Abstract
Coronavirus disease 2019 (Covid‐19) is the illness caused by severe acute respiratory syndrome coronavirus 2, first identified in Wuhan, China at the end of 2019. On March 11, 2020, the World Health Organization declared Covid‐19 a global pandemic. The objective of this study is to determine the role of interleukin (IL) inhibitors in the treatment of Covid‐19. By the majority of the reported clinical studies, the use of tocilizumab in Covid‐19 infection appears promising in specific cases of the cytokine storm. Conflicting results prevent the recommendation of IL inhibitors against Covid‐19 infection by many health organizations. However, many low‐case fatality rate countries, with more advanced therapeutic approaches, uniformly include the use of tocilizumab in case of cytokine storms in addition to the standard treatment for severe cases which includes antivirals. Neglecting the other components is likely an explanation for the contradictory results found in the literature.
Keywords: Covid‐19, IL‐1, IL‐6, immunosuppressive agents, interleukin Inhibitors, tocilizumab
Highlights
Tocilizumab use in Covid‐19 infection is here reviewed.
By the majority of clinical studies, Tocilizumab helps in specific cases of cytokine storm.
However, conflicting results exist preventing recommendations by many health organizations.
The use of Tocilizumab for cytokine storms as part of treatment which includes antivirals generally positive.
Neglecting the administration of antivirals is likely an explanation for contradictory results.
1. INTRODUCTION
Tocilizumab, a recombinant humanized monoclonal anti‐interleukin (IL)‐6 antibody targeting the human IL‐6 receptor (IL‐6R), is a promising anti‐inflammatory reagent in the treatment of coronavirus disease 2019 (Covid‐19) but the results are mixed results from clinical trials. A randomized, double‐blind, placebo‐controlled trial 1 shows that tocilizumab has no efficacy for improving statement of treatment in moderately hospitalized patients infected with severe acute respiratory syndrome coronavirus 2. 2 Tocilizumab and sarilumab, an IL‐6R humanized monoclonal antibody, have been evaluated in clinical trials 3 , 4 , 5 by decreasing IL‐6 level to decrease the risk of mortality caused by Covid‐19. 6
IL inhibitors restrain the action of IL which is a group of cytokines that are synthesized by lymphocytes, monocytes, macrophages, and other cells. The IL controls the immune system. Patients with serious Covid‐19 infections may suffer from a release of cytokines which may damage the lung tissues. IL inhibitors may reduce this damage. 7 A severe Covid‐19 cytokine storm is characterized by the release of IL‐1, IL‐6, IL‐12, and IL‐18, tumor necrosis factor‐α (TNF‐α), and other inflammatory mediators. The amplified pulmonary inflammatory response may result in enlarged alveolar‐capillary gas exchange making oxygenation difficult. 7
Many works have argued in favor of IL inhibitors' use for severe Covid‐19 infection. Zhang et al. 8 are one of the first works arguing for this use from China (published in March 2020). Severe Covid‐19 infection patients suffer from cytokine release syndrome (CRS). The IL‐6R antagonist tocilizumab is proposed for the specific case. According to Arnaldez et al., 9 acute respiratory distress syndrome (ARDS) is often accompanied by a pathological immune reaction, with a significant increment of serum cytokines, especially interferon‐γ, TNF‐α, IL‐17, IL‐8, and IL‐6, administration to critically ill patients of cytokine‐modulatory therapies, mostly IL‐6 inhibitors, is warranted.
Similarly, Nasonov and Samsonov 10 notice as hyperimmune activation is a key factor in severe Covid‐19 infection. As IL‐6 increase is associated with poor prognosis and progression, they argue that IL‐6 inhibition may improve the patients conditions. Liu et al. 11 suggest therapeutic targeting of CRS, and reducing key cytokines such as IL‐6 may, thus, help with CRS.
As nonsurvival in ARDS is linked to elevated IL‐6 and IL‐1 concentration, thus, anticytokine therapy may help with intrapulmonary macrophage activation and pulmonary vascular disease. 12 IL‐6, along with some other inflammatory cytokines, including IL‐1β, IL‐8, and TNF‐α, as well as inflammatory chemokines, can significantly contribute to fever, lymphopenia, coagulation, lung injury, and multiorgan failure. 13
Here, we summarize the use of IL inhibitors for Covid‐19 infection based on the few peer‐reviewed, accepted, and published papers at the time of reviewing.
