Abstract
The coronavirus disease 2019 (COVID‐19) pandemic has particularly affected countries with weakened health services in Latin America, where proper patient management could be a critical step to address the epidemic. In this study, we aimed to characterize and identify which epidemiological, clinical, and paraclinical risk factors defined COVID‐19 infection from the first confirmed cases through the first epidemic wave in Venezuela. A retrospective analysis of consecutive suspected cases of COVID‐19 admitted to a sentinel hospital was carried out, including 576 patient cases subsequently confirmed for severe acute respiratory syndrome coronavirus 2 infection. Of these, 162 (28.1%) patients met the definition criteria for severe/critical disease, and 414 (71.2%) were classified as mild/moderate disease. The mean age was 47 (SD 16) years, the majority of which were men (59.5%), and the most frequent comorbidity was arterial hypertension (23.3%). The most common symptoms included fever (88.7%), headache (65.6%), and dry cough (63.9%). Severe/critical disease affected mostly older males with low schooling (p < 0.001). Similarly, higher levels of glycemia, urea, aminotransferases, total bilirubin, lactate dehydrogenase, and erythrocyte sedimentation rate were observed in severe/critical disease patients compared to those with mild/moderate disease. Overall mortality was 7.6% (44/576), with 41.7% (28/68) dying in hospital. We identified risk factors related to COVID‐19 infection, which could help healthcare providers take appropriate measures and prevent severe clinical outcomes. Our results suggest that the mortality registered by this disease in Venezuela during the first epidemic wave was underestimated. An increase in fatalities is expected to occur in the coming months unless measures that are more effective are implemented to mitigate the epidemic while the vaccination process is ongoing.
Keywords: clinical characteristics, COVID‐19, epidemiology, SARS‐CoV‐2, Venezuela
Highlights
To the best of our knowledge, our study is the first to assess the epidemiological, clinical, and paraclinical characteristics of COVID‐19 patients in Venezuela.
Regarding the occupation, 15% of the patients were healthcare workers.
Patients with more year's smoking, bilateral crackles and altered state of consciousness were associated with severe/critical disease.
Only 42% of the patients with severe/critical disease criteria were hospitalized, of which 41.7% died.
1. INTRODUCTION
Latin America is one of the regions most affected by the coronavirus disease 2019 (COVID‐19) pandemic, contributing significantly to the global burden of disease and showing the highest death tolls. 1 Currently, mortalities attributable to the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in the region amount to almost 2.5 million people. As the deployment of vaccines continues to advance at a slower rate than that of developed countries, the pandemic will continue to affect and generate excess morbidity and mortality in those countries with weakened health services, economic and political instabilities, humanitarian crises, and deep social inequalities. 2 , 3
The impact of COVID‐19 in Venezuela is expected to be high given the complex humanitarian crisis that the country is experiencing. 4 , 5 However, this same socioeconomic crisis has influenced the epidemic to initially develop slowly while displaying a geographically fragmented pattern. 6 A combination of various factors, such as previous air traffic restrictions, severe gasoline deficiency, and the establishment of an early quarantine determined a low initial inflow of imported cases, which significantly impacted mobility within the country, something that delayed the spread of the virus and mitigated its effect during the first epidemic wave that peaked in mid‐August 2020. 6 As of October 28, 2021, 401 259 cases and 4822 deaths from COVID‐19 had been reported in Venezuela. Although these figures are among the lowest reported in Latin America, the limited diagnostic capacity that has governed the management of the epidemic since its inception suggests that this epidemic is 5 to 7 times more intense than what is officially reported. 6 At the regional level, Venezuela is one of the countries with the lowest diagnostic coverage (<25 tests per 1000 inhabitants), and with the lowest vaccination coverage (<22%), which raises doubts about the country's capacity to carry out an adequate surveillance and intervention program of SARS‐CoV‐2 transmission in the near future. 6 , 7 This low coverage is more related to the slow response of the country to obtain an adequate supply of vaccines 7 than to a low vaccination intention. 8 Currently, the incidence of the disease continues to be underestimated, although the health system has begun to overflow under the unfolding of a second major epidemic wave that is now progressing more intensely throughout the country. The inability of the public health system in Venezuela to face the demands of the COVID‐19 pandemic is evidenced by the reduction in the number of hospital beds for critical patient care according to estimates from the National Survey of Venezuelan Hospitals. 9 The massive exodus of Venezuelans to neighboring countries due to the humanitarian crisis has increased as a consequence of the current pandemic. 10 , 11 Similarly, thousands of these migrants have been forced to return when the pandemic has hit the region's economy, 12 adding to the continuous unregistered informal crossing that occurs across borders from Colombia and Brazil. 13 This massive and continuous transit of people can enhance introduction and transmission events, while the uncontrolled spread of the virus can further complicate the management of the pandemic in already vulnerable countries across the region. 5 , 7 , 14
The first epidemic wave of COVID‐19 in Venezuela displayed significant geographic heterogeneity, with most cases concentrated in the north‐central region. 6 The first cases were notified on March 13, 2020, almost three weeks later Brazil detected the first case in the region 15 and one week after the first report from Colombia. 16 The first diagnosed case was that of a 41‐year‐old woman returning from Europe who attended the University Hospital located in Caracas (capital city of the country). As of August 2, 2020, 44 899 cases and 462 deaths had been reported in Caracas with these figures representing around 15% and 13% of the total number of cases and deaths reported in Venezuela, respectively.
