Abstract
Many aspects of the humoral immune response to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), such as its role in protection after natural infection, are still unclear. We evaluated IgA and IgG response to spike subunits 1 and 2 (S1 and S2) and Nucleocapsid proteins of SARS‐COV‐2 in serum samples of 109 volunteers with viral RNA detected or seroconversion with different clinical evolution (asymptomatic, mild, moderate, and severe coronavirus disease 2019), using the ViraChip® Test Kit. We observed that the quantification of antibodies to all antigens had a positive correlation to disease severity, which was strongly associated with the presence of comorbidities. Seroreversion was not uncommon even during the short (median of 77 days) observation, occurring in 15% of mild‐asymptomatic cases at a median of 55 days for IgG and 46 days for IgA. The time to reach the maximal antibody response did not differ significantly among recovered and deceased volunteers. Our study illustrated the dynamic of anti‐S1, anti‐N, and anti‐S2 IgA and IgG antibodies, and suggests that high production of IgG and IgA does not guarantee protection to disease severity and that functional responses that have been studied by other groups, such as antibody avidity, need further attention.
Keywords: COVID‐19, nucleocapsid, SARS‐CoV‐2, spike, ViraChip®
Highlights
Symptomatic SARS‐CoV‐2 infection generally elicits strong humoral immune response.
IgA and IgG titers to three viral antigens (S1, S2 and N) correlate to severity of COVID‐19 disease.
Seroreversion is not uncommon and may occur few months after SARS‐CoV‐2 infection.
Elucidation of functional characteristics of antibodies are necessary to better understand disease pathogenesis and may guide vaccine boosting strategies.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) is caused by the novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS‐CoV‐2]). Until August of 2021, there have been 206 958 371 confirmed cases, including 4 357 179 deaths in the world; Brazil accounts for 20 350 142 cases and more than 568 788 deaths reported to the WHO. 1 The impact of this pandemic turns laboratory markers of disease, such as antibody tests, into a potential instrument to help in the monitoring of the epidemic. 2
SARS‐CoV‐2 expresses, among others, three antigens that are highly immunogenic and capable of inducing humoral immune response: S1 and S2 (subunits of spike) and N (nucleocapsid) glycoproteins.³ Spike is a transmembrane protein that binds at angiotensin‐converting enzyme 2 or CD147 receptor expressed on the host cell's surface, through its receptor‐binding protein (RBD), present in the S1 subunit, whilst S2 subunit mediates the fusion between viral and cellular membranes. N protein binds to the RNA and acts on virion assembly. 3 , 4
COVID‐19 presents a wide clinical spectrum that ranges from asymptomatic to mild disease of the upper respiratory tract, or moderate to severe disease with respiratory distress and multiple organ failure requiring intensive care and organ support. 5 , 6 The role of antibody titer and of immunoglobulin classes involved in immune response may be useful to better elucidate the humoral features of COVID‐19, as it might support clinical management and may tailor vaccine development. Our study aimed to evaluate the IgA and IgG response against S1, S2, and N proteins in individuals with confirmed SARS‐CoV‐2 infection (positive viral RNA) according to different clinical presentations (asymptomatic, mild, moderate, and severe COVID‐19).
2. MATERIAL AND METHODS
2.1. Study population and ethics statement
The study was approved by the institutional ethical committees, CAAE: 31924420.8.0000.0059 (Adolfo Lutz Institute) and 35589320.6.3001.0075 (Institute of Infectology Emilio Ribas). Informed written consent was provided for all subjects. One hundred and nine individuals provided 253 serum samples; all of them were collected at the São Paulo State, Brazil, in 2020. The volunteers included health workers from the Institute Adolfo Lutz of São Paulo (IAL/SP), Santo André Regional Center (IAL/SA), and Santo André Infectious Diseases Outpatient Clinic and patients from the Institute of Infectology Emilio Ribas (IIER). The subjects were grouped according to clinical evolution as (i) asymptomatic without symptoms at the day of sample collection; (ii) mild cases who had two or more symptoms (Supplementary material 1) 7 but did not present disease progression; (iii) moderate or; (iv) severe clinical cases, all hospitalized patients, classified using the criteria set by the Brazilian Ministry of Health (Supplementary material 2). 8
2.2. SARS‐CoV‐2 molecular diagnosis
SARS‐CoV‐2 RNA was obtained for quantitative reverse‐transcription polymerase chain reaction (RT‐qPCR) from nasopharyngeal and/or oropharyngeal secretions either by regular swab collection method, 9 or gargle throat wash, 10 and diagnosis was based on Charité protocol. 11 The samples with amplification in one or more of the three viral targets (E, RdRP, and N) with cycle thresholds (CTs) up to 37 were considered positive.
