Abstract
Objectives
To analyse the outcomes of COVID‐19 vaccination by vaccine type, age group eligibility, vaccination strategy, and population coverage.
Design
Epidemiologic modelling to assess the final size of a COVID‐19 epidemic in Australia, with vaccination program (Pfizer, AstraZeneca, mixed), vaccination strategy (vulnerable first, transmitters first, untargeted), age group eligibility threshold (5 or 15 years), population coverage, and pre‐vaccination effective reproduction number () for the SARS‐CoV‐2 Delta variant as factors.
Main outcome measures
Numbers of SARS‐CoV‐2 infections; cumulative hospitalisations, deaths, and years of life lost.
Results
Assuming = 5, the current mixed vaccination program (vaccinating people aged 60 or more with the AstraZeneca vaccine and people under 60 with the Pfizer vaccine) will not achieve herd protection unless population vaccination coverage reaches 85% by lowering the vaccination eligibility age to 5 years. At = 3, the mixed program could achieve herd protection at 60‒70% population coverage and without vaccinating 5‒15‐year‐old children. At = 7, herd protection is unlikely to be achieved with currently available vaccines, but they would still reduce the number of COVID‐19‐related deaths by 85%.
Conclusion
Vaccinating vulnerable people first is the optimal policy when population vaccination coverage is low, but vaccinating more socially active people becomes more important as the declines and vaccination coverage increases. Assuming the most plausible of 5, vaccinating more than 85% of the population, including children, would be needed to achieve herd protection. Even without herd protection, vaccines are highly effective in reducing the number of deaths.
Keywords: COVID‐19, Infectious diseases, Epidemics, Nonlinear dynamics, Epidemiologic measurements, Vaccine preventable disease, Health policy
The known: The Delta variant of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is more transmissible than the original Wuhan strain, making achievement of herd protection difficult.
The new: Prioritising the vaccination of older, more vulnerable people leads to fewer deaths than first vaccinating young, more socially active people; this is the optimal strategy when population vaccination coverage is below 70%. Herd protection will not be achieved with the current Australian vaccination strategy, but it will prevent a substantial number of deaths.
The implications: Herd protection is unlikely unless vaccination is extended to younger age groups or combined with other mitigation measures. Delivering the Pfizer vaccine to people aged 12‒40 years should be a priority.
On 2 July 2021, the National Cabinet announced a four‐step plan for transitioning from a strategy of suppressing community transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in Australia to one of preventing serious illness, allowing a return to normal social and economic activity. 1 Before moving to a more liberal setting (increased number of arrivals from overseas, reduced use of lockdowns, simplified quarantine procedures for vaccinated people 1 ), the plan requires that 70% of Australians aged 16 years or more be fully vaccinated to minimise the numbers of coronavirus disease 2019 (COVID‐19)‐related hospitalisations and deaths through both direct and herd protection. However, the herd protection threshold is higher for the Delta variant of SARS‐CoV‐2 than for earlier variants, 2 and current vaccines are slightly less effective at preventing symptomatic infection by the Delta variant. 3 , 4 , 5
We have previously investigated herd protection thresholds and optimal vaccination strategies for the original Wuhan strain of SARS‐CoV‐2. 6 In this article, we update this analysis for the Delta variant in Australia.
The Delta variant is about twice as infectious as the original Wuhan strain of SARS‐CoV‐2. 7 However, given the widespread implementation of mitigation measures, its pre‐vaccination effective reproduction rate (expected number of new infections caused by one typical infected person, in the absence of a vaccination program) — — is unclear. For our primary analysis, we assume a value of 5, which is at the lower end of estimated values in an analysis by the Population Interventions Unit at the University of Melbourne (5–6), 8 the central value in the Grattan report (4–6), 9 and higher than that used in the Doherty Institute model (3.6). 10
The model outcomes are sensitive to the value of , which is highly uncertain, as it is influenced by changing mitigation strategies, including contact tracing, mask use, and mobility changes, as well as by viral evolution. We therefore considered outcomes for values ranging from 3 to 7. We also developed a flexible online tool that allows the user to explore the sensitivity of model outputs to variations in a range of input assumptions.