Proper management of a patient's critical, chronic, and comorbid conditions is a critical step in tackling this epidemic. Hence, characterizing and identifying people with a higher risk for developing critical and severe COVID‐19 is crucial for the design of strategies seeking to protect or selectively vaccinate. As an example, public health interventions to modify comorbidities or treatment of COVID‐19 early in the course of the disease could have a positive impact on viral infection overall outcome in vulnerable countries of the Latin America region. 17 To date, reports on the epidemiological and clinical characteristics of this pandemic in the region is very limited. 18 , 19 , 20 , 21 , 22 For the case of Venezuela, there is no data integrated into a national surveillance system that allows identifying the characteristics or results of hospital admissions for COVID‐19 and the impact that the pandemic has had since its origin on the national system of health. The main objective of this study is to characterize and identify which epidemiological, clinical, and paraclinical risk factors are related to COVID‐19 infection in the first confirmed cases in Venezuela. For this, a retrospective analysis of consecutive suspected cases of COVID‐19 admitted to the University Hospital of Caracas was carried out, including 576 patient cases subsequently confirmed for SARS‐CoV‐2 infection by reverse‐transcriptase polymerase chain reaction (RT‐PCR).
2. MATERIALS AND METHODS
2.1. Study design and population
We retrospectively analyzed the confirmed COVID‐19 (according WHO guidelines) 23 cases at the Infectious Diseases Department of the University Hospital of Caracas, Venezuela, between March 4 and July 24, 2020.
2.2. Epidemiological and clinical evaluation
After the patients were interviewed and considered to meet criteria for suspected COVID‐19 cases, a standardized questionnaire was applied by trained postgraduate residents to collect demographic and epidemiological information (including recent travel history or contact with confirmed/suspected COVID‐19 cases), medical comorbidities, and data on 25 symptoms were collected. A detailed physical examination (including pulse and respiratory rate, blood pressure, and temperature) was performed. Follow‐up calls to evaluate the clinical outcome of the patients were performed during 28 days by health professionals not linked to this project as part of their standard of care treatment for patients with COVID‐19. The severity of the disease was established as mild, moderate, severe, and critical, according to the National Institute of Health guidelines. 24 For analysis purposes, cases of mild/moderate disease (oxygen saturation ≥ 94%) and severe/critical cases (oxygen saturation <94%) were grouped.
2.3. Paraclinical evaluation
According to guidelines of the Infectious Diseases Department of the University Hospital of Caracas, a 5‐ml sample of blood by venipuncture was taken from each patient, which was distributed in a tube containing ethylenediaminetetraacetic acid (3 ml) and a tube without anticoagulant (2 ml) for completing blood count and blood chemistry (urea, creatinine, glycemia, electrolytes, aminotransferases, and lactic dehydrogenase), respectively. Radiological evaluation was limited due to a lack of technical equipment during the study period. The nasopharyngeal samples collected were stored at 2–8°C and processed by the diagnostic reference laboratory in the country.
2.4. Statistical analyses
Data analysis considered descriptions of the characteristics of the sample studied by using central tendency and dispersion measures (mean, standard deviation, median, and interquartile range). The distribution of the parameters was evaluated using the Kolmogorov–Smirnov test. Parametric tests were used and when normality was not evident the data were subjected to nonparametric tests. The data were analyzed by Student's t‐test, Mann–Whitney U test, Pearson's χ 2 test, and Fisher's exact test, as appropriate. A p < 0.05 was considered significant. The analyses were performed with the statistical software Statistical Package for the Social Sciences version 25 (International Business Machines Corporation). The bar chart was generated using Microsoft® Excel® version 2019 (Microsoft).
3. RESULTS
3.1. Epidemiological characteristics
During the study period, a total of 4538 patients were evaluated, and 1190 nasopharyngeal swabs were performed. Six hundred thirty‐three patients were confirmed with SARS‐CoV‐2 infection by nucleic acid amplification tests (RT‐PCR). We excluded 57 patients who had incomplete epidemiological, clinical, and paraclinical data. Out of the 576 patients included in the analysis, 162 (28.1%) patients met the criteria for severe/critical disease, and 414 (71.9%) were classified as mild/moderate cases. The mean age was 47 (SD —standard deviation— 16; range: 12–87) years and most of the cases were men (59.5%, n = 343). Four hundred seventy‐three (82.1%) patients were domiciled in the Capital District. Regarding the occupation, most (42%, n = 343) were employees followed by independent (33.3%, n = 192) and 88 (15.3%) were healthcare workers. The most frequent type of exposure was unknown (492; 85.4%); whereas only 59 (10.2%) and 25 (4.3%) patients identified a confirmed or suspected contact with another COVID‐19 infected person. A significant association was found between exposure with a confirmed case and mild/moderate disease (p = 0.03) (Table 1).
Table 1.