2.3. Serology tests
Specific antibodies to SARS‐CoV‐2 were detected using two different commercial kits: Electrochemiluminescence assay (ECLIA) Elecsys® Anti‐SARS‐CoV‐2 (Roche Diagnostics), which uses N as antigen, and chemiluminescence assay (CLIA) VITROS® Anti‐SARS‐CoV‐2 (Ortho Clinical Diagnostics), which uses S1 as antigen, both detect IgG antibodies. Assays were performed following the manufacturer's instructions and used to select PCR‐negative cases.
The SARS‐CoV‐2 ViraChip® Test Kit is a microarray based on an enzyme‐immunoassay for the detection of IgG or IgA antibodies against SARS‐CoV‐2 antigens in human serum. This test uses purified S1, S2, and N antigens of SARS‐CoV‐2 spotted at defined positions on nitrocellulose membrane fixed at the bottom of each well of a standard plate. 12 The assay was performed following the manufacturer's instructions. Scanning was performed using SensoSpot® Colorimetry MicroArray Analyzer (Sensovation) and analyzed using the ViraChip® Software (ViraMed Biotech). Quantification is measured in ViraChip® units. According to manufacturer criteria, a sample is positive for SARS‐CoV‐2 ViraChip® IgG when one or more spots are more than or equal to 100 ViraChip® units; and for SARS‐CoV‐2 ViraChip® IgA, when two or more spots are more than or equal to 100 ViraChip® units. Readings less than 70 are considered negative and from 70 to 99, inconclusive.
2.4. Statistical analysis
Categorical variables were compared by the Pearson's χ 2 test or Fisher's exact test as appropriate. Continuous variables were presented as range, median, and interquartile range (IQR 25th–75th). Mann–Whitney or Kruskal–Wallis tests were used to compare groups. A two‐sided p value less than or equal to 0.05 was considered significant. Statistical analysis was performed using the software STATA version 13 (StataCorp LP) and GraphPad Prism version 5 (GraphPad Softwares Inc).
3. RESULTS
3.1. Study population and demographics
A total of 109 volunteers were included, 51% female, from 22 to 83 years old (median 51, IQR 42–58). Laboratory confirmation for SARS‐CoV‐2 by RT‐qPCR was positive in 93 (85%) of the subjects, three of them were only referred by the volunteer, and negative in 14. However, all these had anti‐SARS‐CoV‐2 positive serology in ECLI or CLI assays, five of them were asymptomatic and 9 had symptoms. Time of symptoms of RNA negative cases ranged from 15 to 90 days, a median of 56 days of symptoms, IQR 30–65. Two additional cases had no RNA or a serology test positive, but referred symptoms compatible with suspected cases of COVID‐19. To allow comparisons between the individuals, the timing of serological determination was defined as days after RNA positivity (DR), ranging among volunteers with diagnosis confirmed by RT‐qPCR from −6 to 74 days (median 15, IQR 2–34).
Eighty‐eight volunteers (81%) reported at least one symptom (dry cough, fever, dyspnea, body pain, headache, taste and smell disorder, throat pain, fatigue, diarrhea, and pneumonia) (the symptoms list is in Supplementary Material 1). Volunteers were categorized according to disease severity: 29 (27%) asymptomatic, that may include one unspecific symptom, 42 (39%) mild, 8 (7%) moderate, and 30 (28%) severe symptomatic (Supplementary Material 2 Clinical classification). Forty‐nine (45%) volunteers did not report any comorbidities (as diabetes, hypertension, obesity, HIV, specific respiratory disease, and/or other chronic diseases), for those with comorbidities, hypertension (n = 39, 36%), obesity (n = 27, 25%), and diabetes (n = 24, 22%), were the most common, followed by respiratory disease (such as asthma or chronic obstructive pulmonary disease, n = 12, 11%).
