Abstract
Over 26 million cases of coronavirus disease 2019 (COVID‐19) have been reported in the United States with over 440 000 deaths. Despite COVID‐19 vaccine approval, pregnant women were excluded from clinical trials. We report a case of immune thrombocytopenia in the first trimester, which occurred 13 days after initiating the COVID‐19 vaccination series. Thorough evaluation, including hematology consultation, established the diagnosis. High‐dose oral corticosteroids were started, and she was discharged home with significant improvement in platelet count on her fourth day of hospitalization with no subsequent complications. We advocate that the benefits of COVID‐19 vaccination outweigh the risk of infection in pregnancy and that pregnant women should be included in clinical trials. Closer post‐vaccination surveillance may be warranted in the pregnant population pending further data.
Keywords: COVID‐19, immune thrombocytopenia, pregnancy, vaccination
Introduction
Based upon the Centers for Disease Control and Prevention (CDC) reports, pregnancy has been established as an independent risk factor for coronavirus disease 2019 (COVID‐19) severity. 1 In a November 2020 Morbidity and Mortality Weekly Review (MMWR) of 409 462 reproductive‐aged women hospitalized for COVID‐19, pregnancy conferred a threefold increased risk for ICU admission and invasive ventilation, 2.4‐fold increased risk for extracorporeal membrane oxygenation (ECMO), and 70% increased risk of death. 2 Therefore, COVID‐19 vaccine development represents a promising and essential strategy toward primary prevention efforts in this high‐risk population. 3
Despite medical literature establishing the high‐risk nature of SARS‐CoV‐2 infection in pregnancy, both pregnant and breastfeeding women were excluded from initial vaccination trials. Since then, multiple medical organizations, including the American College of Obstetrics and Gynecology (ACOG) and the Society for Maternal‐Fetal Medicine (SMFM), have firmly advocated for the inclusion of pregnant and lactating women in vaccination trials. Moreover, both societies recommend that the COVID‐19 vaccine should not be withheld from pregnant and lactating women who desire the vaccine and have no contraindications. 3
While the COVID‐19 vaccination trials have demonstrated high efficacy and low adverse event rates within the general population, to date, there have been no published reports of side effects following the COVID‐19 vaccine in pregnant women. We report a case of immune thrombocytopenia (ITP) in a healthy pregnant patient after COVID‐19 vaccination.
Case
A 32‐year‐old healthy G1P0 patient at 8 6/7 weeks of gestation was admitted for hospitalization in the setting of acute‐onset bruising and petechiae. The patient received the first injection of the Moderna mRNA‐1273 vaccine through her workplace 13 days before admission, with development of a petechial rash 11 days after vaccine administration (Figure 1). The patient has no prior medical conditions and is up to date on CDC recommended vaccinations with no previous vaccine adverse events. Additionally, she has no known personal or familial history of autoimmune disease.
Figure 1.
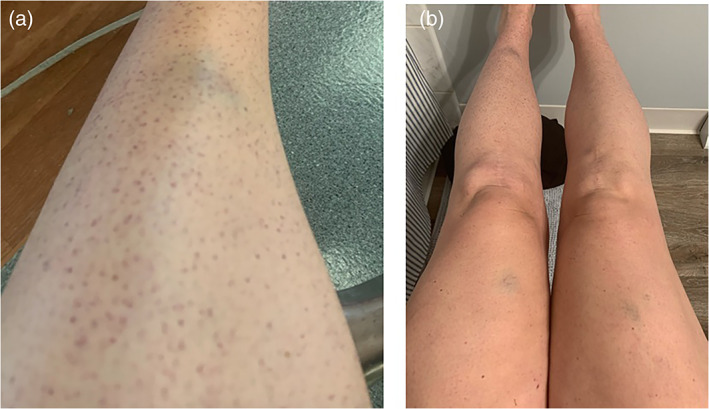
Petechial rash and bruising of the (a) left lower extremity and (b) bilateral lower extremities.
On admission, the patient was found to have a platelet count of 1000/mL. Three months prior, her platelets were 268 000/mL, white blood cell count was 8900/mL, hemoglobin was 13.8 g/dL, and hematocrit was 41.1%. On admission, her white blood cell count was 8000/mL, hemoglobin was 13.1 g/dL, and hematocrit was 38.2%. Comprehensive laboratory evaluation including vitamin B12, folate, reticulocyte count, haptoglobin, d‐dimer, prothrombin time (PTT), activated partial thromboplastin time (aPTT), and fibrinogen were within normal limits. A peripheral smear was obtained, which was notable only for thrombocytopenia on hematologic review. An upper abdominal ultrasound demonstrated a normal size and sonographic appearance of the spleen. Hematologic specialists were consulted; given her negative thrombocytopenia work‐up, ITP was suspected as a diagnosis of exclusion. For management, oral prednisone was started at a dose of 1 mg/kg/day (85 mg).