Age‐specific vaccination strategies have characterised the COVID‐19 response in Australia. 11 Published age‐specific contact matrices indicate that young adults are more socially active than other age groups in Australia, with a marked decline in contacts beyond age 55, 12 whereas estimated COVID‐19 fatality rates are highest for older people. We therefore assessed two vaccination strategies, assuming fixed, limited vaccine supplies: the first focuses on vaccinating the most vulnerable (people aged 55 years or more), the second prioritises vaccinating the most socially active (people under 55). We considered using the AstraZeneca vaccine (Vaxzevria) only, the Pfizer vaccine (Comirnaty) only, and the current mixed program recommended by the Australian Technical Advisory Group on Immunisation (ATAGI); that is, vaccinating people under 60 years of age with the Pfizer vaccine and older people with the AstraZeneca vaccine. 11
Methods
Vaccine program and eligibility
Given the availability of vaccines in Australia, we considered three COVID‐19 vaccine program options:
only the Pfizer vaccine is used;
only the AstraZeneca vaccine is used;
people under 60 years receive the Pfizer vaccine, older people receive the AstraZeneca vaccine (mixed program: the current ATAGI recommendation 11 ).
We consider two age thresholds for vaccination eligibility — 5 and 15 years — and three age‐specific coverage strategies for the eligible population:
the “vulnerable first” strategy: people aged 55 years or more (more vulnerable to severe disease) are vaccinated first, then people under 55 years of age;
the “transmitters first” strategy: people under 55 years of age (the more socially active) are vaccinated first, then people aged 55 years or more;
the “untargeted” strategy: undifferentiated vaccination of people in all eligible age groups.
Vaccination uptake
We considered different maximum levels of vaccination uptake that corresponded to its acceptance by eligible vaccination groups. Our main analysis assumed a maximum uptake of 90% by all eligible age groups.
Vaccine efficacy, mechanisms, and model assumptions
We defined V a as the efficacy of the vaccine for reducing susceptibility to infection with SARS‐CoV‐2 (derived from the relative rates of asymptomatic and all PCR‐positive infections in a vaccinated population), V s as its efficacy for reducing the proportion of infected people who develop symptomatic disease (leading to both reduced severity of disease and reduced infectiousness of the vaccinated infected person), V m as its efficacy for preventing hospitalisation and death, and V t as its efficacy for reducing the probability of disease transmission by an infected person. We derived the reduction in risk of symptomatic COVID‐19 (overall vaccine efficacy, V e), specific to the Delta strain of SARS‐CoV‐2, from published values for clinical trials; some values for V s and V m were derived from published data on earlier viral variants. 13 The three parameters are related by the formula, 1 – V e = (1 – V a)(1 – V s) (Box 1).
Box 1. Model inputs for vaccine efficacy against the SARS‐CoV‐2 Delta variant, by effect.
| Efficacy | Vaccine | |
|---|---|---|
| Pfizer | AstraZeneca | |
| Susceptibility to infection (V a) | 0.76* | 0.48 † , 20 |
| Symptomatic COVID‐19 in infected people (V s) | 0.5 ‡ , 19 , 21 | 0.37 † , 19 , 20 |
| Symptomatic COVID‐19 (V e) | 0.88 5 , 13 | 0.67 5 , 13 , 22 |
| Disease transmission by an infected person (V t) | 0.5 23 | 0.5 23 |
| Hospitalisation and death (V m) | 0.5 24 | 0.8 24 |
COVID‐19 = coronavirus disease 2019; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus 2.
Estimated by solving the expression 1 – V e = (1 – V a)(1 – V s), for Va .
These values are chosen to achieve vaccine efficacy of 0.67 in symptomatic infection and 0.22 for asymptomatic infection reported in reference 20.