Thirty‐eight (35%) were hospitalized patients, age 34–82 years (median 57, IQR 48–63); 33 of them (87%) had some comorbidity and the majority was male (61%). The time of symptoms reported by hospitalized volunteers was 1–23 days (median 8, IQR 6–12) before diagnostic. The most frequent symptoms in this group were fever (84%), cough (82%), difficulty in breathing (74%), and myalgia (50%). The main referred comorbidities, hypertension, obesity, and diabetes, all correlated with hospitalization with statistical significance (Pearson's χ 2): p = 0.0235; p < 0.0001; p = 0.0246, respectively.
Out of the 38 hospitalized volunteers, 28 were discharged and 10 were deceased. Mortality was higher among men (8/53 vs. 2/56, p = 0.05), although the number of hospitalized men were higher than women, the difference was not significant (43% vs. 27%, p = 0.07), with 2/15 women (13%) and 8/23 men (35%) deceased (p = 0.26). Men had 53–83 years and women were 64 and 66 years old.
Seventy‐one participants in the study did not require hospital care. Most of the individuals in this group were female (58%) from 22 to 66 years (median 48, IQR 39–54) the majority referring no comorbidity (62%). The time of symptoms of volunteers in the mild group was 30 days (IQR 15–45) before SARS‐CoV‐2 positive RNA. The most frequent symptoms reported were headache (39%), fatigue (33%), anosmia and/or dysgeusia (30%), and cough (28%).
The patients' information is shown in Table 1.
Table 1.
Demographic and clinical characteristics according to severity symptoms of 109 volunteers with COVID‐19 in São Paulo, Brazil
| All | Asymptomatic | Mild | Moderate | Severe | |
|---|---|---|---|---|---|
| No. volunteers (%) | 109 (100) | 29 (27) | 42 (39) | 8 (7) | 30 (28) |
| Male (%) | 53 (49) | 13 (45) | 17 (40) | 3 (38) | 20 (67) |
| Age | 51 (42–58) | 49 (41–54) | 48 (38–54) | 48 (41–56) | 60 (50–68) |
| Comorbiditya (%) | 60 (55) | 12 (41) | 15 (36) | 6 (75) | 27 (90) |
| DRb (days) | 15 (2–34) | 33 (12–51) | 24 (15–35) | 14 (4–28) | 2 (0–10) |
| Symptomsc | |||||
| Cough (%) | 49 (43) | 2 (7) | 18 (43) | 7 (87) | 24 (80) |
| Fever/febrile (%) | 43 (38) | NA | 11 (26) | 8 (100) | 24 (80) |
| Shortness of breath (%) | 36 (32) | NA | 8 (19) | 7 (87) | 21 (70) |
| Myalgia (%) | 35 (31) | NA | 16 (38) | 5 (62) | 14 (47) |
| Headache (%) | 35 (31) | 2 (7) | 26 (62) | 2 (25) | 7 (23) |
| Anosmia/dysgeusia (%) | 24 (21) | NA | 21 (50) | 3 (37) | NE |
| Sore throat (%) | 13 (11) | 2 (7) | 11 (26) | 2 (25) | NE |
| Fatigue (%) | 26 (23) | 2 (7) | 22 (52) | 2 (25) | 2 (7) |
| Diarrhea (%) | 14 (12) | NA | 9 (21) | 3 (37) | 2 (7) |
| Days of symptomsd | 17 (11–33) | 15 (8–42) | 30 (15–45) | 20 (17–34) | 12 (7–17) |
| Hospitalized patients | |||||
| Days of hospitalizatione | 20 (12–43) | NA | NA | 13 (10–42) | 22 (14–44) |
| Intubated (%) | 5 (5) | NA | NA | NA | 5 (100) |
| Death (%) | 10 (9) | NA | NA | NA | 10 (100) |
Note: Absolute number of cases, percentage, and median interquartile (IQR) 25–75th.
Abbreviations: COVID‐19, coronavirus disease 2019; IQR, interquartile range.
Comorbidities include diabetes, hypertension, obesity, HIV, specific respiratory disease, and/or other chronic diseases.
Days after RNA positive test.
Some asymptomatic cases referred unspecific and isolated symptoms.
Days on symptoms referred.
Time of hospitalization. NA: Not applicable. NE: Anosmia and sore throat were not evaluated in severe cases.