Characteristics of 576 Venezuelan patients with confirmed SARS‐CoV‐2 infection
| Demographic characteristics | All (N = 576) | Mild/moderate disease (N = 414) | Severe/critical disease (N = 162) | p‐value |
|---|---|---|---|---|
| Sex, men/women (%) | 343/233 (59.5/40.5) | 277/187 (54.8/45.2) | 116/46 (71.6/28.4) | <0.001*, ‡ |
| Age, mean (SD), years | 46.9 (15.9) | 42.6 (14.6) | 57.9 (13.4) | <0.001† |
| Provenance by state and municipality, n (%) | 0.5* | |||
| Capital District, Libertador | 473 (82.1) | 334 (80.7) | 139 (85.8) | |
| Miranda | 99 (17.2) | 77 (18.6) | 22 (13.6) | |
| Sucre | 41 (41.4) | 35 (45.5) | 6 (27.3) | |
| Baruta | 21 (21.2) | 16 (20.8) | 5 (22.7) | |
| El Hatillo | 9 (9.1) | 6 (7.8) | 3 (13.6) | |
| Urdaneta | 6 (6.1) | 4 (5.2) | 2 (9.1) | |
| Chacao | 5 (5.1) | 4 (5.2) | 1 (4.5) | |
| Others | 17 (17.2) | 12 (15.6) | 5 (22.7) | |
| Other states | 4 (0.7) | 3 (0.7) | 1 (0.6) | |
| Instruction, n (%) | <0.001*, § | |||
| None | 5 (0.9) | 0 (0.0) | 5 (3.1) | |
| Primary school | 165 (28.6) | 97 (23.4) | 68 (42) | |
| High school | 219 (38) | 167 (40.3) | 52 (32.1) | |
| University/Technical | 187 (32.5) | 150 (36.2) | 37 (22.8) | |
| Marital status, n (%) | <0.001*, || | |||
| Single | 378 (65.6) | 294 (71) | 84 (51.9) | |
| Married | 170 (29.5) | 106 (25.6) | 64 (39.5) | |
| Divorced | 15 (2.6) | 9 (2.2) | 6 (3.7) | |
| Widower | 13 (2.3) | 5 (1.2) | 8 (4.9) | |
| Occupation, n (%) | <0.001*, ¶ | |||
| Employee | 242 (42) | 188 (45.4) | 54 (33.3) | |
| Independent | 192 (33.3) | 121 (29.2) | 71 (43.8) | |
| Healthcare worker | 88 (15.3) | 78 (18.8) | 10 (6.2) | |
| Unemployed/Retired | 43 (7.5) | 16 (3.9) | 27 (16.7) | |
| Student | 11 (1.9) | 11 (2.7) | – | |
| Exposure type, n (%) | 0.03*, ** | |||
| Unknown | 492 (85.4) | 344 (83.1) | 148 (91.4) | |
| With confirmed case | 59 (10.2) | 51 (12.3) | 8 (4.9) | |
| With suspicious case | 25 (4.3) | 19 (4.6) | 6 (3.5) |
Abbreviations: SD, standard deviation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Pearson's χ 2 test.
Student's t‐test.
Post hoc analysis: Significant association only between male and severe/critical disease (standardized residual = 2).
Post hoc analysis: Significant association only between severe/critical disease with no instruction (standardized residual = 3) and primary school (standardized residual = 3.2).
Post hoc analysis: Significant association only between severe/critical disease with married and widowed (standardized residual = 2.3; for both).
Post hoc analysis: Significant association only between severe/critical disease with unemployed/retired (standardized residual = 4.3) and independent occupation (standardized residual = 2.3).
Post hoc analysis: Significant association only between exposure with a confirmed case and mild/moderate disease (standardized residual = 1.9).
Compared with mild/moderate disease, severe/critical disease patients were often elderly males with no schooling or only elementary school‐level, married or widowed, and economically independent or unemployed (p < 0.001).
3.2. Pathological background and smoking habits
The most frequent comorbidity was arterial hypertension (23.3%, n = 134), followed by asthma (8.2%, n = 47), and diabetes mellitus (7.3%, n = 42); whereas six patients had human immunodeficiency virus. Severe/critical disease patients exhibited a higher proportion of comorbidities, such as hypertension, diabetes, and chronic obstructive pulmonary disease (p < 0.001). In relation to disease severity, there were no significant differences between those who smoke or smoked and those who had never smoked. However, within the group that smoke or smoked, a greater number of cigarettes per day and more year's smoking was associated with severe/critical disease (10 [IQR —interquartile range— 15]) versus 4 [IQR 8] cigarettes per day, p = 0.001; 18.5 [IQR 20] versus 10 [IQR 15] years smoking, p = 0.022; respectively) (Table 2).
Table 2.
Pathological background and smoking habits of 576 Venezuelan patients with confirmed SARS‐CoV‐2 infection
| All (N = 576) | Mild/moderate disease (N = 414) | Severe/critical disease (N = 162) | p‐value | |
|---|---|---|---|---|
| Pathological background, n (%) | ||||
| Systemic arterial hypertension | 134 (23.3) | 68 (16.4) | 66 (40.7) | <0.001 |
| Asthma | 47 (8.2) | 35 (8.5) | 12 (7.4) | 0.68 |
| Mellitus diabetes | 42 (7.3) | 14 (3.4) | 28 (17.3) | <0.001 |
| COPD | 9 (1.6) | 1 (0.2) | 8 (4.9) | <0.001 |
| Cancer | 9 (1.6) | 7 (1.7) | 2 (1.2) | 0.69 |
| HIV | 6 (1) | 6 (1.4) | – | 0.12 |
| Smoking habit, yes/no (%) | 120/456 (20.8/79.2) | 82/332 (19.8/80.2) | 38/124 (23.5/76.5) | 0.33* |
| Cigarettes per day, median [IQR] | 5 [8] | 4 [8] | 10 [15] | 0.001† |
| Habit duration, median [IQR], years | 12 [15] | 10 [15] | 18.5 [20] | 0.02† |
| Pack‐year index, median [IQR] | 3 [8.75] | 2 [6.7] | 6.75 [17.5] | 0.06† |
Abbreviations: COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IQR, interquartile range; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Pearson's χ 2 test.
Median test.
3.3. Clinical characteristics
Fever (88.7%, n = 511), headache (65.6%, n = 378), dry cough (63.9%, n = 368), chills (59.7%, n = 344), and asthenia (56.9%, n = 328) were the most frequent symptoms in all patients; fewer common symptoms included abdominal pain (18.8%, n = 108), dysphonia (14.2%, n = 82), dysphagia (9.9%, n = 57), and otalgia (7.5%, n = 43). A higher proportion of dyspnea and wet cough was found in severe/critical disease patients compared with mild/moderate disease (79% vs. 38.2%, p < 0.001; and 25.9% vs. 17.4%, p < 0.05; respectively). However, headache, anosmia, rhinorrhea, nasal congestion, odynophagia, and sneezing were symptoms more frequently found in mild/moderate disease patients than in severe/critical diseased patients (Figure 1). On physical exam, median heart rate, median respiratory rate, and median systolic blood pressure were significantly higher in severe/critical disease patients than in those with mild/moderate disease; while median oxygen saturation was significantly higher in mild/moderate disease patients than in severe/critical disease cases (97 [IQR 2] vs. 89 [IQR 9], p < 0.001). Among the chest pathological findings, a statistically significant association was found between bilateral crackles and severe/critical disease (p < 0.001). Altered state of consciousness was more frequent in severe/critical disease patients (p < 0.001) (Table 3).
