Table 1 shows the characteristics of patients among the waves. The median age of patients was similar in the first and second wave [64.5 years (IQR: 54–74.7) vs. 65 years (IQR: 54–73), p = 0.65, respectively]. Both groups were also similar regarding gender [129 male patients (56.6%) in the first wave vs. 391 male patients (57.4%) in the second wave, p = 0.87]. One hundred and fifty‐five patients (68%) in the first wave and 486 patients (71.4%) in the second wave had at least one comorbidity (p = 0.35). The most common comorbidity in both waves was hypertension (43.4% in the first wave vs. 46.5% in the second wave), followed by diabetes mellitus (31.1% in the first wave vs. 34.2% in the second wave), and ischemic heart disease (11.9% in the first wave vs. 14.1% in the second wave). The frequency of chronic renal disease was higher in the second wave than in the first wave (5.3% vs. 7%, p = 0.03, respectively). There were no statistically significant differences regarding other comorbidities between the waves. Median CRP levels on admission were found to be significantly higher in the second wave compared with the first wave [109 mg/L (65–157) vs. 87 mg/L (39–140), p < 0.001, respectively]. When patients were stratified by sex, there were no differences regarding age, gender, comorbidities, and CRP levels between the waves (data not shown).
Table 1.
Baseline characteristics of COVID‐19 patients with severe/critical illness in the first versus second wave
| Variable | First wave (n = 228) | Second wave (n = 681) | p Value |
|---|---|---|---|
| Age (years) | 64.5 (54–74.7) | 65(54–73) | 0.65 |
| Male, n (%) | 129 (56.6) | 391 (57.4) | 0.87 |
| Comorbidities, n (%) | |||
| Any comorbidity | 155 (68) | 486 (71.4) | 0.35 |
| Hypertension | 99 (43.4) | 317 (46.5) | 0.44 |
| Diabetes mellitus | 71 (31.1) | 233 (34.2) | 0.41 |
| Asthma | 18 (7.9) | 55 (8.1) | NS |
| COPD | 17 (7.5) | 49 (7.2) | 0.22 |
| Ischemic heart disease | 27 (11.9) | 96 (14.1) | 0.43 |
| Hyperlipidemia | 10 (4.4) | 42 (6.2) | 0.41 |
| Chronic renal disease | 12 (5.3) | 48(7) | 0.03 |
| Malignity | 5 (2.2) | 19 (2.8) | 0.81 |
| Congestive heart failure | 15 (6.6) | 52 (7.6) | 0.66 |
| Rheumatologic disease | 3 (1.3) | 21 (3.1) | 0.23 |
| Neurologic disease | 15 (6.6) | 42 (6.2) | 0.87 |
| C‐reactive protein (mg/L)a | 87 (39–140) | 109 (65–157) | <0.001 |
| Length of hospitalization (days) | 11 (7.2–17) | 10 (7–15) | 0.04 |
| Time from hospitalization to ICU admission (days) | 5 (3–7.7) | 3 (0‐6) | 0.01 |
| Admission to ICU, n (%) | 64 (28.1) | 191 (28) | NS |
| IMV in ICU, n (%) | 48 (21.1) | 137 (20.1) | 0.76 |
| Mortality, n (%) | 46 (20.2) | 149 (21.9) | 0.64 |
Note: Those with significant p values are indicated in bold (p values < 0.05 indicated that the difference was statistically significant). All continuous variables were reported as median and interquartile range.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IMV, invasive mechanical ventilation; NS, not significant (p = 1.00); SD, standard deviation.
CRP normal range is between 0 and 5 mg/L.