3.2. Antibodies response against SARS‐CoV2
Two classes of immunoglobulins, G (IgG) and A (IgA), were analyzed to assess the immune response to three SARS‐CoV‐2 antigens (S1, N, and S2). In total, 253 samples were collected, 218 from volunteers with documented RNA diagnosis (n = 90) and 35 from volunteers without confirmed or documented RNA diagnosis (n = 19). The proportion of IgG and IgA positivity according to days after RNA is shown in Table 2. From volunteers who only have previous serology or clinical diagnosis for COVID‐19, 16/19 were IgG positive and 1/19 was IgA positive.
Table 2.
SARS‐CoV‐2® Test antibodies response according to days after RNA confirmation and clinical severity
| Days after RNA (DR) for SARS‐CoV‐2 | |||||
|---|---|---|---|---|---|
| All | 1–7 | 8–13 | 14–31 | ≥32 | |
| No. samples | 218 | 86 | 22 | 59 | 51 |
| No. positive for IgG (%) | 178 (82) | 78 (91) | 21 (96) | 51 (86) | 28 (55) |
| No. positive for IgA (%) | 105 (48) | 65 (76) | 12 (55) | 27 (46) | 1 (2) |
| All | Asymptomatic | Mild | Moderate | Severe | |
| No. samples | 218 | 42 | 56 | 25 | 95 |
| No. positive for IgG (%) | 178 (82) | 25 (60) | 34 (61) | 24 (96) | 95 (100) |
| No. positive for IgA (%) | 105 (48) | 2 (5) | 8 (9) | 11 (44) | 84 (88) |
Note: Percentage of samples by days after RNA according to test result (one spot or more ≥70 Virachip® units for IgG and two spots or more for IgA).
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.3. Seroconversion and seroreversion
In our study, seventy‐five individuals have two or more samples tested, allowing to verify seroconversion or seroreversion. All the seroconversions happened between the first and second blood collection.
To better elucidate these data, the volunteers were categorized as nonhospitalized (asymptomatic and mild), and hospitalized (moderate and severe). The latter group was followed during the hospitalization, therefore, they entered the study presenting symptoms, and their sampling occurred more frequently (median of 6 days between collections, IQR 5–12) and during a shorter time, as they were not followed after discharge. In this group, 28 volunteers (80%) remained IgG positive throughout the study, none seroreverted and 7 (20%) seroconverted to IgG (median of 5 days, IQR 4–6). For IgA, 29 (82%) were positive in all collections, none seroreverted and 6 (17%) seroconverted (median of 6 days, IQR 4–6).
The blood collection of nonhospitalized volunteers in a longer period (median of 56 days between collections, IQR 49–90), so they presented higher chances to seroconvert and serorevert during the study. In this group, 14 (35%) were IgG positive in all collections, 12 (30%) remained negative, 8 (20%) seroconverted (median of 56 days, IQR 37–78), and 6 (15%) seroreverted (median of 55 days, IQR 46–58). For IgA, 2 (5%) were positive in all collections, 29 (73%) remained negative, 2 (5%) seroconverted (12 and 78 days), and 7 (18%) seroreverted (median of 46 days, IQR 21–52).
3.4. S1, S2, and N IgA and IgG against SARS‐CoV‐2
To assess the IgG and IgA separated responses to these antigens (S1, S2, or N) we evaluated the higher quantification of ViraChip® units. It was verified that the antibody level increased along with severity, as shown in Figure 1. The severe group presented higher quantifications than the other groups (p < 0.0001 for all parameters). If we compare the recognition of the antigens in each group, there is no statistical difference in IgA response. Considering IgG response, there is no difference in asymptomatic volunteers, but in the mild and moderate groups, there is a superiority of IgG‐S1 compared with IgG‐S2 (p < 0.01 and p < 0.05, respectively), and in severe volunteers, IgG‐S1 was superior to IgG‐N and IgG‐S2 (p < 0.01 for both). Death occurred among 10 of the hospitalized volunteers, as shown in Figure 2. There is no statistical difference when these two groups are compared.
Figure 1.
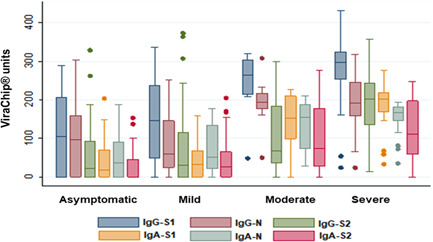
Quantification of ViraChip® units of IgA/IgG anti‐S1, N, and S2 antigens according to disease severity. Analysis based on the maximal quantification obtained for each parameter
Figure 2.