Figure 1.
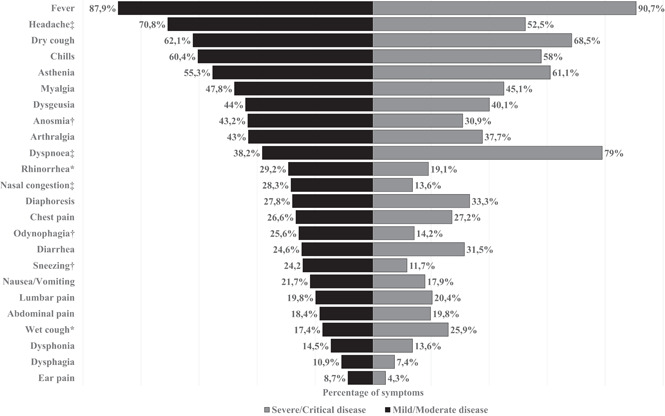
Symptoms of 576 Venezuelan patients with confirmed SARS‐CoV‐2 infection. Data are graphed as percentage. *p < 0.05; † p < 0.01; ‡ p < 0.001 (p‐values by χ 2)
Table 3.
Findings from a physical exam of 576 Venezuelan patients with confirmed SARS‐CoV‐2 infection
| Physical exam | All (N = 576) | Mild/moderate disease (N = 414) | Severe/critical disease (N = 162) | p‐value |
|---|---|---|---|---|
| Hemodynamic parameters | ||||
| Heart rate, median [IQR], bpm | 89 [21] | 87 [20] | 98 [24] | <0.001* |
| Breathing frequency, median [IQR], rpm | 18 [4] | 16 [2] | 22 [6] | <0.001* |
| Systolic blood pressure, median [IQR], mmHg | 118 [20] | 115 [18] | 120 [20] | 0.04* |
| Diastolic blood pressure, median [IQR], mmHg | 72 [10] | 72 [10] | 73 [10] | 0.99* |
| Oxygen saturation, median [IQR], % | 96 [5] | 97 [2] | 89 [9] | <0.001* |
| Altered chest physical exam, yes/no (%) | 250/318 (44.8/55.2) | 127/287 (30.7/69.3) | 131/31 (80.9/19.1) | <0.001† |
| Pathological findings, n (%) | <0.001†, ‡ | |||
| Breathing sounds decreased bilaterally | 83 (32.2) | 46 (36.2) | 37 (28.2) | |
| Bilateral crackles | 62 (24) | 11 (8.7) | 51 (38.9) | |
| Decreased right breath sounds | 27 (10.5) | 20 (15.7) | 7 (5.3) | |
| Bilateral roncus | 27 (10.5) | 18 (14.2) | 9 (6.9) | |
| Crackling rights | 22 (8.5) | 12 (9.4) | 10 (7.6) | |
| Crackles left | 12 (4.7) | 7 (5.5) | 5 (3.8) | |
| Decreased left breath sounds | 10 (3.9) | 9 (7.1) | 1 (0.8) | |
| Bilateral wheezing | 5 (1.9) | 1 (0.8) | 4 (3.1) | |
| Intercostal pull | 4 (1.6) | 1 (0.8) | 3 (2.3) | |
| Roncus and bilateral crackles | 3 (1.2) | 1 (0.8) | 2 (1.5) | |
| Roncus rights | 3 (1.2) | 1 (0.8) | 2 (1.5) | |
| Altered neurological status, n (%) | 9 (1.6) | 2 (0.5) | 7 (4.3) | 0.001† |
Abbreviations: IQR, interquartile range; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Median test.
Pearson's χ 2 test.
Post hoc analysis: Significant association only between bilateral crackles with severe/critical disease (standardized residual = 3.5).
3.4. Paraclinical findings
Table 4 shows paraclinical findings. Hemoglobin levels and platelet count means were similar in both groups. White blood cell count and absolute neutrophil value medians were higher in severe/critical disease patients compared with mild/moderate disease, while absolutes lymphocytes, monocytes, and basophils values medians were higher in mild/moderate disease patients compared with severe/critical disease. In blood chemistry, higher levels of glycemia, urea, aminotransferases (AST —aspartate aminotransferase— and ALT —alanine aminotransferase—), total bilirubin, lactate dehydrogenase (LDH), erythrocyte sedimentation rate (ESR) were observed in severe/critical disease patients compared with mild/moderate disease, which was statistically significant (Table 4). No significant differences were found in alkaline phosphatase, cardiac biomarkers (CK —creatine kinase— and CKMB —creatine kinase MB—), creatinine, and total and fractionated proteins (data not shown). Finally, there were no relevant alterations in electrolytes except for chlorine, showing lower levels in severe/critical disease patients compared with mild/moderate disease (p = 0.003) (Table 4).
Table 4.