This value is chosen to achieve vaccine efficacy of 0.9 for symptomatic infection and 0.8 for asymptomatic infection reported in reference 19 (based on non‐Delta variants).
Mathematical methods
The full mathematical methodology is described in the online Supporting Information and has also been published elsewhere. 6 Briefly, we combine inferred daily rates of age‐specific contacts 12 with differences in susceptibility and infection by age 14 to build a table of expected transmission events over each individual’s infectious lifetime (the next‐generation matrix, K̃ij 15 ). This table enables us to calculate the total number of infected individuals in each age and vaccination group over the course of an epidemic wave, using the final‐size equation: 16
where z̃i is the fraction of people in group i (which indexes both age and vaccination status) who are infected during the course of the epidemic, and Ñi is the total number of people in this group.
Next, we combine age‐specific estimates of the infection fatality rate 17 , 18 and life expectancy with vaccine efficacy estimates (Box 1), enabling us to calculate the cumulative number of infections, hospitalisations, deaths, and years of life lost for each vaccination strategy.
Our default assumption for the effective reproduction number for the Delta strain before vaccination () was 5, but we explored values from 3 to 7. We define coverage as the number of complete vaccine courses (two doses) divided by the total Australian population (irrespective of minimum vaccination age or uptake proportion).
Results
Impact on number of infections and herd protection
For all values and vaccination programs, the number of infections is lowest with the “transmitters first” strategy; reducing the threshold age for vaccination from 15 to 5 years reduces the number of infections in the Pfizer only and mixed programs for = 5 or 7, but not for = 3 (Box 2, A).
Box 2. The impact on the numbers of infections and years of life lost of the Pfizer only, AstraZeneca only, and mixed vaccine programs, by strategy, age eligibility threshold, target coverage, and R eff( v̅ ) value.
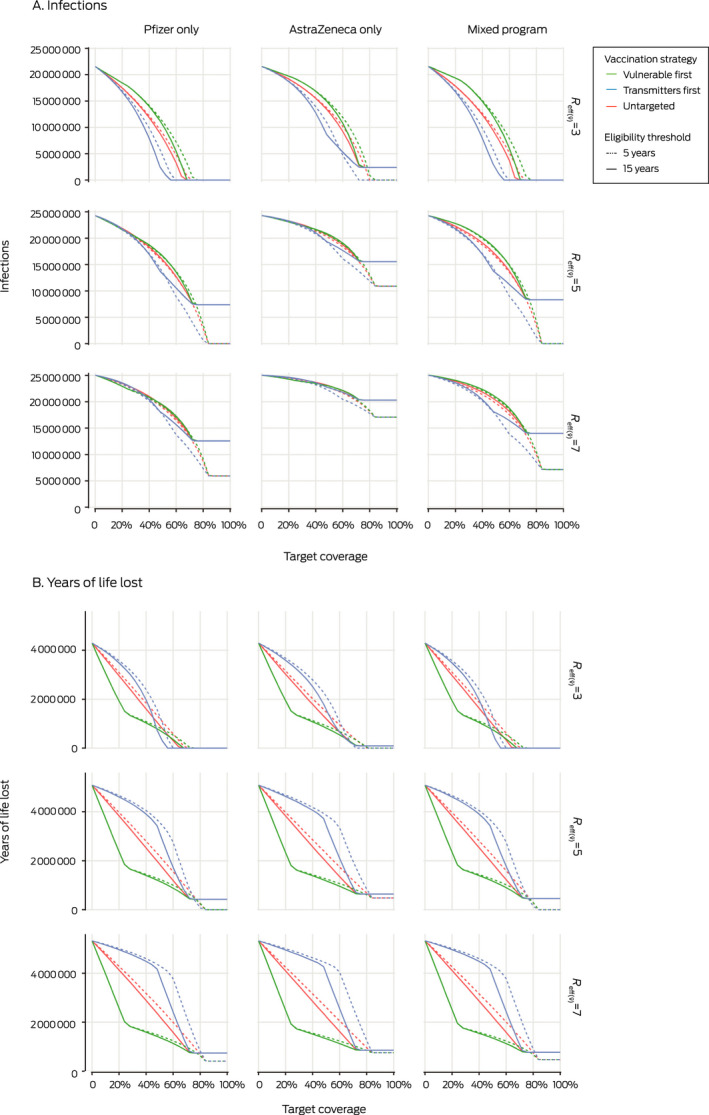
When = 7, no program or strategy achieves herd immunity (in this model: indicated by zero infections), regardless of age of eligibility. For = 5, herd protection can be achieved with the Pfizer or mixed programs only if the minimum vaccination age is 5 years. If = 3, herd protection is achieved at 60% vaccination coverage (regardless of age of eligibility) with the Pfizer only or mixed programs and the “transmitters first” strategy, or 70% coverage with the “vulnerable first” or “untargeted” strategies. Herd protection is not achieved with the AstraZeneca only program with any combination of , strategy, and eligibility inputs (Box 2, A).