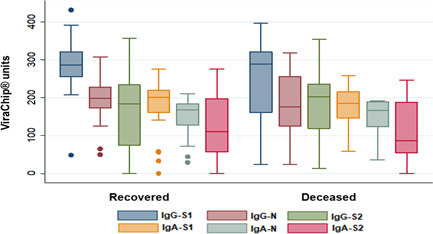
Quantification of ViraChip® units IgA/IgG anti‐S1, S2, and N antigens in 38 hospitalized volunteers according to patient's outcome (recovered or deceased). Analysis based on the maximal quantification obtained for each parameter
The deceased volunteers achieved the maximum antibody response slightly earlier than the recovered volunteers, however, there is no statistical difference between the groups. The deceased volunteers took 7 days (IQR 4–9) to reach maximal quantification for all parameters, while the recovered group took 9 days (IQR 6–12) to achieve maximum IgG‐S1, IgG‐N, IgA‐N, and IgA‐S2, and 8 days (IQR 6–12) for IgG‐S2 and IgA‐S1.
4. DISCUSSION
Several studies had been conducted to describe the immune response of patients exposed to SARS‐CoV‐2, which helps to understand the immunopathogenesis of the disease. The immune response seems to vary extensively among COVID‐19 patients and immunologic surveys are important to elucidate this aspect. Currently, two major hypotheses to explain the relationship between the immune response and the COVID‐19 have been raised: the individual immune response to SARS‐CoV‐2, according to the host's genetic background, age and comorbidities, for example, would determine the clinical course after the infection 13 ; on the other hand, the severity of the disease, marked by higher viral load and longer time of viral shedding, could lead to excessive inflammation and impaired immune response, hampering the disease control. 6 , 14 , 15 However, both scenarios may converge. A compromised initial response, by innate cells and mucosa immunity, that fails in orchestrating the adaptative response and controlling the infection in the upper respiratory tract, would be followed by persistent viremia. 16 , 17 Therefore, it is possible that a continuous synergism between the host's immune system and SARS‐CoV‐2 infection define the disease presentation. 13
We assessed the IgA and IgG responses to S1, N, and S2 antigens of SARS‐CoV‐2 of four different groups (asymptomatic, mild, moderate, or severe COVID‐19). Although it was not the main objective of this study, we verified statistical significance in hospitalized patients presenting comorbidities, as the literature describes. 18 Obesity was strongly associated with hospitalization (p < 0.0001). A different study that enrolled IIER patients confirmed the result. 19 In COVID‐19, obesity increases the chance of the patient being hospitalized in intensive care units, it is usually associated with other aggravating factors, like hypertension and diabetes; and has been related to immunological impairments, like increased inflammation and decrease of Treg activity, which may contribute to inadequate immune response. 20 This information highlights the need to observe comorbidities in the risk assessment of COVID‐19 patients.
When the absolute number of positive samples was analyzed, regardless of the severity classification (Table 2), we verified that IgG response increases in the first week after RNA confirmation, reaching a peak between 8 and 13 days and starting to decrease after it. For IgA, the peak happened during the first week and then started to decrease, being almost not detected after 30 days. Even though these data comprised the whole study population, despite each group's particularities, these results agree with the literature. 2 Unfortunately, due to differences in sampling time, we could not construct four different curves to directly compare the kinetics of each group.
The majority of hospitalized patients (80%) had positive serology during the whole study. It can be related to their time of observation, but also to increased viral loads and prolonged viral shedding, which have been documented among hospitalized cases 21 and associated with common comorbidities. 22 , 23
Seroreversion has been observed in COVID‐19, however, results vary from 90 to 180 days to decrease the antibody quantity into nondetectable levels, not to mention the difference between techniques used. 24 The short period of sampling during hospitalization limited the chances of observing seroreversion in the moderate and severe groups, but in the asymptomatic and mild groups, we observed seroreversion in 15% of volunteers, at a median of 55 days to serorevert for IgG and 46 days for IgA. The literature describes different times for IgG seroreversion, varying from 66 days to 164 days. 25 , 26 IgA usually decays after 30 days; even though there is a report of 70 days to serorevert, but the majority part of the population in that study had severe COVID‐19, which probably lead to higher antibodies titers that would take a longer time to decay. 2 , 27 As our observation was limited to a median of 77 days, we can expect higher reversion rates with longer observational periods.