3.1.1. Comparison of outcomes of patients with severe/critical illness in the first versus second wave
The outcomes of patients among the waves are depicted in Table 1. The median length of hospitalization was longer in the first wave than in the second wave [11 days (IQR: 7.2–17) vs. 10 days (IQR: 7–15), p = 0.04]. The proportion of the patients admitted to ICU was similar in both waves [(28.1%) vs. (28%); p = NS]. However, the median time from hospitalization to ICU admission was shorter in the second wave compared with the first wave [3 days (IQR: 0–6) vs. 5 days (IQR: 3–7.7), p = 0.01, respectively]. The frequencies of patients who required invasive mechanical ventilation were 21.1% in the first wave and 20.1% in the second wave (p = 0.76). A total of 195 patients had died during two waves. Forty‐six patients were in the first wave and 149 were in the second wave. The mortality rate did not differ in both waves (first vs. second wave: 20.2% vs. 21.9%, p = 0.64). When patients were stratified by sex, there were no differences regarding outcomes between the waves (data not shown).
3.1.2. Comparison of characteristics and outcomes of deceased patients in the first versus second wave
Table 2 shows the characteristics and outcomes of deceased patients among the waves. In the second wave, there were more male and younger patients among the deceased patients. However, the differences were not significant. There was no difference in terms of having any comorbidity between the waves [first wave vs. second wave: 36 patients (78.3%) vs. 123 patients (82.6%), p = 0.51]. The most common comorbidity of the deceased patients in both waves was hypertension (58.7% in the first wave vs. 56.4% in the second wave), followed by diabetes mellitus (45.7% in the first wave vs. 34.9% in the second wave), and ischemic heart disease (17.4% in the first wave vs. 19.5% in the second wave). The median level of CRP was found to be higher in the first wave than in the second wave [104 mg/L (IQR: 63‐156) vs. 134 mg/L (IQR: 82‐192), p = .04, respectively]. The median length of hospitalization and the median time from hospitalization to ICU admission were similar in the first wave with the second wave [11.5 days (IQR: 7–23) vs. 13 days (IQR: 7–19); 5 days (IQR: 2–7) vs. 4 days (IQR: 1–6.7, respectively] (Table 2). When we stratified our patients into age groups by decade, there were no differences between the waves regarding mortality (Figure 1). When patients were stratified by sex, there were no differences regarding characteristics and outcomes between the waves (data not shown).
Table 2.
Baseline characteristics of deceased patients in the first versus second wave
| Variable | First wave (n = 46) | Second wave (n = 149) | p Value |
|---|---|---|---|
| Age (years) | 70.6 ± 13.2 | 68.4 ± 12.2 | 0.29 |
| Male, n (%) | 24 (52.2) | 90 (60.4) | 0.39 |
| Comorbidities, n (%) | |||
| Any comorbidity | 36 (78.3) | 123 (82.6) | 0.51 |
| Diabetes mellitus | 21 (45.7) | 52 (34.9) | 0.22 |
| Hypertension | 27 (58.7) | 84 (56.4) | 0.86 |
| Chronic renal failure | 3 (6.7) | 15 (10.1) | 0.76 |
| Congestive heart failure | 5 (10.9) | 14 (9.4) | 0.77 |
| Ischemic heart disease | 8 (17.4) | 29 (19.5) | 0.83 |
| Hyperlipidemia | 4 (8.7) | 10 (6.7) | 0.74 |
| Asthma | 3 (6.5) | 10 (6.7) | NS |
| COPD | 1 (2.2) | 17 (11.4) | 0.07 |
| Malignity | 2 (4.3) | 10 (6.7) | 0.73 |
| Rheumatologic disease | 0 (0) | 6 (4) | 0.33 |
| Neurologic disease | 5 (10.9) | 15 (10.1) | NS |
| C‐reactive protein (mg/L)a | 104 (63–156) | 134 (82–192) | 0.04 |
| Length of hospitalization (days) | 11.5 (7–23) | 13 (7–19) | 0.97 |
| Time from hospitalization to ICU admission (days) | 5 (2–7) | 4 (1‐6.7) | 0.2 |
| Admission to ICU, n (%) | 41 (89.1) | 140 (94) | 0.32 |
| IMV in ICU, n (%) | 39 (97.5) | 118 (87.4) | 0.18 |
Note: Those with significant p values are indicated in bold (p values < 0.05 indicated that the difference was statistically significant). All continuous variables other than age were reported as median and interquartile range.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IMV, invasive mechanical ventilation; NS, not significant (p = 1.00); SD, standard deviation.