2. METHOD
Literature review of the published literature regarding the use of Tocilizumab for COVID‐19 infection.
3. RESULTS
Few works have been proposed in the literature reporting about the use of IL inhibitors for Covid‐19 infection. These works are here subdivided into IL‐6 and IL‐1 or IL‐7.
Most of the works cited in Medscape 7 deal with IL‐6. This is a pleiotropic proinflammatory cytokine. Covid‐19 infection provokes the production of IL‐6 from bronchial epithelial cells. 14 As of August 2020, the National Institutes of Health (NIH) 15 was not for or against the use of IL‐6 inhibitors because of a lack of data. In April 2021, the statement has not been updated. However, the NIH has recently (March 2021) issued a positive recommendation about the use of the IL‐6 inhibitor tocilizumab 16 as discussed later.
Regeneron and Sanofi 17 started in March 2020 a randomized, double‐blind, placebo‐controlled phase 2/3 trial of the IL‐6 inhibitor sarilumab (Kevzara). The first part recruited patients with severe Covid‐19 infection to evaluate the effect of sarilumab on fever and the need for supplemental oxygen. The second part of the trial evaluated improvement in longer‐term outcomes, including preventing death and reducing the need for mechanical ventilation, supplemental oxygen, and/or hospitalization. 17 Minor positive trends statistically insignificant were observed in critical patients on sarilumab 400 mg who were mechanically ventilated at baseline. These were countered by negative trends in a subgroup of critical patients who were not mechanically ventilated at baseline. 18 The US‐based trial was stopped, including a cohort of patients receiving 800 mg. 19
Tocilizumab (Actemra), another IL‐6 inhibitor, was part of other randomized, double‐blind, placebo‐controlled phase 3 clinical trials. These trials were the REMDACTA, COVACTA, and EMPACTA trials. Tocilizumab plus standard of care was compared to placebo plus standard of care in patients with severe Covid‐19 pneumonia.
In the EMPACTA trial, patients who received tocilizumab in the first 2 days of intensive care unit (ICU) admission had a lower risk of mortality. The 30‐day mortality was 27.5% in the tocilizumab‐treated patients and 37.1% in the non‐tocilizumab‐treated patients. 20 In the BACC Bay trial, tocilizumab was not effective in preventing intubation or death in patients with moderate Covid‐19 infection, but the confidence interval was too wide, to infer any proper conclusion. 21 In the COVACTA trial, both improved clinical status or reduced mortality in patients with Covid‐19 pneumonia were not reached. There was, however, a positive trend in time to discharge from hospital with tocilizumab. 22
Patients with severe Covid‐19 infection in an observational study conducted at Yale were treated with a standardized algorithm that comprised tocilizumab to deal with cytokine release disease. Tocilizumab‐treated patients were 90% of those with severe disease. Their survival rate was similar to those of patients with the nonsevere disease. The survival rate was 75% in tocilizumab‐treated patients necessitating mechanical ventilation. While some oxygenation and inflammatory biomarkers improved, others increased significantly. 23
A single 400‐mg iv dose of tocilizumab reduced inflammation, oxygen requirements, vasopressor support, and mortality in a very small compassionate study. 24
Tocilizumab was associated with a 45% reduction in the hazard of death in a small study 25 of Covid‐19 patients requiring mechanical ventilation. An increased incidence of superinfections was associated with tocilizumab. There was no difference in the 28‐day case fatality rate of tocilizumab‐treated patients with or without superinfection. 25
In a small study, solid organ transplant recipients received tocilizumab. Mortality at 90 days was significantly higher for those who received tocilizumab compared to control. 26
An observational study in New Jersey found an improved survival rate of patients who received tocilizumab compared with those who did not receive it. 27
An observational study in tertiary care centers of northern Italy concluded that tocilizumab may reduce the risk of invasive mechanical ventilation or death in patients with severe Covid‐19 pneumonia. 28 In another contemporary study from Italy, tocilizumab did not reduce severe respiratory symptoms, intensive care, or death compared with standard care. 29
In a small open‐label trial in France, tocilizumab‐treated patients did not reduce the World Health Organization‐combined positive score scores lower than 5 at Day 4. On Day 14, marginally fewer patients in noninvasive ventilation or mechanical ventilation died in the tocilizumab group. No difference in death at Day 28 was recorded between tocilizumab and usual care. 30
In an open‐label, noncontrolled, non‐peer‐reviewed study conducted in China, patients with severe Covid‐19 improved with lower oxygen requirements, lymphocyte counts returned to normal after tocilizumab treatment. 31
In a small retrospective review of patients with severe Covid‐19 who received tocilizumab plus antivirals, there was a decline in inflammatory markers, radiological improvement, and reduced ventilatory support. 32 No controls were used in this study.