Paraclinical findings of 576 Venezuelan patients with confirmed SARS‐CoV‐2 infection
| Paraclinical findings | All (N = 576) | Mild/moderate disease (N = 414) | Severe/critical disease (N = 162) | P‐value |
|---|---|---|---|---|
| Hemoglobin, median [IQR], g/dL | 13.9 [2.1] | 13.9 [2] | 13.7 [2.4] | 0.24* |
| Hematocrit, median [IQR], % | 42.5 [6.2] | 42.6 [6.6] | 41.85 [6.2] | 0.16* |
| White blood cells, median [IQR], ×103/ml | 5.73 [2.9] | 5.33 [2.4] | 7.18 [4.52] | <0.001* |
| Neutrophils, median [IQR], ×103/ml | 3.76 [2.85] | 3.3 [2.1] | 5.5 [4.29] | <0.001* |
| Lymphocytes, median [IQR], ×103/ml | 1.28 [0.95] | 1.49 [0.93] | 0.88 [0.56] | <0.001* |
| Monocytes, median [IQR], ×103/ml | 0.29 [0.19] | 0.31 [0.2] | 0.26 [0.18] | 0.038* |
| Eosinophils, median [IQR], ×103/ml | 0.04 [0.06] | 0.05 [0.07] | 0.04 [0.05] | 0.072* |
| Basophils, median [IQR], ×103/ml | 0.02 [0.02] | 0.02 [0.02] | 0.01 [0.01] | <0.001† |
| Platelets, median [IQR], ×103/ml | 190 [80] | 191 [77.5] | 186 [85] | 0.77* |
| Glycemia, median [IQR], mg/dL | 89 [29] | 86 [22] | 108 [55] | <0.001* |
| Urea, mean (SD), mg/dL | 31.23 (22.58) | 27.77 (17.87) | 41.26 (30.6) | <0.001† |
| Creatinine, median [IQR], mg/dL | 0.8 [0.3] | 0.8 [0.3] | 0.9 [0.4] | 0.08* |
| AST, median [IQR], U/L | 27 [20] | 25 [14] | 48 [40] | <0.001* |
| ALT, mean (SD), U/L | 38.85 (37.35) | 31.96 (29.18) | 58.52 (49.55) | <0.001† |
| Albumins, mean (SD), g/L | 4.31 (0.55) | 4.47 (0.47) | 3.82 (0.47) | 0.48† |
| Total bilirubin, mean (SD), mg/dL | 0.62 (0.33) | 0.58 (0.29) | 0.78 (0.41) | 0.003† |
| Chloride, mean (SD), mEq/L | 101.56 (4.54) | 102.23 (3.03) | 99.54 (7.12) | 0.003† |
| LDH, median [IQR], U/L | 265 [175] | 241.5 [80] | 426 [160] | <0.001* |
| ESR, median [IQR], mm/h | 26 [43] | 20 [26] | 60 [50] | <0.001* |
| CRP, median [IQR], mg/L | 2 [17] | 0.5 [15] | 22 [6] | 0.08* |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; ERS, erythrocyte sedimentation rate; IQR, interquartile range; LDH, lactate dehydrogenase; SD, standard deviation.
Median test.
Student's t‐test.
3.5. Rate of hospitalizations and mortality
Of the 162 patients with severe/critical disease criteria, only 68 (42%) patients were hospitalized in the University Hospital of Caracas, of which 28 died (in‐hospital mortality of 41.7%), and 94 (58%) were referred to another institution, due to lack of bed availability. Finally, a total of 44 of the 576 (7.6%) that were followed during the study period died.
4. DISCUSSION
To the best of our knowledge, our study is the first to assess the epidemiological, clinical, and paraclinical characteristics of COVID‐19 patients in Venezuela. We retrospectively analyzed data from 576 patients with confirmed SARS‐CoV‐2 infection by RT‐PCR, who attended a main reference hospital of Caracas with COVID‐19 during the first 5 months of the pandemic.
Here, the mean age and male predominance of patients confirmed with SARS‐CoV‐2 infection were similar to those reported by other authors in Ecuador, 21 Peru, 25 Chile, 26 Mexico, 27 Europe, 28 and China. 29 , 30 Likewise, advanced age was associated with a worse outcome of the disease, in accordance with other studies. 31 , 32 , 33 We found a significant association between lower levels of schooling and severe/critical disease, suggesting that the educational level could be a determining factor in the knowledge of the disease and the consultation time in the presence of suspicious symptoms of COVID‐19. This could also be evidenced in bigger cohort studies from the United States where the mortality in people without a high school degree and black residents was higher when compared with the rest of the population. 34 This notorious difference could be based mainly on the possibility of access to a healthcare institution and education regarding the alarming symptoms, which the patient should be aware of to get medical attention. 34 , 35
In our study, severe/critical disease patients had a higher proportion of systemic arterial hypertension and diabetes mellitus, consistent with recent publications, 29 , 30 , 31 , 33 , 36 , 37 , 38 , 39 , 40 suggesting that patients with these comorbidities are at increased risk of developing severe/critical disease. According to previous studies in the United States, 41 Europe, 42 , 43 and Asia, 29 , 30 , 31 , 37 , 44 , 45 , 46 , 47 predominant symptoms of SARS‐CoV‐2 disease were fever, dry cough, and asthenia, while gastrointestinal symptoms were rare. Unlike these studies, we found that headache was frequent, which is consistent with a recent report in Chile, 48 where the predominant symptom was a headache. This may suggest a potential neurotropic and neurovirulent effect of SARS‐CoV‐2. A study carried out in China, 49 reported that, compared to mild disease patients, severe disease patients had a higher incidence of dyspnea, similar to what was found in our study. Dyspnea is related to severe alveolar damage in the severe/critical group, and the appearance of this symptom could help health personnel identify and anticipate disease severity in clinical practice.