CRP normal range is between 0 and 5 mg/L.
Figure 1.
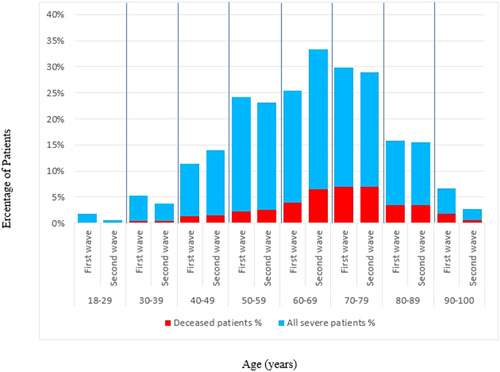
Distribution by age intervals of all patients with severe/critical illness and deceased patients in the first and second waves
4. DISCUSSION
In this study, we included only hospitalized COVID‐19 patients with severe/critical illness, and we compared their demographic characteristics, comorbidities, CRP levels on admission, and outcomes between the first and second wave. Both waves were similar in terms of age, gender, and comorbidities other than chronic kidney disease. CRP levels on admission were significantly higher in the second wave than in the first wave. Unlike previous studies that reported lower mortality in the second wave, we found a similar mortality rate between the waves. This finding contradicted previous studies, in which patients with COVID‐19 pneumonia at all clinical stages were analyzed and revealed a favorable outcome in the second wave. 3 , 6 , 7
Several studies have been reported that male patients experience higher disease severity. 8 , 9 , 10 , 11 In addition, patients with a fatal course were more likely to be male. 11 , 12 According to our national data, 6062 (62%) of 9799 cases with COVID‐19 who died from COVID‐19 from the beginning of the pandemic (March 11, 2020) to October 25, 2020 were men. 2 In our study, the proportion of male individuals among the patients with severe/critical illness was similar in the two waves however, there were more male patients among the deceased patients in the second wave than in the first wave. To explain this finding, patients were stratified by sex and we found no differences regarding age, comorbidities, and CRP levels between the waves. A comparative study from Spain reported that gender was associated with mortality in the second wave but not in the first wave. 3 Although the authors did not provide the type of gender, that study and ours showed a gender difference in the mortality of COVID‐19 patients in the second wave.
The aforementioned prospective study comparing characteristics of two waves in all hospitalized patients in Spain reported that patients who were hospitalized in the second wave were younger, and had lower mortality rates. 3 According to another study conducted in 53 different countries, the mortality rate in the second wave of the pandemic has been reported to be decreased sharply. 6 Finally, the last comparative study of two waves again observed lower mortality rates in the second wave and the patients in the second wave were younger with fewer comorbidities as stated in these articles. 7 As one reason, the increased testing capacities over time were allowing physicians to diagnose less severe patients, and this seems to be one of the leading reasons for the reduced mortality rate in the second wave. The reason we included only patients with severe/critical illness in this study was to evaluate whether favorable outcomes also existed in these populations. The other reason we did not include all hospitalized patients was that hospitalization criteria have been revised in the second wave. We hospitalized all patients over 50 years old or those with any comorbidities irrespective of their severity in the first wave. However, in the second wave, we hospitalized only patients with a severe or critical illness. However, despite the change in hospitalization criteria, definitions of severe and critical illness remained unchanged in the second wave. When we compared the patients with similar disease severity among the waves, the mortality rate did not appear to decrease in the second wave despite the advances in treatment strategies and growing experience in the management of COVID‐19. However, it still needs to be clarified whether new mutant/variant strains led to an increase in mortality rate in the second wave. Unfortunately, we could not provide data on the circulating virus strains since the variant analysis was not routinely performed in Turkey during the study period.