Impact on numbers of years of life lost, deaths, and hospitalisations
When = 5 or 7, the number of years of life lost is generally lowest with the “vulnerable first” strategy; but at 75–82% vaccination coverage, the “transmitter first” strategy marginally outperforms the other two strategies with the Pfizer only or mixed programs if the minimum vaccination age is reduced to 5 years (Box 2, B).
When = 3, the “transmitter first” strategy is superior to the “vulnerable first” strategy for reducing the number of years of life lost with the Pfizer only and mixed programs at 50–70% vaccination coverage; below 50% coverage, the “vulnerable first” strategy is superior (Box 2, B). The impacts on hospitalisations and deaths were similar (Supporting Information, figure 2). The impacts of the “transmitter first”, “vulnerable first”, and “untargeted” strategies on the numbers of years of life lost, deaths, and hospitalisations were the same when vaccination coverage reached the level of maximum acceptance, reflecting identical distribution of vaccines (Box 2, B; Supporting Information, figure 2).
The impact on number of years of life lost at any level of vaccination coverage was generally reduced by lowering the eligibility age to 5 years, until vaccination coverage reached the uptake maximum for people aged 15 years or more (the coverage at which the solid lines plateau in Box 2, B). Beyond this point, vaccinating children aged 5–15 years further reduced the number of years of life lost (Box 2, B).
For all combinations of , strategy, coverage, and age eligibility, the Pfizer only and mixed vaccination programs were each superior to the AstraZeneca only program. The differences were much more pronounced for infections (Box 2, A) than for years of life lost (Box 2, B) and hospitalisations or deaths (Supporting Information, figure 2).
We estimated the expected numbers of COVID‐19‐related deaths of vaccinated and unvaccinated people with the mixed vaccination program by vaccination strategy and coverage, and for eligibility thresholds of 5 and 15 years. At full vaccination coverage and 90% maximum uptake, herd protection can be achieved if the vaccination eligibility age is 5 years, but not if it is 15 years. At 60% vaccination coverage or higher, a substantial minority of deaths will be of vaccinated people with any combination of vaccination strategy and age eligibility (Box 3).
Box 3. Numbers of deaths with the mixed vaccine program (R eff( v̅ ) = 5, uptake = 90%), by vaccination strategy and vaccination coverage.
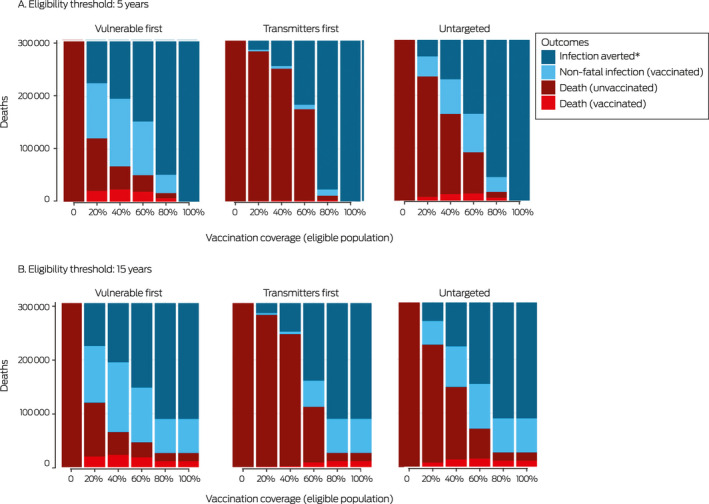
* Through personal immunity or herd protection (vaccinated and unvaccinated people).