ViraChip® tests provide quantitative results. Analyzing the maximum ViraChip® units, we verified that the two antibodies classes (IgA and IgG) to three antigens tested (S1, N, and S2) increased according to disease severity (Figure 1). COVID‐19 patients showed higher IgA and IgG levels in severe cases when compared with asymptomatic and mild patients, which is also seen in Carsetti et al. 16 study.
Some studies described an N‐biased IgG response in hospitalized patients of COVID‐19, which would reflect the persistent viremia, because of the higher quantity of N antigen incorporated into the virion. 14 , 28 However, in our study, the IgG‐S1 parameter was detected in statistically higher quantity compared with IgG‐N and IgG‐S2 in the severe group (p < 0.01); in asymptomatic, mild, and moderate groups this parameter overcame the others, as well. The S protein is one of the main immunogenic antigens of SARS‐CoV‐2, therefore, its dominance on the humoral response can be expected. 3
This quantification analysis does not reflect the actual functionality of antibodies. For example, anti‐RBD has been related to IgA and IgG neutralization capacity, 29 , 30 and needed to decrease the viral load of severe patients in the Silva et al. 15 study. Comparing decease and recovery outcomes, Atyeo et al. 14 pointed an S‐biased response in the recovered group.
The time to reach the maximum antibody quantity has been investigated, but disagreeing results were seen in the literature: the indication of fatality in Hashem et al. 28 was reaching higher anti‐S1 and anti‐N IgG responses at the beginning of symptoms; while in Lucas et al. 31 deceased patients presented a delayed antibody response. In this study, the deceased patients reached the higher humoral response within 7 days, slightly earlier than the recovered ones, which varied from 8 to 9 days. However, disagreeing results in the literature suggest that the kinetics of humoral response as an indicator of the outcome should be further investigated.
Interestingly, some of the hospitalized patients presented low maximum antibody quantification (Figure 2) and were able to recover from COVID‐19. We cannot properly evaluate the particularities that lead to it without further investigations, but it has been suggested that different arms of immune response participate in the control of the disease: the quality of innate immunity, with activation of Natural Killer (NK) cells, has been related with resistance to infection 32 ; phagocytosis and complement‐fixing activity were remarkable features of recovered patients 14 ; convalescent patients with almost undetectable antibody titers presented T cell immune response to S and Membrane proteins 33 ; higher frequencies of Treg cells were found in nonhospitalized patients, compared with hospitalized cases. 34 These studies indicate that the humoral response, especially antibody titers, despite its importance, should not be the only goal for immunity against SARS‐CoV‐2. Although the information in B cell memory is not available, the functional characteristics of antibodies have been investigated. The avidity index, measuring the strength of binding between the paratope and epitope, suggests an affinity maturation of the immune response with time. 35 Studies following COVID‐19 patients point to overall low avidity of antibodies. 19 The incomplete avidity maturation followed by a decay in antibodies titers is common in coronaviruses response and could explain reinfections and repetitive outbreaks. 36 Considering it all, it is important to highlight that positive serology following COVID‐19 by itself is not a definitive marker of protection.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest regarding the publication of this article. The support of NL Diagnóstica did not interfere with study design or result interpretation.
AUTHOR CONTRIBUTIONS
Conception and design were performed by Marisa Ailin Hong, Elizabeth De Gaspari, and Luís Fernando de Macedo Brígido. Volunteers recruitment and data collection were performed by Valéria Oliveira Silva, Cintia Mayumi Ahagon, Elaine Monteiro Matsuda, Elaine Lopes de Oliveira, Edilene Peres Real da Silveira, Ana Kesia de Souza Lima, José Angelo Lauletta Lindoso, Ivana Barros de Campos, and Marisa Ailin Hong. Execution of laboratory tests were performed by Amanda Izeli Portilho, Valéria Oliveira Silva, Cintia Mayumi Ahagon, Elaine Lopes de Oliveira, Edilene Peres Real da Silveira, and Marisa Ailin Hong. Samples management was performed by Elaine Lopes de Oliveira and Marisa Ailin Hong. Statistical analysis was performed by Luís Fernando de Macedo Brígido. The manuscript was written by Amanda Izeli Portilho and Valéria Oliveira Silva. All authors contributed to the study, commented on previous versions of the manuscript, and approved the final manuscript.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to thank NL Diagnóstica for support in the IgA/IgG ViraChip® Test procedures. This study was partially funded by CNPq/MS‐DIAHV N° 24/2019 Process: 442776/2019‐5; CNPq Process: 131412/2019‐1; FESIMA CAF: 011/2021. NL Diagnóstica (São Paulo, SP, Brazil) donated the IgA/IgG ViraChip® Test kits (ViraMed Biotech) used in this study for Dr. Elizabeth De Gaspari (Center of Immunology, Institute Adolfo Lutz).
