ETHICS STATEMENT
Informed consent was obtained from patients, for publication of medical images.
CONFLICT OF INTEREST
I declare that there is no financial support or conflict of interest in this work. Informed consent was obtained from patients, for publication of medical images.
TO THE EDITOR
I read an interesting letter by Boston et al published in February 2021, where the authors had reported herpes zoster in a 78 years‐old man (with multiple comorbidities) 5 days after he had the inactivated COVID‐19 vaccine. 1 This is the first report from India, on two healthy individuals who presented with herpes zoster infection within 1 week of obtaining the first dose of COVID‐19 vaccine.
Currently, two vaccines are widely used in India to prevent the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) disease transmission. One is the ChAdOx1‐nCOV vaccine 19 (Covishield®) and BBV‐152 (Covaxin®). 2
ChAdOx1‐nCOV 19 vaccine is a replication‐deficient chimpanzee adenoviral vector containing the sequence for SARS‐CoV‐2 structural surface glycoprotein antigen. 2 It is one of the vaccines, which is authorized by the World Health Organization for emergency use. 2 , 3 The common side‐effects reported with ChAdOx1‐nCOV 19 vaccine are fatigue, malaise, muscle ache, headaches, fever, chills, and pain at the site of the injection. 3 Rare occurrences of immune‐mediated thrombotic thrombocytopenia have also been reported with the vaccine. 4
The first patient is a 25 years‐old woman with a 2 days history of vesicles on the back of the right thigh (Figure 1). She was inoculated with the first dose of the ChAdOx1‐nCOV 19 vaccine, 4 days before the lesions started. Following the injection, she reported minimal body pain and pain on the injection site. On the fourth day, she developed pain on the back of the right thigh, and the following day she noticed red, vesicular spots on the same area. Dermatological examination showed confluent, vesicular lesions on an erythematous base on the back of the right thigh corresponding to S1, S2 dermatome, clinically suggestive of herpes zoster infection. She was commenced on acyclovir topical cream and oral valaciclovir thrice daily for 1 .
FIGURE 1.
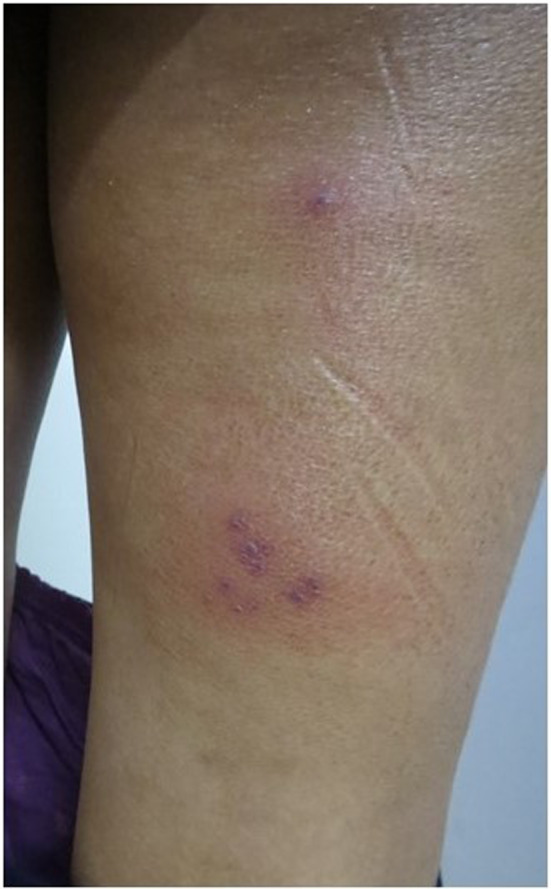
A 25 years‐old Female with tiny, confluent vesicles on an erythematous base on the back of the right thing
Similarly, a 55 years‐old woman presented with four days history of painful, crusty, hemorrhagic rashes on the left upper thigh and upper buttock region (Figure 2). She had the first dose of the ChAdOx1‐nCOV 19 vaccine one week before the rashes started. This lady was prescribed topical acyclovir for the rashes and a combination of vitamin B12 and pregabalin to control the pain.