Another clinical phase 3 trial (COV‐AID) with IL‐6 inhibitors (tocilizumab, siltuximab), an IL‐1 inhibitor (anakinra), or combining both is ongoing. 33 Another anti‐IL‐6R monoclonal antibody study is under development. 34
In between the observational studies, 35 reports observational data on IL‐6 receptor inhibitors (IL6ri). 35 The IL6ri therapy is associated with improved outcomes. The greatest benefits occurred with early therapy. According to Sinha et al., 35 IL6ri therapy is superior to treatment with remdesivir and dexamethasone.
Opposite conclusions are drawn from the observational study. 36 The incidence of fungal infections was significantly higher in patients who were given tocilizumab. Tocilizumab did not decrease in‐hospital mortality in the cohort.
Mixed results were proposed in the observational data. 37 More patients in the tocilizumab group reported fever, cough, and shortness of breath. There was nonstatistically significantly lower mortality in the tocilizumab group. When excluding intubated patients, there was statistically significant lower mortality in patients treated with tocilizumab. Bacteremia was more common in the control group, while fungemia was similar for both.
Crisafulli et al. 38 warn as the available clinical evidence for IL‐6 inhibitors are mostly limited to tocilizumab, and still not fully comprehensive. More robust clinical data are needed to support the efficacy of IL‐6 inhibitors. Crisafulli et al. 38 also warn about their potential toxicity.
The NIH has recently (March 2021) 16 issued a positive recommendation about the use of the IL‐6 inhibitor tocilizumab. On the basis of evidence from the REMAP‐CAP and RECOVERY trials, the use of tocilizumab in combination with dexamethasone in certain hospitalized patients who are exhibiting rapid respiratory decompensation. These patients are recently hospitalized, admitted to ICU within the prior 24 h and who require invasive mechanical ventilation, noninvasive mechanical ventilation or high‐flow nasal cannula oxygen, or not in the ICU with rapidly increasing oxygen needs who require noninvasive mechanical ventilation or high‐flow nasal cannula oxygen and have significantly increased markers of inflammation. The use for other patients is still controversial.
Medscape 7 also mentions IL‐1 inhibitors. Endogenous IL‐1 levels are elevated in individuals with Covid‐19 and other conditions, such as severe severe chimeric antigen receptor (CAR)‐T‐cell‐mediated cytokine‐release syndrome. The previously mentioned anakinra has been used for this indication. As of June 2020, the NIH notes insufficient data to recommend for or against the use of IL‐1 inhibitors. 39 The statement has not been updated up to April 2021.
A small retrospective study in Italy of patients with Covid‐19 and moderate‐to‐severe ARDS managed with noninvasive ventilation outside of the ICU evidenced some benefits in the use of anakinra plus standard treatment, at the time hydroxychloroquine and lopinavir/ritonavir. At 21 days, reductions in serum C‐reactive protein levels and progressive improvements in respiratory function, fewer patients on mechanical ventilation, and fewer patients died versus the standard treatment group. 40
A small study from France showed benefits in patients who were given anakinra in terms of admission to the ICU for invasive mechanical ventilation or death. 41
In another IL‐1 study, the phase 3 CAN‐COVID proposing canakinumab plus standard of care, the primary endpoint of a greater chance of survival without invasive mechanical ventilation, or the key secondary endpoint of reduced Covid‐19 mortality, were not met compared with standard of care. 42
Medscape 7 also mentions the opportunity of using IL‐7 inhibitors. The recombinant IL‐7 inhibitor, CYT107 (RevImmune), increases T‐cell production addressing immune exhaustion. Several phase‐2 clinical trials have been performed in France, Belgium, and the UK. 43 , 44 , 45
According to Laterre et al., 45 IL‐7 can be administered to critically ill patients without increasing inflammation or pulmonary injury addressing the dramatic reduction of lymphocytes associated with severe Covid‐19 infection. The IL‐7 reestablishment of lymphocyte numbers improves the activity of antiviral agents. 45
4. DISCUSSION
While IL inhibitors such as IL‐6 may certainly help to diminish hyperinflammation processes in patients with severe Covid‐19 infection, they may also have contraindications. This explains the contradictory results in terms of clinical outcomes.