Portilho AI, Silva VO, Ahagon CM, et al. Humoral response to spike S1 and S2 and nucleocapsid proteins on microarray after SARS‐CoV‐2 infection. J Med Virol. 2021;94:178‐185. 10.1002/jmv.27290
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. World Health Organization . WHO coronavirus disease (COVID‐19) dashboard. Accessed August 16, 2021. https://covid19.who.int/
- 2. West R, Kobokovich A, Connell N, Gronvall GK. COVID‐19 Antibody tests: a valuable public health tool with limited relevance to individuals. Trends Microbiol. 2021;29(3):214‐223. 10.1016/j.tim.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai L, Gao GF. Viral targets for vaccines against COVID‐19. Nat Rev Immunol. 2021;21(2):73‐82. 10.1038/s41577-020-00480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Assadiasl S, Fatahi Y, Zavvar M, Nicknam MH. COVID‐19: significance of antibodies. Hum Antibodies. 2020;28(4):287‐297. 10.3233/HAB-200429 [DOI] [PubMed] [Google Scholar]
- 5. Liu YC, Kuo RL, Shih SR. COVID‐19: The first documented coronavirus pandemic in history. Biomed J. 2020;43(4):328‐333. 10.1016/j.bj.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silva VO, de Oliveira EL, Castejon MJ, et al. Prevalence of antibodies against SARS‐CoV‐2 in professionals of a public health laboratory at São Paulo, SP, Brazil. bioRxiv. 2020. 10.1101/2020.10.19.20213421 [DOI] [Google Scholar]
- 8. Ministério da Saúde do Brasil . Guia de vigilância epidemiológica ‐ COVID‐19. Accessed July 14, 2021. https://www.gov.br/saude/pt-br/coronavirus/publicacoes-tecnicas/guias-e-planos/guia-de-vigilancia-epidemiologica-covid-19/view
- 9. Brazil . Boletim Epidemiológico Especial n01 do Ministério da Saúde: Infecção Humana pelo Novo Coronavírus (2019‐nCoV). Accessed June 10, 2020 . http://portalarquivos2.saude.gov.br/images/pdf/2020/janeiro/28/Boletim-epidemiologico-SVS-28jan20.pdf
- 10. Lopez‐Lopes GI, Ahagon C, Benega MA, et al. Throat wash as a source of SARS‐CoV‐2 RNA to monitor community spread of COVID‐19. medRxiv. 2020. 10.1101/2020.07.29.20163998 [DOI] [Google Scholar]
- 11. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ViraMed Biotech AG. SARS‐CoV‐2 ViraChip® IgG, SARS‐CoV‐2 ViraChip® IgA. Accessed September 5, 2020. https://www.viramed.de/produkte/virachip-immunoblots/virachip-infektionsdiagnostik/sars-virachip%C2%AE-igg-iga.html
- 13. Maggi E, Canonica GW, Moretta L. COVID‐19: unanswered questions on immune response and pathogenesis. J Allergy Clin Immunol. 2020;146(1):18‐22. 10.1016/j.jaci.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Atyeo C, Fischinger S, Zohar T, et al. Distinct early serological signatures track with SARS‐CoV‐2 survival. Immunity. 2020;53(3):524‐532 e4. 10.1016/j.immuni.2020.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silva J, Lucas C, Sundaram M, et al. Saliva viral load is a dynamic unifying correlate of COVID‐19 severity and mortality. medRxiv. 2021. 10.1101/2021.01.04.21249236 [DOI] [Google Scholar]
- 16. Carsetti R, Zaffina S, Piano Mortari E, et al. Different innate and adaptive immune responses to SARS‐CoV‐2 infection of asymptomatic, mild, and severe cases. Front Immunol. 2020;11(December):1‐16. 10.3389/fimmu.2020.610300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS‐CoV‐2 infection. Mucosal Immunol. 2021;14(2):305‐316. 10.1038/s41385-020-00359-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fang X, Li S, Yu H, et al. Epidemiological, comorbidity factors with severity and prognosis. Aging. 2020;12(13):12493‐12503. 10.18632/aging.103579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moura A, Costa HH, Correa V, et al. Serological assessment of COVID‐19 patients in Brazil: levels, avidity, and subclasses of IgG against RBD. Res Sq. 2021. 10.21203/rs.3.rs-131195/v1 [DOI] [Google Scholar]