FIGURE 2.
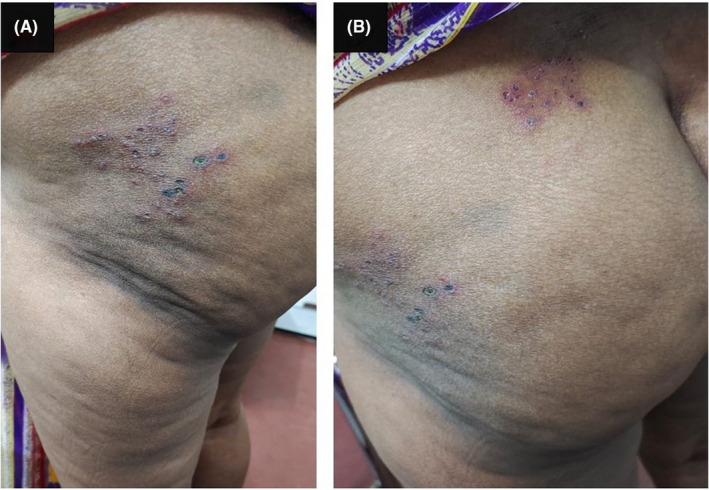
A 55 years‐old lady with crusty, hemorrhagic blisters on (A) upper left thigh and (B) upper buttock region
Varicella (chickenpox) is a common childhood illness caused by the varicella‐zoster virus, a human alphaherpesvirus. 5 Post‐infection, the varicella virus can remain latent in the dorsal root ganglia for years, before it could be reactivated with aging or in the immunosuppressive state, like pregnancy, HIV infection, cancer, or being on steroid medications, etc. 5 Varicella in childhood presents with scattered, vesicular lesions on the body with prodromal symptoms. However, VZV reactivation causes painful blisters or vesicular lesions on a particular dermatome. This is referred to as herpes zoster or shingles. 5
A weakened VZV‐specific T‐cell‐mediated immunity in immunocompromised individuals is linked with herpes zoster reactivation. 6 Similarly concomitant infection, with other pathogens such as herpes simplex 1 or disseminated non‐tuberculous mycobacteria, can reactivate the latent VZV virus. 6 It is unclear if other specific antigens can increase the likelihood of VZV reactivation. 6 However, there are previous reports of VZV infection post‐vaccination with inactivated hepatitis virus vaccine, influenza virus vaccine and rabies vaccine have been described. 7
Both the above‐reported patients are healthy individuals with no comorbidities. Hence, the VZV infection post‐COVID vaccination in these patients could not be by chance. Thus, as Bostal et al indicated, COVID vaccination could trigger a cascade of antigenic‐mediated immunological events triggering latent VZV reactivation. 1 However, further studies are required to establish a direct relationship between herpes zoster reactivation and COVID‐19 vaccination.
Palanivel JA. Herpes zoster after COVID‐19 vaccination—Can the vaccine reactivate latent zoster virus? J Cosmet Dermatol. 2021;20:3376–3377. 10.1111/jocd.14470
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20(6):1566‐1567. 10.1111/jocd.14035 [DOI] [PubMed] [Google Scholar]
- 2. Rather RA, Islam T, Rehman IUL, Pandey D. Development of vaccine against coronavirus disease 2019 ( Covid‐19) India. Asian J Med Sci. 2021;3(2):13‐21. [Google Scholar]
- 3. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV‐19 vaccine against SARS‐CoV‐2: a preliminary report of a phase 1/2, single‐blind, randomised controlled trial. Lancet. 2020;396(10249):467‐478. 10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384(22):2092‐2101. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freer G, Pistello M. Varicella‐zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41(2):95‐105. [PubMed] [Google Scholar]
- 6. Gerada C, Campbell TM, Kennedy JJ, et al. Manipulation of the innate immune response by varicella zoster virus. Front Immunol. 2020;11:1. 10.3389/fimmu.2020.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet.. 1999;353(9155):810. 10.1016/S0140-6736(99)00623-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