Scherger et al. 46 notice as observational data support IL‐6 inhibition, as elevated IL‐6 levels are associated with poor clinical outcomes. However, IL‐6 also affects the response to infection. Clinical experiences with IL‐6 inhibitors, such as tocilizumab, show an increase in other infections.
Covid‐19 induces both impairment and hyperactivation of the immune system, 47 IL inhibitors may help or not almost in a case‐by‐case situation. Early viral clearance is a key to prevent further viral replication, and ultimately the cytokine storm, and as IL inhibitors are not antivirals, they are only part of a therapeutic approach. It depends on specific situations when IL inhibitors help or not, and they should be better used in conjunction with antiviral therapies. The recent (March 2021) 16 positive recommendation by the NIH about the use of the IL‐6 inhibitor tocilizumab demonstrates consensus about the specific cases where there is consensus about the use. In other cases, for example, hospitalized patients with hypoxemia who require conventional oxygen supplementation, and are treated with remdesivir, dexamethasone plus remdesivir, or dexamethasone alone, the addition of tocilizumab is still controversial. 16
In the best countries for therapeutic approaches, where the case fatality rate is much smaller also because of therapies, there are specific, successful, targeted uses of Tocilizumab. In Bahrein, 48 cases fatality rate 0.37%, they often use for severe pneumonia, or ARDS the “Triple Antiviral Therapy: Kaletra + Ribavirin + Interferon beta‐1b” or favipiravir. In these cases, tocilizumab is considered if fitting the criteria. It is suggested to avoid using tocilizumab and interferon in combination. Tocilizumab can be given in the presence of a severe cytokine storm. In the United Arab Emirates, 49 case fatality rate 0.30%, tocilizumab is also an option for severe pneumonia and ARDS, where they generally also use favipiravir + camostat ± nebulized interferon‐α or interferon‐β, or lopinavir–ritonavir ± ribavirin + nebulized interferon, or even remdesivir. Tocilizumab is to be considered in case of a cytokine storm.
There is drug‐induced hepatotoxicity to consider. Although tocilizumab, reversed liver injury during CRS that was induced by noninfectious means (i.e. CAR‐T cell therapy), 50 , 51 liver enzyme elevations are predominantly mild to moderate in severity and infrequently lead to treatment discontinuation. 52 The use of hepatotoxic drugs, including tocilizumab, has been established as a risk factor for liver dysfunction. 53 Tocilizumab has also been associated with abnormal AST and ALT levels in this setting. 54 , 55 According to the recent American Association for the Study of Liver Diseases guidelines, regular monitoring of liver function should be considered in all hospitalized Covid‐19 patients, in particular in those treated with tocilizumab, irrespective of the baseline value of liver biochemistry. 56 Furthermore, tocilizumab and other immunosuppressants used in the treatment of Covid‐19 can potentially induce liver injury via hepatitis B virus reactivation in patients with occult infections. 57 , 58 In respect to drug‐induced hepatotoxicity in Covid‐19, Muhović et al. 59 reported a 40‐fold rise in transaminases following two doses of tocilizumab which regressed 10 days later. Morena et al. 60 also reported elevated liver enzymes in 29% of patients who were receiving tocilizumab.
Tocilizumab‐induced immune‐related functional pathways are discussed in different references such as Catanzaro et al. 61 or Sullivan and Weber. 62 The pathways of Catanzaro et al. 61 are summarized in Figure 1.
Figure 1.