Sensitivity analysis
We conducted sensitivity analyses that included variations in the following input parameters: the relative infectiousness of people with asymptomatic and symptomatic infections, overall vaccine efficacy against symptomatic infection (V e), relative lethality of Delta and earlier original SARS‐CoV‐2 strains, and the choice of age‐specific contact matrix. In all scenarios, our general conclusions regarding the achievability of herd protection with vaccination, including the relative performance of each vaccination strategy, were confirmed (online Supporting Information). The sensitivity of outcomes to , coverage, uptake, vaccine choice and age eligibility cut‐off can be evaluated directly using our online tool. 25
Discussion
If for the Delta variant of SARS‐CoV‐2 is as high as 7, herd protection is unlikely to be achieved in Australia. On the other hand, if its value can be constrained to below 3.0 by measures other than vaccination, herd protection is achievable with the AstraZeneca only, Pfizer only, and mixed programs.
Our modelling indicates that vaccinating more vulnerable (older) age groups first, as undertaken in Australia, is the optimal strategy for reducing the numbers of hospitalisations, deaths, and years of life lost to COVID‐19 caused by a highly infectious SARS‐CoV‐2 variant. Herd protection could be achieved with a program including the AstraZeneca vaccine for older people and the Pfizer vaccine for younger people. However, if the for the Delta strain is 5 or higher, at least 85% of the Australian population, including children aged 5 years or more, would need to be vaccinated. The current plan to vaccinate 80% of people aged 16 years or more, or about 65% of the total population, will consequently fall short of achieving herd protection. Nevertheless, vaccination averts a considerable number of deaths and years of life lost by both reducing the number of infections of vaccinated and unvaccinated people, and by directly reducing the severity of disease in vaccinated infected persons.
For lower values, reflecting the situation in an Australian city with partial lockdowns or other transmission mitigation measures, we found that targeting more socially active people first could achieve herd protection at lower vaccination coverage rates than untargeted strategies or strategies targeting the vulnerable older age groups.
The Doherty model projections by R eff, 10 derived using inputs similar to ours, 6 are generally consistent with our findings. Some of the divergences are probably attributable to differences in the plausible R eff values considered in the context of multiple public health interventions. In particular, we found that teenagers and younger children need to be vaccinated to achieve herd protection and to avoid substantial loss of life if is slightly higher than the value in the Doherty model (3.6).
Our model was based on the pre‐vaccination effective reproduction number (), taking into account all public health and individual mitigation strategies apart from vaccination. This value changes with public health responses; with partial lockdowns, we expect a value below 3, and our model would favour vaccinating high transmitters as the priority. Our baseline value was 5, corresponding to the relaxation of all general mitigation measures and reliance on a specific public health response. We also modelled a fully unmitigated epidemic ( = 7), which would preclude achieving herd protection with available vaccines.
The effects of values and other variables on model outputs can be further evaluated with our online tool, 25 which allows users to vary model parameters as new evidence emerges, including , the choice of several vaccines, and the extent of population immunity attributable to natural infection (ie, seroprevalence of SARS‐CoV‐2 antibodies). The open source code is publicly available (https://github.com/michaeltmeehan/covid19/tree/main/immunization_australia).