Regarding laboratory findings, the differential white blood cell counts proved to be an excellent, fast, and inexpensive parameter, to discriminate patients with mild/moderate disease from severe/critical due to COVID‐19. 50 , 51 In severe/critical disease patients, there is a tendency for neutrophilia and lymphopenia, as demonstrated in our study. Lymphocyte counts less than 0.8 × 109/L have been associated with disease severity; conversely, neutrophil counts greater than 3.5 × 109/L have been associated with a worse prognosis for COVID‐19 patients in China, 50 , 52 , 53 , 54 as well as in Latin American countries, like Brazil 39 and Mexico. 55 In limited‐resource settings, the white blood cell count can be a key prognostic factor in the evaluation of COVID‐19 patients. In our study, higher levels of blood glucose, urea, aminotransferases (AST and ALT), total bilirubin, LDH, and ESR were observed in severe/critical disease patients compared to mild/moderate disease, similar to other studies in patients with severe disease in China. 29 , 56 , 57 Elevation of LDH has been identified as a marker of lung damage in previous studies, 58 with a direct relationship between the decrease in LDH and the clearance of viral mRNA, associating its decrease with a better prognosis when faced with disease, 53 and its increase as a risk factor for death in COVID‐19 patients. 55 Likewise, the elevation of liver enzymes has been linked to damage to other organs and mediation of systemic response in severe disease. 29 Hyponatremia, hypernatremia, hypokalemia, hypocalcemia, and hypochloremia are the most common electrolyte disorders among COVID‐19 patients, 59 each one associated with higher intensive care unit requirement, mechanical ventilation requirement, and mortality. 59 The study of these laboratory parameters not only guides us in the diagnosis and therapy but also allows inferring disease prognosis. 54
Allocating healthcare resources for severe/critical disease patients has represented a difficult ethical dilemma during this pandemic as shortages for beds or equipment has been increasing worldwide. 21 , 60 , 61 Here we provided evidence that less than half of the patients requiring care were admitted to the Caracas University Hospital. Unfortunately, it was not possible to ensure admission to other institutions after being referred; therefore, many severe/critical disease patients achieved admission only after several attempts or in some cases had to return home, which could have influenced a higher mortality rate on those patients. Sentinel hospitals such as the Caracas University Hospital should reflect on their capacity through Venezuela's first wave and find ways to improve preparedness despite scarce resources. The overall in‐hospital mortality was 41.7%, consistent with studies in the region, such as Colombia (51%), 62 Honduras (42.8%), 63 and Brazil (38%). 22 However, our reported in‐hospital mortality was higher than mortality in high‐income countries such as the United Kingdom (26%), 64 Germany (22%), 65 and the United States (21.4%) 66 ; these results are most possibly related to the direct association between low availability of hospital resources and in‐hospital mortality. 67 On July 24, 2020, according to the nationally televised presidential address, Venezuela documented 14 263 cases, with 4861 hospitalized patients and 134 deceased of COVID‐19, reporting overall mortality of 0.9%. 68 In contrast, of the 576 patients who were diagnosed by the Caracas University Hospital, forty‐four patients died (7.6%), representing almost a third of the deaths nationwide, suggesting a significant underreporting of deaths in Venezuela.
This study has several important limitations. First, our results are based on a relatively small number of COVID‐19 patients coming from one hospital; however, this number represented an important proportion of the cases in the country by the time of the study. Additionally, the University Hospital of Caracas has been the main sentinel hospital attending COVID‐19 patients in the country's capital, which accumulates the highest number of cases to date. Second, the patient's daily clinical and long‐term follow‐up, consecutive RT‐PCR tests to assess viral clearance was not performed. Finally, due to the retrospective study design, not all laboratory tests were done in all patients and the number of radiological studies was limited.
5. CONCLUSIONS
This report describes the clinical and epidemiological characteristics of the first confirmed COVID‐19 cases in Caracas, Venezuela. Male sex, advanced age, low education, and presence of hypertension or diabetes, are factors associated with severe/critical disease in the patients studied. In addition, the presence of dyspnea and wet cough, low levels of oxygen saturation, bilateral crackles on pulmonary auscultation, as well as higher levels of glycemia, urea, aminotransferases, total bilirubin, lactate dehydrogenase, and erythrocyte sedimentation rate were associated with worse clinical outcome. Our data help to understand the impact the pandemic has had on the country during its first epidemic wave. In addition, to assist future analysis of the COVID‐19 response in the first wave of the pandemic in countries worst affected by this pandemic. One relevant finding is that mortality recorded in this study suggests a significant underreporting of deaths at the national level. While the pandemic is still raging and vaccines rollout are underway in Venezuela, infections and deaths continue to increase and health infrastructure has started to strain. In October 2021, the number of deaths due to respiratory infections reported in public hospitals almost doubled that observed during the first peak in August 2020 (unpublished data). We caution that unless steps are taken to restructure the failing public health infrastructure in Venezuela and the vaccine campaign be accelerated, the death toll due to COVID‐19 is expected to increase in the following months.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
The study protocol was reviewed and approved by the National Center for Bioethics (CENABI, in Spanish) of Venezuela (CIBI‐CENABI‐08/2020). The information was collected according to the Helsinki Convention and the Venezuelan regulations for this kind of research.