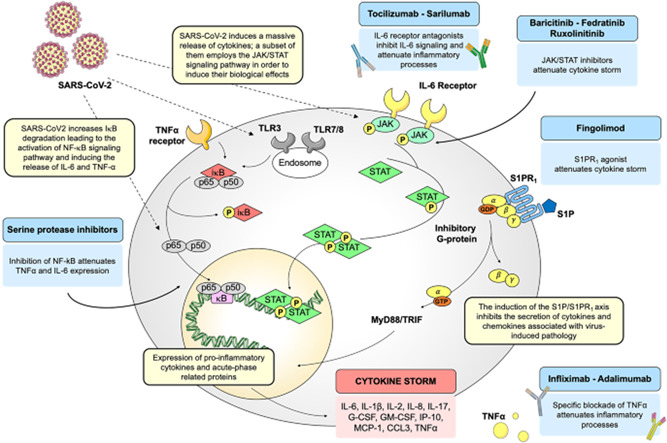
Schematic representation of SARS‐CoV‐2‐driven signaling pathways and potential drug targets. Images reproduced from Catanzaro et al. 61 This article is licensed under a creative commons attribution 4.0 international license. http://creativecommons.org/licenses/by/4.0/. GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; IL‐6, interleukin 6; JAK, Janus kinase; NF‐κB, nuclear factor‐κB; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; STAT, signal transducer and activator of transcription; TNF‐α, tumor necrosis factor‐α
5. CONCLUSION
It is written that tocilizumab has not been proven to be safe and effective against Covid‐19 infection, and the data are insufficient to recommend the use. Though, it must be noted as the data are also insufficient to recommend against the use of Tocilizumab. It is, however, improper to consider tocilizumab a cure for Covid‐19 infection. Tocilizumab is only a therapy helpful to inhibit ILs adjunctive to antivirals. Tocilizumab has had so far a very specific, positive application to specific cases of a cytokine storm; however, better when in conjunction with other therapies and not in isolation. This at least partially explains the inconsistent results found in the literature. Physicians should independently use the available evidence as a guide and be contingent on clinical and scientific judgment individualizing the care. When this happens, tocilizumab has its field of application within an ICU setting.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Alberto Boretti and Bimal Banik contributed to the writing of the manuscript.
Boretti A, Banik B. Modulation of Covid‐19 cytokine storm by tocilizumab. J Med Virol. 2022;94:823‐828. 10.1002/jmv.27380
DATA AVAILABILITY STATEMENT
The manuscript reviews published information. No new data has been generated during this study.
REFERENCES
- 1.Efficacy of tocilizumab on patients with COVID‐19. https://clinicaltrials.gov/ct2/show/NCT04356937 Accessed October 1, 2021.
- 2. Stone JH, Frigault MJ, Serling‐Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383(24):2333‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treatment of COVID‐19 patients with anti‐interleukin drugs (COV‐AID). https://clinicaltrials.gov/ct2/show/NCT04330638. Accessed October 1, 2021
- 4.Anti‐IL6 and corticosteroid monotherapy vs combination in COVID‐19. https://clinicaltrials.gov/ct2/show/NCT04486521 Accessed October 1, 2021.
- 5.Efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID‐19 pneumonia. https://clinicaltrials.gov/ct2/show/NCT04329650 Accessed October 1, 2021.
- 6. Castelnovo L, Tamburello A, Lurati A, et al. Anti‐IL6 treatment of serious COVID‐19 disease: a monocentric retrospective experience. Medicine. 2021;100:e23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medscape. What is the role of interleukin (IL) inhibitors in the treatment of coronavirus disease 2019 (COVID‐19)? November 25, 2020. www.medscape.com/answers/2500114-197455/what-is-the-role-of-interleukin-il-inhibitors-in-the-treatment-of-coronavirus-disease-2019-covid-19. Accessed November 29, 2020.
- 8. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arnaldez FI, O'Day SJ, Drake CG, et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin‐6 signaling in COVID‐19‐related systemic inflammatory response. J Immunother Cancer. 2020;8(1):e000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasonov E, Samsonov M. The role of Interleukin 6 inhibitors in therapy of severe COVID‐19. Biomed Pharmacother. 2020;131:110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGonagle D, Sharif K, O'Regan A, Bridgewood C. Interleukin‐6 use in COVID‐19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020;19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abbasifard M, Khorramdelazad H. The bio‐mission of interleukin‐6 in the pathogenesis of COVID‐19: a brief look at potential therapeutic tactics. Life Sci. 2020;257:118097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wagner JL, Veve MP, Barber KE. Using IL‐6 inhibitors to treat COVID‐19. ContagionLive. August 17, 2020. www.contagionlive.com/publications/contagion/2020/august/using-il6-inhibitors-to-treat-covid19?ekey=c2NiZXJnbWFuQG5lYnJhc2thbWVkLmNvbQ. Accessed November 29, 2020.
- 15.Interleukin‐6 inhibitors. COVID‐19 treatment guidelines. NIH. June 11, 2020. www.covid19treatmentguidelines.nih.gov/immune-based-therapy/interleukin-6-inhibitors/. Accessed November 29, 2020.