CRP, which is one of the most important biomarkers showing the severity of COVID‐19, is an independent discriminator of severe illness. 13 , 14 In our study, we found that median CRP levels on admission were significantly higher in the second wave compared with the first wave. This finding showed that patients with severe/critical illness who were hospitalized in the second wave had more severe disease compared with the first wave. According to the COVID‐19 Guide published by The Turkish Ministry of Health, patients had to be hospitalized to initiate favipiravir treatment and we had to hospitalize patients with comorbidities or those with >50 years of age regardless of the disease severity in the first wave. Non‐severe patients were hospitalized for at least 10 days to see if the disease has worsened and discharge criteria were more strict in the first wave than in the second wave. In the second wave, our national guideline had changed and we were able to administer favipiravir treatment to outpatients with mild to moderate pneumonia, and hospitalized patients who worsened despite outpatient management. 8 In other words, we did not hospitalize non‐severe patients and prescribed favipiravir to all outpatients in the second wave. This could explain why median CRP levels were higher in patients in the second wave than in the first wave and the median length of hospitalization was longer in the first wave than in the second wave because we did not hospitalize non‐severe patients in the first wave and we started to hospitalize patients with severe disease at admission or outpatients who progressed under favipiravir treatment in the second wave. However, although we hospitalized more severe patients in the second wave, the mortality rate, the proportions of patients admitted to the ICU, and those who required invasive mechanical ventilation did not increase in the second wave, which may be explained by the fact that we used more intensive treatment regimens including higher doses of corticosteroids, tocilizumab, anakinra and therapeutic doses of enoxaparin. The median time from hospitalization to ICU admission was shortened in the second wave, which can also be due to the fact that we hospitalized more severe patients in the second wave. Another explanation may be that we had more sufficient resources in the second wave and patients requiring intensive care had an opportunity to be admitted to ICU.
5. LIMITATIONS
This study has several limitations. First, our study was a single‐center and retrospective study. Second, prognostic factors related to the severity of the disease, including the extent of radiological involvement and laboratory parameters such as D‐dimer were not evaluated. Third, we did not collect data on secondary infections. Early initiation and more frequent use of corticosteroid therapy could cause more secondary infections, which could lead to increased mortality in the second wave. Fourth, data on causes of death, such as venous thromboembolism, which affects the course of the disease and shows seasonal characteristics were not collected. Finally, previous studies from Turkey have reported mortality rates in the first wave varying from 4.2% to 75.8%. 15 , 16 , 17 , 18 , 19 This variation was possibly driven by differences in study settings and patient population characteristics. However, we are not aware of any studies from Turkey that reported a mortality rate of the second wave or compared mortality rates between the first and second waves. This and the lack of detailed information provided by national authorities were the reasons we could not compare our results with previous findings from Turkey.
6. CONCLUSIONS
Although the previous studies which include patients at all clinical stages have observed a decrease in the mortality rate in the second wave compared with the first wave, demographic characteristics and outcomes of hospitalized COVID‐19 patients with severe or critical illness were similar in the first and second waves.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki on Ethical Principles and was approved by the ethics committee of Gaziosmanpasa Research and Training Hospital, University of Health Sciences, Istanbul, Turkey (approval number: 203/12.2020).
AUTHOR CONTRIBUTIONS
Elif Sargin Altunok was responsible for the organization and coordination of the trial. All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by Elif Sargin Altunok, Celal Satici, Sinem Nihal Esatoglu, and Veysel Dinc. The first draft of the manuscript was written by Elif Sargin Altunok and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We thank Tansu Altunok for his support in creating the figure. In addition, our sincere thanks to all healthcare professionals for their brave efforts in COVID‐19 treatment, prevention, and control.
Sargin Altunok E, Satici C, Dinc V, et al. Comparison of demographic and clinical characteristics of hospitalized COVID‐19 patients with severe/critical illness in the first wave versus the second wave. J Med Virol. 2021;94:291‐297. 10.1002/jmv.27319
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19) dashboard. https://covid19.who.int. Accessed August 12, 2021.
- 2. The Ministry of Health of the Republic of Turkey . COVID‐19 status report. https://covid19.saglik.gov.tr/TR-68443/covid-19-durum-raporu.html. Accessed August 12, 2021.