AUTHOR CONTRIBUTIONS
David A. Forero‐Peña, Fhabián S. Carrión‐Nessi, Daniela L. Mendoza‐Millán, Óscar D. Omaña‐Ávila, and María E. Grillet conceived and designed the study. David A. Forero‐Peña, Daniela L. Mendoza‐Millán, Óscar D. Omaña‐Ávila, Mario D. Mejía‐Bernard, Natasha A. Camejo‐Ávila, David M. Flora‐Noda, Viledy L. Velásquez, Fabián R. Chacón‐Labrador, Juan M. Doval‐Fernández, Andrea L. Maricuto, Verónica I. Rodríguez, and Mariana B. Contreras collected clinical data. David A. Forero‐Peña, FSC‐N, Daniela L. Mendoza‐Millán, Óscar D. Omaña‐Ávila, Mario D. Mejía‐Bernard, Natasha A. Camejo‐Ávila, María E. Grillet, Juan V. Hernández‐Villena, María F. Vincenti‐González, Alberto E. Paniz‐Mondolfi, and José Orejas analyzed and interpreted the data. David A. Forero‐Peña, Fhabián S. Carrión‐Nessi, Daniela L. Mendoza‐Millán, Óscar D. Omaña‐Ávila, Mario D. Mejía‐Bernard, Natasha A. Camejo‐Ávila, María E. Grillet, Juan V. Hernández‐Villena, María F. Vincenti‐González, Alberto E. Paniz‐Mondolfi, and José Orejas wrote the manuscript. María E. Grillet, Juan V. Hernández‐Villena, María F. Vincenti‐González, Alberto E. Paniz‐Mondolfi, José Orejas, Rafael N. Guevara, Martín Carballo, Jocays Caldera, María C. Redondo, and María E. Landaeta critically reviewed the manuscript. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank patients, healthcare workers from Infectious Disease Department, Caracas University Hospital, and research staff involved in the work. This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Forero‐Peña DA, Carrión‐Nessi FS, Mendoza‐Millán DL, et al. First wave of COVID‐19 in Venezuela: Epidemiological, clinical, and paraclinical characteristics of first cases. J Med Virol. 2022;94:1175‐1185. 10.1002/jmv.27449
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Karlinsky A, Kobak D. Tracking excess mortality across countries during the COVID‐19 pandemic with the World Mortality Dataset. eLife. 2021:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The L. COVID‐19 in Latin America‐emergency and opportunity. Lancet. 2021;398(10295):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Litewka SG, Heitman E. Latin American healthcare systems in times of pandemic. Dev World Bioeth. 2020;20(2):69‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grillet ME, Hernández‐Villena JV, Llewellyn MS, et al. Venezuela's humanitarian crisis, resurgence of vector‐borne diseases, and implications for spillover in the region. Lancet Infect Dis. 2019;19(5):e149‐e161. [DOI] [PubMed] [Google Scholar]
- 5. Paniz‐Mondolfi AE, Sordillo EM, Márquez‐Colmenarez MC, Delgado‐Noguera LA, Rodriguez‐Morales AJ. The arrival of SARS‐CoV‐2 in Venezuela. Lancet. 2020;395(10236):e85‐e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lampo M, Hernández‐Villena JV, Cascante J, et al. Signatures of the Venezuelan humanitarian crisis in the first wave of COVID‐19: fuel shortages and border migration. Vaccines. 2021;9(7):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loyo ESL, González MJ, Esparza J. Venezuela is collapsing without COVID‐19 vaccines. Lancet. 2021;397(10287):1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Urrunaga‐Pastor D, Bendezu‐Quispe G, Herrera‐Añazco P, et al. Cross‐sectional analysis of COVID‐19 vaccine intention, perceptions and hesitancy across Latin America and the Caribbean. Travel Med Infect Dis. 2021;41:102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salud Mpl. Encuesta Nacional de Hospitales. Accessed May 14, 2020. https://www.encuestanacionaldehospitales.com/
- 10. Daniels JP. Venezuelan migrants "struggling to survive" amid COVID‐19. Lancet. 2020;395(10229):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The L. The unfolding migrant crisis in Latin America. Lancet. 2019;394(10213):1966. [DOI] [PubMed] [Google Scholar]
- 12. Fernández‐Niño JA, Cubillos‐Novella A, Bojórquez I, Rodríguez M. Recommendations for the response against COVID‐19 in migratory contexts under a closed border: The case of Colombia. Biomedica. 2020;40(Supl. 2):68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazuera‐Arias R, Albornoz‐Arias N, Cuberos M‐A, Vivas‐García M, Morffe Peraza MÁ. Sociodemographic Profiles and the Causes of Regular Venezuelan Emigration. International Migration. 2020;58(5):164‐182. [Google Scholar]
- 14. Patiño LH, Ballesteros N, Muñoz M, et al. SARS‐CoV‐2 in transit: characterization of SARS‐CoV‐2 genomes from Venezuelan migrants in Colombia. Int J Infect Dis. 2021;110:410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodriguez‐Morales AJ, Gallego V, Escalera‐Antezana JP, et al. COVID‐19 in Latin America: the implications of the first confirmed case in Brazil. Travel Med Infect Dis. 2020;35:101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramírez JD, Florez C, Muñoz M, et al. The arrival and spread of SARS‐CoV‐2 in Colombia. J Med Virol. 2021;93(2):1158‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Whittaker C, Watson OJ, Alvarez‐Moreno C, et al. Understanding the potential impact of different drug properties on SARS‐CoV‐2 transmission and disease burden: a modelling analysis. Clin Infect Dis. 2021:ciab837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Forero‐Peña DA, Carrión‐Nessi FS, Camejo‐Ávila NA, Forero‐Peña MJ. COVID‐19 en Latinoamérica: una revisión sistemática de la literatura y análisis bibliométrico. Rev Salud Pública. 2020;22(2):1‐7.e216. [DOI] [PubMed] [Google Scholar]
- 19. de Souza WM, Buss LF, Candido DDS, et al. Epidemiological and clinical characteristics of the COVID‐19 epidemic in Brazil. Nat Hum Behav. 2020;4(8):856‐865. [DOI] [PubMed] [Google Scholar]
- 20. Clark A, Jit M, Warren‐Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID‐19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003‐e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ortiz‐Prado E, Simbaña‐Rivera K, Barreno LG, et al. Epidemiological, socio‐demographic and clinical features of the early phase of the COVID‐19 epidemic in Ecuador. PLoS Negl Trop Dis. 2021;15(1):e0008958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ranzani OT, Bastos LSL, Gelli JGM, et al. Characterisation of the first 250,000 hospital admissions for COVID‐19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir Med. 2021;9(4):407‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance: Geneva: January 28, 2020.