- 16.The COVID‐19 treatment guidelines panel's statement on the use of tocilizumab for the treatment of COVID‐19. NIH. March 5, 2021. https://www.covid19treatmentguidelines.nih.gov/statement-on-tocilizumab/. Accessed April 9, 2021.
- 17.Regeneron and Sanofi Begin Global Kevzara (sarilumab) clinical trial program in patients with severe COVID‐19. Regeneron/Sanofi. March 16, 2020. investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-begin-global-kevzarar-sarilumab-clinical. Accessed November 29, 2020.
- 18.Regeneron and Sanofi provide update on U.S. phase 2/3 adaptive‐designed trial of Kevzara (sarilumab) in hospitalized COVID‐19 patients. Regeneron. investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-us-phase-23-adaptive. Accessed November 29, 2020.
- 19.Regeneron and Sanofi provide update on Kevzara (sarilumab) phase 3 US trial in COVID‐19 patients. Regeneron. July 2, 2020. investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-provide-update-kevzarar-sarilumab-phase-3. Accessed November 29, 2020.
- 20. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID‐19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181:24‐31. 10.1001/jamainternmed.2020.6615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone JH, Frigault MJ, Serling‐Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383:2333‐2344. 10.1056/NEJMoa2028836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Genentech provides an update on the phase III COVACTA trial of Actemra in hospitalized patients with severe COVID‐19 associated pneumonia. Genentech. July 28, 2020. www.gene.com/media/press-releases/14867/2020-07-28/genentech-provides-an-update-on-the-phas. Accessed November 29, 2020.
- 23. Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID‐19 patients: survival and clinical outcomes. Chest. 2020;158:1397‐1408. 10.1016/j.chest.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jordan SC, Zakowski P, Tran HP, et al. Compassionate use of tocilizumab for treatment of Covid19 pneumonia. Clin Infect Dis. 2020;71:3168‐3173. 10.1093/cid/ciaa812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID‐19. Clin Infect Dis. 2021;73:e445‐e454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pereira MR, Aversa MM, Farr MA, et al. Tocilizumab for severe COVID‐19 in solid organ transplant recipients: a matched case‐control study. Am J Transplant. 2020;20:3198‐3205. 10.1111/ajt.16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ip A, Berry DA, Hansen E, et al. Hydroxychloroquine and tocilizumab therapy in COVID‐19 patients—an observational study. PLoS One. 2020;15:e0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guaraldi G, Meschiari M, Cozzi‐Lepri A, et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474‐e484. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roche rheumatoid arthritis drug fails to help COVID‐19 patients in Italian study. Reuters. June 17, 2020. www.reuters.com/article/us-health-coronavirus-roche-hldg-idUSKBN23O3GG. Accessed November 29, 2020.
- 30. Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID‐19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2020;181:32‐40. 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. National Health Commission (NHC) of the People's Republic of China. The Diagnosis and Treatment Guide of COVID‐19 Pneumonia Caused by New Coronavirus Infection. 7th ed., 2020. [Google Scholar]
- 32. Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;92:2042‐2049. 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treatment of COVID‐19 patients with anti‐interleukin drugs (COV‐AID). July 9, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04330638. Accessed November 29, 2020.
- 34.Tiziana Life Sciences PLC to expedite development of its fully human anti‐interleukin‐6‐receptor monoclonal antibody, a potential treatment of certain patients infected with coronavirus COVID‐19. Tiziana Life Sciences. March 11, 2020. www.tizianalifesciences.com/news-item?s=2020-03-11-tiziana-life-sciences-plc-to-expedite-development-of-its-fully-human-anti-interleukin-6-receptor-monoclonal-antibody-a-potential-treatment-of-certain-patients-infected-with-coronavirus-covid-19. Accessed November 29, 2020.
- 35. Sinha P, Mostaghim A, Bielick CG, et al. Early administration of Interleukin‐6 inhibitors for patients with severe Covid‐19 disease is associated with decreased intubation, reduced mortality, and increased discharge. Int J Infect Dis. 2020;99:28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maeda T, Obata R, Rizk DOD, Kuno T. The association of interleukin‐6 value, interleukin inhibitors, and outcomes of patients with COVID‐19 in New York City. J Med Virol. 2020;93:463‐471. 10.1002/jmv.26365 [DOI] [PubMed] [Google Scholar]
- 37. Rojas‐Marte G, Khalid M, Mukhtar O, et al. Outcomes in patients with severe COVID‐19 disease treated with tocilizumab: a case–controlled study. Int J Med. 2020;113(8):546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crisafulli S, Isgrò V, La Corte L, Atzeni F, Trifirò G. Potential role of anti‐interleukin (IL)‐6 drugs in the treatment of COVID‐19: rationale, clinical evidence and risks. BioDrugs. 2020;34(4):415‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Interleukin‐1 inhibitors. COVID‐19 treatment guidelines. NIH. June 11, 2020. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/interleukin-1-inhibitors/. Accessed November 29, 2020.