The patient did not exhibit any other adverse effects throughout hospital admission. Her ITP symptoms remained mild, limited to bruising and petechiae. The patient did not have any episodes of spontaneous bleeding and, as such, did not undergo platelet transfusion. On hospital day 2, her platelet count improved to 6000/mL. On hospital day 3, her platelet count improved to 26 000/mL, and she was discharged home to continue a week of full dose steroids, followed by a 6‐week taper. A telehealth visit was performed 5 days after diagnosis, and the patient denied any additional symptoms. If the patient's laboratory or clinical values are refractory to corticosteroid treatment, a plan for intravenous immunoglobulin (IVIG) initiation will be enacted. Patient had repeat laboratory evaluation 19 days after vaccination and was found to have recovery of her platelets to 303 000/mL (Figure 2).
Figure 2.
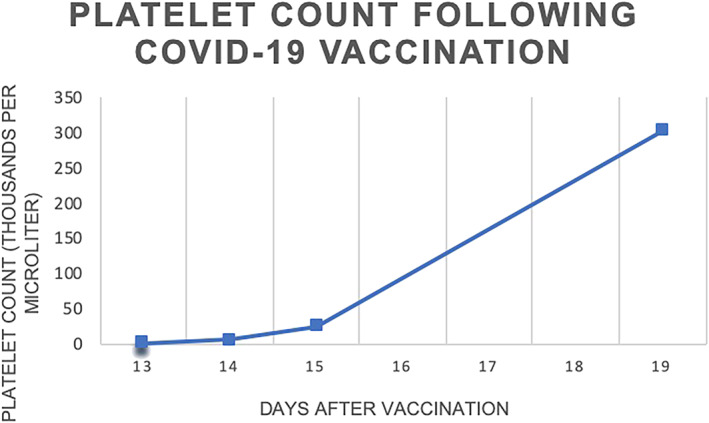
Platelet count (thousands per microliter) following COVID‐19 vaccination.
Comment
The expeditious development of vaccinations against SARS‐CoV‐2 infection represents one of the most remarkable scientific achievements of our time. Although large‐scale randomized trials demonstrated the safety of the COVID‐19 vaccination in the general population, pregnant and lactating women were noticeably excluded from these studies. In this report, we describe a case of ITP following COVID‐19 vaccination.
Important maternal immunologic adaptations occur in pregnancy to accommodate the presence of fetal paternal antigens. Specifically, there is a shift from cell‐mediated immunity toward humoral immunity, dominated by Th2 lymphocytes, which secrete IL‐4 and IL‐10 and enhance B‐cell immunity. 4 The pathogenesis of ITP involves autoantibodies produced by a patient's B cells that target platelet membrane glycoproteins, specifically GpIIb/IIIa. While the exact immunological mechanism of ITP remains undetermined, the production of anti‐platelet antibodies leads to T‐cell‐mediated platelet destruction and impaired megakaryocyte function. 5
ITP has been described in the literature following measles, mumps, and rubella (MMR) vaccination at an estimated incidence of 1–3 per 100000. 6 This usually presents within 6 weeks of vaccination and in most cases is mild and self‐limiting, with symptoms limited to bruising and petechiae, resolving within days to weeks. 6 Platelet counts are typically higher than in nonvaccine‐associated ITP. 6 Notably, the prognosis is better than viral‐related ITP, which may progress to a chronic state in up to 28% of patients compared to only 10% of patients following vaccination. 7 Of note, most studies reporting the incidence of ITP following MMR vaccination have been conducted on pediatric populations.
One case of ITP diagnosed in a 22‐year‐old male 3 days after receiving the Pfizer‐BioNTech BNT16B2b2 mRNA vaccine was recently published. 8 The patient presented with spontaneous oral bleeding and a platelet count of 2000/mL. His platelet count improved to 28000/mL 6 days later after receiving platelet transfusion, dexamethasone, and IVIG. By day 11, the patient's platelet count returned to normal. 8 There has also been a report in the press citing the death of a 56‐year‐old male secondary to ITP 16 days after receiving Pfizer COVID‐19 vaccination. 9
Similar to these reported cases of ITP following vaccination, our patient presented within a short interval following vaccination. Additionally, her symptoms were limited to cutaneous findings and were self‐limiting. Further, her symptoms resolved within days to weeks, consistent with prior reports. Differing from reports of ITP following MMR vaccination, however, our patient had a drastic decline in her platelet count to 1000/mL.