- 20. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):1‐17. 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu K, Chen Y, Yuan J, et al. Factors associated with prolonged viral RNA shedding in patients with COVID‐19. Clin Infect Dis. 2020;71(15):799‐806. 10.1093/cid/ciaa351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buetti N, Trimboli P, Mazzuchelli T, et al. Diabetes mellitus is a risk factor for prolonged SARS‐CoV‐2 viral shedding in lower respiratory tract samples of critically ill patients. Endocrine. 2020;70(3):454‐460. 10.1007/s12020-020-02465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu L, Gong N, Liu B, et al. Coronavirus Disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol. 2020;77(6):748‐754. 10.1016/j.eururo.2020.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poland GA. Antibody dynamics, seroreversion and persistence after SARS‐CoV‐2: another answer. Clin Infect Dis. Published online March 21, 2021. 10.1093/cid/ciab243 [DOI] [PMC free article] [PubMed]
- 25. Self WH, Tenforde MW, Stubblefield WB, et al. Decline in SARS‐CoV‐2 antibodies after mild infection among frontline health care personnel in a multistate hospital network — 12 states, April–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(47):1762‐1766. 10.15585/mmwr.mm6947a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papasavas P, Olugbile S, Wu U, et al. Seroprevalence of SARS‐CoV‐2 antibodies, associated epidemiological factors and antibody kinetics among healthcare workers in Connecticut. J Hosp Infect. 2021;114:117‐125. 10.1016/j.jhin.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iyer AS, Jones FK, Nodoushani A, et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS‐CoV‐2 spike protein in COVID‐19 patients. Sci Immunol. 2020;5(52):1‐8. 10.1126/sciimmunol.abe0367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashem AM, Algaissi A, Almahboub SA, et al. Early humoral response correlates with disease severity and outcomes in COVID‐19 patients. Viruses. 2020;12(12):1390. 10.1101/2020.09.21.20198309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oliveira JR, Machado RRG, Arcuri HA, et al. Immunodominant B cell epitope in a hotspot mutation site and mechanism of immune escape for SARS‐CoV‐2. medRxiv. 2021. 10.1101/2021.03.11.21253399 [DOI] [Google Scholar]
- 30. Moriyama S, Adachi Y, Sato T, et al. Temporal maturation of neutralizing antibodies in COVID‐19 convalescent individuals improves potency and breadth to circulating SARS‐CoV‐2 variants. Immunity. 2021;54(8):1841‐1852. 10.1016/j.immuni.2021.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lucas C, Klein J, Sundaram ME, et al. Delayed production of neutralizing antibodies correlates with fatal COVID‐19. Nat Med. 2021;27:1178‐1186. 10.1038/s41591-021-01355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castelli EC, Castro MVDe, Naslavsky MS, Scliar MO, Nayane S. Immunogenetics of resistance to SARS‐CoV‐2 infection in discordant couples. medRxiv. 2021. 10.1101/2021.04.21.21255872 [DOI] [Google Scholar]
- 33. Schwarzkopf S, Krawczyk A, Knop D, et al. Cellular immunity in COVID‐19 convalescents with PCR‐confirmed infection but with undetectable SARS‐CoV‐2‐specific IgG. Emerg Infect Dis. 2021;27(1):33058753. 10.3201/eid2701.203772 [DOI] [PubMed] [Google Scholar]
- 34. Meckiff BJ, Ramírez‐Suástegui C. Fajardo V, et al. Imbalance of regulatory and cytotoxic SARS‐CoV‐2‐Reactive CD4+ T cells in COVID‐19. Cell. 2020;183(5):1340‐1353. 10.1016/j.cell.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gaspar EB, De Gaspari E. Avidity assay to test functionality of anti‐SARS‐CoV‐2 antibodies. Vaccine. 2021;39(10):1473‐1475. 10.1016/j.vaccine.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bauer G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS‐CoV‐2. Int J Infect Dis. 2021;106:61‐64. 10.1016/j.ijid.2021.01.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