- 40. Cavalli G, DeLuca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325‐e331. 10.1016/S2665-9913(20)30127-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID‐19: a cohort study. Lancet Rheumatol. 2020;2:e393‐e400. 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novartis provides update on CAN‐COVID trial in hospitalized patients with COVID‐19 pneumonia and cytokine release syndrome (CRS). Novartis. November 6, 2020. www.novartis.com/news/media-releases/novartis-provides-update-can-covid-trial-hospitalized-patients-covid-19-pneumonia-and-cytokine-release-syndrome-crs. Accessed November 29, 2020.
- 43.Interleukin‐7 to improve clinical outcomes in lymphopenic patients with COVID‐19 infection FR BL cohort (ILIAD‐7‐FR). ClinicalTrials.gov. June 22, 2020. clinicaltrials.gov/ct2/show/NCT04407689. Accessed November 29, 2020.
- 44.Interleukin‐7 (CYT107) to improve clinical outcomes in lymphopenic patients with COVID‐19 infection UK cohort (ILIAD‐7‐UK). ClinicalTrials.gov. May 15, 2020. clinicaltrials.gov/ct2/show/NCT04379076. Accessed November 29, 2020.
- 45. Laterre PF, François B, Collienne C, et al. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID‐19). JAMA Netw Open. 2020;3(7):e2016485. 10.1001/jamanetworkopen.2020.16485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scherger S, Henao‐Martínez A, Franco‐Paredes C, Shapiro L. Rethinking interleukin‐6 blockade for treatment of COVID‐19. Med Hypotheses. 2020;144:110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID‐19? Cytokine and anti‐cytokine interventions. Autoimmun Rev. 2020;19(7):102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. ghc.sa/ar-sa/Documents/Bahrain.pdf. Accessed October 1, 2021.
- 49. www.doh.gov.ae/-/media/7BD7B077D8F846B48A70C5872902DD1C.ashx. Accessed October 1, 2021.
- 50. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brudno JN, Kochenderfer JN. Recent advances in CAR T‐cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sultan S, Altayar O, Siddique SM, et al. AGA institute rapid review of the gastrointestinal and liver manifestations of COVID‐19, meta‐analysis of international data, and recommendations for the consultative management of patients with COVID‐19. Gastroenterology. 2020;159(1):320‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020;72(2):389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ferm S, Fisher C, Pakala T, et al. Analysis of gastrointestinal and hepatic manifestations of SARS‐CoV‐2 infection in 892 patients in Queens, NY. Clin Gastroenterol Hepatol. 2020;18(10):2378‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta‐analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther. 2020;52(4):584‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72(1):287‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shiota G, Harada KI, Oyama K, et al. Severe exacerbation of hepatitis after short‐term corticosteroid therapy in a patient with “latent” chronic hepatitis B. Liver. 2000;20(5):415‐420. [DOI] [PubMed] [Google Scholar]
- 58. Hammond A, Ramersdorfer C, Palitzsch KD, Schölmerich J, Lock G. Fatal liver failure after corticosteroid treatment of a hepatitis B virus carrier. Deutsche Medizinische Wochenschrift. 1999;124(22):687‐690. [DOI] [PubMed] [Google Scholar]
- 59. Muhović D, Bojović J, Bulatović A, et al. First case of drug‐induced liver injury associated with the use of tocilizumab in a patient with COVID‐19. Liver Int. 2020;40(8):1901‐1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morena V, Milazzo L, Oreni L, et al. Off‐label use of tocilizumab for the treatment of SARS‐CoV‐2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID‐19: addressing a pharmacological challenge by targeting pathways triggered by SARS‐CoV‐2. Signal Transduct Target Ther. 2020;5(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sullivan RJ, Weber JS. Immune‐related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov. 2021. 10.1038/s41573-021-00259-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript reviews published information. No new data has been generated during this study.