These cases highlight the need for continued research, evidence gathering, and inclusion of our pregnant population in clinical trials to ascertain efficacy and safety. ACOG and SMFM have maintained that pregnant and lactating women should be empowered to make their own decisions regarding vaccine administration based on available evidence, and barriers should not be put in place to hinder access. Further, ACOG and SMFM encourage agencies to collect and report data regarding vaccine use in this population to guide counseling. 10 Conversely, the World Health Organization published a document on January 25, 2021, recommending against COVID‐19 vaccination in pregnancy unless these patients were at high risk of exposure. 11 This stance later changed, stating vaccination is reasonable within this population following consultation with healthcare providers. 12
In these unprecedented times, excluding pregnant women from clinical trials for a preventative vaccination that may decrease their morbidity and mortality is ethically problematic. While healthcare providers advocate that women are at increased risk of severe disease following infection with SARS‐CoV‐2, we are at odds with conflicting guidance from professional associations, disseminated false information, and patient vaccine hesitancy. 13 This is a call to action to establish a culture where clinical trials are prioritized during pregnancy and transparent data reporting is upheld. Establishing an effective safety profile will promote evidence‐based shared decision making between women's health providers and our patients.
To conclude, in this case report, we describe a case of ITP following COVID‐19 vaccination in a pregnant patient in the first trimester, with resolved symptoms following treatment with oral corticosteroids. At present, a causal relationship between COVID‐19 vaccination and ITP has not been established, but providers must remain vigilant to infrequent complications. Nonetheless, we advocate that the benefits of COVID‐19 vaccination outweigh the risks of SARS‐CoV‐2 infection during pregnancy.
Disclosure
The authors have no financial disclosures.
Author Contributions
Carrie Bennett: Conceptualization; investigation; data curation; writing – original draft. Laura M. Chambers: Conceptualization; writing – original draft; writing – review and editing; supervision. Ji Son: Conceptualization; writing – review and editing. Oluwatosin Goje: writing – review and editing; supervision.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status – United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zambrano LD, Ellington S, Strid P, Galang RR, Oduyebo T, Tong VT, et al. Update: characteristics of symptomatic women of reproductive age with laboratory‐confirmed SARS‐CoV‐2 infection by pregnancy status – United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Society for Maternal‐Fetal Medicine . SARS‐CoV‐2 vaccination in pregnancy [cited 2021 Jan 29]. Available from: https://s3.amazonaws.com/cdn.smfm.org/media/2591/SMFM_Vaccine_Statement_12-1-20_(final).pdf
- 4. Cunningham FG, Leveno KJ, Bloom SL, Dashe JS, Hoffman BL, Casey BM, et al. Williams obstetrics. 25th ed. New York, NY: McGraw Hill; c2018. Chapter 64, Infectious diseases. [Google Scholar]
- 5. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. [Google Scholar]
- 6. Cecinati V, Principi N, Brescia L, Giordano P, Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum Vaccin Immunother. 2013;9(5):1158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113:2386–93. [DOI] [PubMed] [Google Scholar]
- 8. Tarawneh OH, Tarawneh HS. Immune thrombocytopenia in a 22‐year‐old post COVID‐19 vaccine. Am J Hematol. 2021;96(5):E133–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grady D, Mazzei P. Doctor's Death After Covid Vaccine Is Being Investigated. The New York Times. [cited 2021 Jan 12]. Available from: https://www.nytimes.com/2021/01/12/health/covid-vaccinedeath.html
- 10. American College of Obstetricians and Gynecologists and Society for Maternal‐ Fetal Medicine . ACOG and SMFM Joint Statement on WHO Recommendations Regarding COVID‐19 Vaccines and Pregnant Individuals [cited 2021 Jan 29]. Available from: https://s3.amazonaws.com/cdn.smfm.org/media/2726/WHO_Response.pdf
- 11. World Health Organization . Interim recommendations for the use of the Moderna mRNA‐1273 vaccine against COVID‐19. 2021. [cited 2021 Jan 29]. Available from: https://www.who.int/publications/i/item/interim-recommendations-for-use-of-the-moderna-mrna-1273-vaccine-against-covid-19
- 12. World Health Organization . The Moderna COVID‐19 (mRNA‐1273) vaccine: what you need to know. 2021. [cited 2021 Jan 29]. Available from: https://www.who.int/news‐room/feature‐stories/detail/the‐moderna‐covid‐19‐mrna‐1273‐vaccine‐what‐you‐need‐to‐know
- 13. Chervenak FA, McCullough LB, Bornstein E, Johnson L, Katz A, McLeod‐Sordjan R, et al. Professionally responsible COVID‐19 vaccination counseling of obstetric/gynecologic patients. Am J Obstet Gynecol. 2021;224(5):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