Limitations
We aggregated the effects of all public health interventions other than vaccination with a single value (), rather than modelling them separately. This value was fixed for each simulation, and we modelled the final size of an epidemic, not dynamic time steps. Our model does not predict the epidemic curve or the impact of sequential interventions, nor the effects of waning immunity, which is currently poorly understood. The number of people affected by “long COVID” could be estimated from our model outputs (age‐specific infection rates) once more precise data are available, but we have not examined this outcome. Contact pattern heterogeneity was considered in our model, but only to the level of 5‐year age groups. We did not model the impact on specific occupations or subgroups of people; we may have overestimated the impact of vaccination if particular subgroups remain unvaccinated or underestimated the effect of targeting subgroups of high transmitters.
Conclusion
By August 2021, people in Australia at greatest risk of COVID‐19, including those over 70 years of age, had the opportunity to be vaccinated, and more than 80% of people in this age group had received their first vaccination dose by early September. 26 The recent focus in Australia has been to vaccinate people aged 60 years or more with the AstraZeneca vaccine and 40–59 year‐old people with the Pfizer vaccine. Our model findings suggest that priorities for the short term future should be to expand vaccination access to all ages for whom the Pfizer vaccine has provisional approval in Australia (people aged 12 years or more). The Pfizer vaccine supply has been the rate‐limiting step for this approach.
We need to determine the national vaccination level required before re‐opening Australia to international travellers and moving from our aggressive domestic control strategy. The current acceptance of COVID‐19 vaccination among Australians, vaccine efficacy, and the infectiousness of the SARS‐CoV‐2 Delta variant mean that achieving herd protection through vaccination alone is improbable. If children are vaccinated and new vaccines that elicit long lasting sterilising immunity become available, this situation could change. However, substantial reductions in the numbers of deaths, hospitalisations, and years of life lost to COVID‐19 can be achieved if we strategically vaccinate most of our older and more vulnerable residents, and then vaccinate high transmitters. Herd protection should be our aim, but not the criterion for transitioning to Phase B of the national response plan. 1
Competing interests
No relevant disclosures.
Supporting information
Supplementary results
Acknowledgements
Emma McBryde is supported by a National Health and Medical Research Council (NHMRC) investigator grant (1195102), James Trauer by an NHMRC Early Career Fellowship (1142638), and Michael Meehan by an Australian Research Council Discovery Early Career Fellowship (210101344). James Trauer and Pavithra Jayasundara are supported by a Medical Research Future Fund Rapid Response Digital Health grant (RRDHI000027). Jamie Caldwell is funded by the United States National Aeronautics and Space Administration Ecological Forecasting program (NNX17AI21G).
References
- 1. Prime Minister of Australia . National plan to transition Australia’s national COVID response [media release]. 2 July 2021. https://www.pm.gov.au/media/national‐cabinet‐statement‐6 (viewed July 2021).
- 2. Meehan MT, Rojas DP, Adekunle AI, et al. Modelling insights into the COVID‐19 pandemic. Paediatr Respir Rev 2020; 35: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffmann M, Hofmann‐Winkler H, Krüger N, et al. SARS‐CoV‐2 variant B.1.617 is resistant to bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep 2021; 36: 109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2‐elicited serum. N Engl J Med 2021; 384: 1466–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid‐19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 2021; 385: 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meehan MT, Cocks DG, Caldwell JM, et al. Age‐targeted dose allocation can halve COVID‐19 vaccine requirements [preprint], version 2. medRxiv 2020.10.08.20208108; 2 Dec 2020. 10.1101/2020.10.08.20208108 (viewed July 2021). [DOI] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Delta variant: what we know about the science. Updated 26 Aug 2021. https://www.cdc.gov/coronavirus/2019‐ncov/variants/delta‐variant.html (viewed Aug 2021).
- 8. Blakely A, Wilson T, Sundararajan V. Population Interventions Unit, University of Melbourne. COVID‐19 pandemic trade‐offs: version 2, July 2021, including border opening. Updated 4 July 2021. https://populationinterventions.science.unimelb.edu.au/pandemic‐trade‐offs‐july‐2021 (viewed Aug 2021).