- 3. Iftimie S, López‐Azcona AF & Vallverdú I et al. First and second waves of coronavirus disease‐19: a comparative study in hospitalized patients in Reus, Spain. PLoS One. 2021;16(3):e0248029. 10.1101/2020.12.10.20246959. Accessed March 31, 2021. [DOI] [PMC free article] [PubMed]
- 4. World Health Organization . The severity of COVID‐19 pneumonia was defined according to the clinical management of COVID‐19: interim guidance. WHO. https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed August 12, 2021.
- 5. The Ministry of Health of the Republic of Turkey . COVID‐19 guide. https://covid19.saglik.gov.tr/TR-66301/covid-19-rehberi.html. Accessed August 12, 2021.
- 6. Fan G, Yang Z, Lin Q, Zhao S, Yang L, He D. Decreased case fatality rate of COVID‐19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2020;68:213‐215. 10.1111/tbed.13819 [DOI] [PubMed] [Google Scholar]
- 7. Saito S, Asai Y, Matsunaga N, et al. First and second COVID‐19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2020;S0163‐4453(20):30693‐30699. 10.1016/j.jinf.2020.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin JM, Bai P, He W, et al. Gender differences in patients with COVID‐19: focus on severity and mortality. Front Public Health. 2020;29(8):152. 10.3389/fpubh.2020.00152. Accessed April 29, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng Y, Wu P, Lu W, et al. Sex‐specific clinical characteristics and prognosis of coronavirus disease‐19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16:e1008520. 10.1371/journal.ppat.1008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vahidy FS, Pan AP, Ahnstedt H, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: crosssectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1):e0245556. 10.1371/journal.pone.0245556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xie J, Tong Z, Guan X, Du B, Qiu H. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open. 2020;3:e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sharma G, Volgman AS, Michos ED. Sex differences in mortality from COVID‐19 pandemic: are men vulnerable and women protected? JACC Case Rep. 2020;2(9):1407‐1410. 10.1016/j.jaccas.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z. Plasma CRP level is positively associated with the severity of COVID‐19. Ann Clin Microbiol Antimicrob. 2020;19:18. 10.1186/s12941-020-00362-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahnach M, Zbiri S, Nejjari S, Ousti F, Elkettani C. C‐reactive protein as an early predictor of COVID‐19 severity. J Med Biochem. 2020;39(4):500‐507. 10.5937/jomb0-27554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esme M, Koca M, Dikmeer A, et al. Older adults with coronavirus disease 2019; a nationwide study in Turkey. J Gerontol A Biol Sci Med Sci. 2021;76(3):e68‐e75. 10.1093/gerona/glaa219.glaa219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kocayiğit H, Özmen Süner K, Tomak Y, et al. Characteristics and outcomes of critically ill patients with covid‐19 in Sakarya, Turkey: a single centre cohort study. Turk J Med Sci. 2021;51(2):440‐447. 10.3906/sag-2005-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aksel G, Islam MM, Algın A, et al. Early predictors of mortality for moderate to severely ill patients with Covid‐19. Am J Emerg Med. 2020;S0735‐6757(20):30770‐30771. 10.1016/j.ajem.2020.08.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ucan ES, Ozgen Alpaydin A, Ozuygur SS, et al. Pneumonia severity indices predict prognosis in coronavirus disease‐2019. Respir Med Res. 2021;79:100826. 10.1016/j.resmer.2021.100826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ucan A, Cerci P, Efe S, et al. Benefits of treatment with favipiravir in hospitalized patients for COVID‐19: a retrospective observational case‐control study. Virol J. 2021;18(1):102. 10.1186/s12985-021-01577-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ak R, Kurt E, Bahadirli S. The comparison of two risk prediction models specific for COVID‐19: the Brescia‐COVID Respiratory Severity Scale versus the Quick COVID‐19 Severity Index. Disaster Med Public Health Prep. 2021:1‐17. 10.1017/dmp.2021.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