- 24. NIH . COVID‐19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines. Accessed December 21, 2020. https://www.covid19treatmentguidelines.nih.gov/
- 25. Munayco C, Chowell G, Tariq A, Undurraga EA, Mizumoto K. Risk of death by age and gender from CoVID‐19 in Peru, March‐May, 2020. Aging. 2020;12(14):13869‐13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashktorab H, Pizuomo A, González NAF, et al. A comprehensive analysis of COVID‐19 impact in Latin America. Res Sq. 2021:rs‐141245. [Google Scholar]
- 27. Ñamendys‐Silva SA, Alvarado‐Ávila PE, Domínguez‐Cherit G, et al. Outcomes of patients with COVID‐19 in the intensive care unit in Mexico: A multicenter observational study. Heart Lung. 2021;50(1):28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl‐Jacobsen R. Sex and age differences in COVID‐19 mortality in Europe. Wien Klin Wochenschr. 2021;133(7‐8):393‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. CDC COVID‐19 Response Team . Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hawkins RB, Charles EJ, Mehaffey JH. Socio‐economic status and COVID‐19‐related cases and fatalities. Public Health. 2020;189:129‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Selden TM, Berdahl TA. COVID‐19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff (Millwood). 2020;39(9):1624‐1632. [DOI] [PubMed] [Google Scholar]
- 36. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. [DOI] [PubMed] [Google Scholar]
- 37. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133(9):1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marcolino MS, Ziegelmann PK, Souza‐Silva MVR, et al. Clinical characteristics and outcomes of patients hospitalized with COVID‐19 in Brazil: Results from the Brazilian COVID‐19 registry. Int J Infect Dis. 2021;107:300‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schönfeld D, Arias S, Bossio JC, Fernández H, Gozal D, Pérez‐Chada D. Clinical presentation and outcomes of the first patients with COVID‐19 in Argentina: Results of 207079 cases from a national database. PLoS One. 2021;16(2):e0246793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical Characteristics of Covid‐19 in New York City. N Engl J Med. 2020;382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Colaneri M, Sacchi P, Zuccaro V, et al. Clinical characteristics of coronavirus disease (COVID‐19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveill. 2020;25(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tomlins J, Hamilton F, Gunning S, Sheehy C, Moran E, MacGowan A. Clinical features of 95 sequential hospitalised patients with novel coronavirus 2019 disease (COVID‐19), the first UK cohort. J Infect. 2020;81(2):e59‐e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang H, Kang Z, Gong H, et al. Digestive system is a potential route of COVID‐19: an analysis of single‐cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69(6):1010‐1018. [Google Scholar]
- 46. Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092‐1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 48. Rodriguez‐Morales AJ, Rodriguez‐Morales AG, Méndez CA, Hernández‐Botero S. Tracing new clinical manifestations in patients with COVID‐19 in Chile and its potential relationship with the SARS‐CoV‐2 divergence. Curr Trop Med Rep. 2020:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest Radiol. 2020;55(6):327‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang T, Du Z, Zhu F, et al. Comorbidities and multi‐organ injuries in the treatment of COVID‐19. Lancet. 2020;395(10228):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID‐19 infected discharged patients. Inflamm Res. 2020;69(6):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pourbagheri‐Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID‐19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Del Carpio‐Orantes L, García‐Méndez S, Contreras‐Sánchez ER, et al. Caracterización clínica y del hemograma de pacientes con neumonía por COVID‐19 en Veracruz. México. Revista de Hematología. 2020;21(4):205‐209. [Google Scholar]
- 56. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China: Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jurisic V, Radenkovic S, Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:115‐124. [DOI] [PubMed] [Google Scholar]
- 59. Pourfridoni M, Abbasnia SM, Shafaei F, Razaviyan J, Heidari‐Soureshjani R. Fluid and electrolyte disturbances in COVID‐19 and their complications. BioMed Res Int. 2021;2021:6667047‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. White DB, Lo B. A framework for rationing ventilators and critical care beds during the COVID‐19 pandemic. JAMA. 2020;323(18):1773‐1774. [DOI] [PubMed] [Google Scholar]
- 61. Carenzo L, Costantini E, Greco M, et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID‐19 outbreak in Italy. Anaesthesia. 2020;75(7):928‐934. [DOI] [PubMed] [Google Scholar]
- 62. García‐Posada M, Aruachan‐Vesga S, Mestra D, et al. Clinical outcomes of patients hospitalized for COVID‐19 and evidence‐based on the pharmacological management reduce mortality in a region of the Colombian Caribbean. J Infect Public Health. 2021;14(6):696‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zuniga‐Moya JC, Norwood DA, Romero Reyes LE, et al. Epidemiology, outcomes, and associated factors of coronavirus disease 2019 (COVID‐19) reverse transcriptase polymerase chain reaction‐confirmed cases in the San Pedro Sula metropolitan area, Honduras. Clin Infect Dis. 2021;72(10):e476‐e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID‐19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fried MW, Crawford JM, Mospan AR, et al. Patient characteristics and outcomes of 11 721 patients with coronavirus disease 2019 (COVID‐19) hospitalized across the United States. Clin Infect Dis. 2021;72(10):e558‐e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Machado FR, Cavalcanti AB, Bozza FA, et al. The epidemiology of sepsis in Brazilian intensive care units (the Sepsis PREvalence Assessment Database, SPREAD): an observational study. Lancet Infect Dis. 2017;17(11):1180‐1189. [DOI] [PubMed] [Google Scholar]
- 68. VTV . Cifra de pacientes positivos de COVID‐19 se ubica en 14.263, de los cuales 8.127 se han recuperado. 2020; https://www.finanzasdigital.com/2020/03/gaceta-oficial-extraordinaria-n6-519-se-decreta-el-estado-de-alarma-en-todo-el-territorio-nacional-por-epidemia-del-coronavirus-covid-19/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