- 9. Duckett S, Wood D, Coates B, et al. Race to 80: our best shot at living with COVID. July 2021. https://grattan.edu.au/wp‐content/uploads/2021/07/Race‐to‐80‐our‐best‐shot‐at‐living‐with‐COVID‐Grattan‐Report.pdf (viewed Aug 2021).
- 10. Doherty Institute . Doherty modelling report for National Cabinet. Revised 10 Aug 2021. https://www.doherty.edu.au/uploads/content_doc/DohertyModelling_NationalPlan_and_Addendum_20210810.pdf (viewed Aug 2021).
- 11. Australian Technical Advisory Group on Immunisation . ATAGI update following weekly COVID‐19 meeting, 7 July 2021. https://www.health.gov.au/news/atagi‐update‐following‐weekly‐covid‐19‐meeting‐7‐july‐2021 (viewed July 2021).
- 12. Prem K, Zandvoort KV, Klepac P, et al; Centre for the Mathematical Modelling of Infectious Diseases COVID‐19 Working Group . Projecting contact matrices in 177 geographical regions: an update and comparison with empirical data for the COVID‐19 era. PLoS Comput Biol 2021; 17: e1009098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer‐BioNTech and Oxford‐AstraZeneca vaccines on covid‐19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case‐control study. BMJ 2021; 373: n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies NG, Klepac P, Liu Y, et al. Age‐dependent effects in the transmission and control of COVID‐19 epidemics. Nat Med 2020; 26: 1205–1211. [DOI] [PubMed] [Google Scholar]
- 15. Diekmann O, Heesterbeek JA, Roberts MG. The construction of next‐generation matrices for compartmental epidemic models. J R Soc Interface 2010; 7: 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreasen V. The final size of an epidemic and its relation to the basic reproduction number. Bull Math Biol 2011; 73: 2305–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age‐specific mortality and immunity patterns of SARS‐CoV‐2. Nature 2021; 590: 140–145. [DOI] [PubMed] [Google Scholar]
- 18. Fisman DN, Tuite AR. Progressive increase in virulence of novel SARS‐CoV‐2 variants in Ontario, Canada [preprint], version 3. medRxiv 2021.07.05.21260050; 4 Aug 2021. 10.1101/2021.07.05.21260050 (viewed Aug 2021). [DOI]
- 19. Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID‐19 vaccine on asymptomatic infection among patients undergoing pre‐procedural COVID‐19 molecular screening. Clin Infect Dis 2021; 10.1093/cid/ciab229 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Voysey M, Clemens SAC, Madhi SA, et al; Oxford COVID Vaccine Trial Group . Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397: 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levine‐Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS‐CoV‐2 viral load after inoculation with the BNT162b2 vaccine. Nat Med 2021; 27: 790–792. [DOI] [PubMed] [Google Scholar]
- 22. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris RJ, Hall JA, Zaidi A, et al. Effect of vaccination on household transmission of SARS‐CoV‐2 in England. N Engl J Med 2021; 385: 759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stowe J, Andrews N, Gower C, et al. Effectiveness of COVID‐19 vaccines against hospital admission with the Delta (B.1.617.2) variant [preprint]. Fiocruz (The Global Health Network); 14 June 2021. https://fiocruz.tghn.org/articles/effectiveness‐covid‐19‐vaccines‐against‐hospital‐admission‐delta‐b16172‐variant (viewed Aug 2021).
- 25. McBryde ES, Meehan MT, Caldwell JM, et al. COVID‐19 vaccination modelling results. https://covid‐19‐aithm.shinyapps.io/vaccine_coverage_analysis (viewed Sept 2021).
- 26. Australian Department of Health . COVID‐19 vaccination: doses by age and sex. Updated 4 Sept 2021. https://www.health.gov.au/resources/publications/covid‐19‐vaccination‐doses‐by‐age‐and‐sex (viewed Sept 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary results


