Abstract
Limited data are available on risks and benefits of anti‐SARS‐CoV2 vaccination in solid organ transplant recipients, and weaker responses have been described. At the Italian National Institute for Infectious Diseases, 61 liver transplant recipients underwent testing to describe the dynamics of humoral and cell‐mediated immune response after two doses of anti‐SARS‐CoV2 mRNA vaccines and compared with 51 healthy controls. Humoral response was measured by quantifying both anti‐spike and neutralizing antibodies; cell‐mediated response was measured by PBMC proliferation assay with IFN‐γ and IL‐2 production. Liver transplant recipients showed lower response rates compared with controls in both humoral and cellular arms; shorter time since transplantation and multi‐drug immunosuppressive regimen containing mycophenolate mofetil were predictive of reduced response to vaccination. Specific antibody and cytokine production, though reduced, were highly correlated in transplant recipients.
Keywords: anti‐spike titre, liver transplant, SARS‐CoV2 vaccination, T‐cell immune response
Abbreviations
- BAU
Binding Arbitrary Units
- CNIs
calcineurin inhibitors
- COVID‐19
coronavirus disease 2019
- eGFR
estimated glomerular filtration rate
- HC
healthy control
- IFN‐γ
interferon‐γ
- IL‐2
interleukin‐2
- IQR
interquartile range
- LTRs
liver transplant recipients
- MMF
mycophenolate mofetil
- MNA
Micro‐Neutralization Assay
- N‐Ab
Neutralizing antibody
- SARS‐CoV2
severe acute respiratory syndrome coronavirus 2
- SOTRs
Solid organ transplant recipients
1. INTRODUCTION
Solid organ transplant recipients (SOTRs) have an increased risk of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV2) infection due to immunosuppression required to prevent rejection. In addition, immunosuppression may reduce the response to vaccination. 1 For this reason, SOTRs are considered at high‐risk to develop severe coronavirus disease‐2019 (COVID‐19) with reported mortality rate up to 20%. 2 A position paper from the European Association for the Study of the Liver, consequently, recommended vaccination for liver transplant recipients (LTRs). 3 Two novel mRNA‐technology‐based anti‐SARS‐CoV2 vaccines, developed by Pfizer‐BioNTech and Moderna, have been safely administered in the general population, with efficacy reaching 94%‐95%. 4 , 5 .
In March 2021, the Italian Health Ministry began vaccination in SOTRs with mRNA vaccines (BNT162b2 or mRNA‐1273) with two doses administered 3 or 4 weeks apart, respectively.
Preliminary studies suggested a poorer response to SARS‐CoV2 vaccination in LTRs with a significantly lower antibody titre and faster decline in antibody levels than the general population. 6 Treatment factors significantly related to non‐response were high‐dose prednisone in the previous 12 months and mycophenolate mofetil (MMF) treatment. 7 , 8 Vaccine protection against SARS‐CoV2 in LTRs is not well defined; in fact, severe cases of COVID‐19 were reported in SOTRs who had received two doses of mRNA vaccine. 9
Definition of anti‐SARS‐CoV2 vaccination efficacy in LTRs includes assessment of serological and cellular responses, and limited data are available. Recently, B and T‐cell responses in a 16 SOTRs, with only four LTRs, were assessed after the second dose of BNT162b2 vaccine. Cellular response rate in SOTRs was 56.2%, and humoral response was significantly lower than in immunocompetent controls. 10
The objective of our study was assessment of humoral and cellular responses after two doses of mRNA anti‐SARS‐CoV2 vaccine in a larger cohort of LTRs, compared with healthy controls, and investigating clinical features associated with non‐response.
2. PATIENTS AND METHODS
Consecutive 61 LTRs who received anti‐SARS‐CoV2 vaccination between March and April 2021 underwent testing for humoral and cell‐mediated immune response at three time points: before 1st dose (T0), 2nd dose (T1) and 2 weeks after 2nd dose (T2). Results were compared with a healthy control (HC) group of hospital employees with no major co‐morbidities who underwent the same protocol. Subjects who tested positive for anti‐nucleoprotein IgG at T0 (indicating previous SARS‐CoV2 natural infection) were excluded.
All subjects tested negative for SARS‐CoV2 anti‐receptor‐binding domain (anti‐Spike) IgG before vaccination. All subjects received either BNT162b2 or mRNA‐1273 anti‐SARS‐CoV2 vaccine.
The study was approved by INMI L. Spallanzani Ethical Committee 3580 (March 17, 2021) and all participants signed a written informed consent.
2.1. Antibody evaluation
A chemiluminescence microparticle antibody assay (ARCHITECT® i2000sr Abbott Diagnostics) was used to detect anti‐spike IgG to quantify response to mRNA vaccination. Positive anti‐spike response was defined as ≥7.2 Binding Arbitrary Units (BAU)/ml.
2.2. Micro‐neutralization assay (MNA)
The assay was performed according to, 6 using SARS‐CoV2/Human/ITA/ PAVIA10734/2020, provided by F. Baldanti, Pavia, as a challenging virus. First, heat‐inactivated and 7 twofold serial diluted sera (starting dilution 1:10) were mixed and incubated at 37°C and 5% CO2 for 30 min with equal volumes of 100 TCID50 SARS‐CoV2. Next, 96‐well tissue culture plates with sub‐confluent Vero E6 cell monolayers were infected with 100 µl/well of virus‐serum mixture and incubated at 37°C and 5% CO2. To standardize inter‐assay procedures, positive control samples showing high (1:160) and low (1:40) neutralizing activity were included in each MNA session. After 48 h, microplates were observed using a light microscope for cytopathic effect. The highest serum dilution inhibiting the cytopathic effect was the neutralization titre and expressed as the reciprocal of serum dilution. Neutralizing antibody (N‐Ab) response was evaluated at T2 only and defined as MNA ≥ 10.
2.3. T‐cell immune response
Peripheral blood was collected in heparin tubes and stimulated with a pool of peptides spanning the Spike antigen (S‐peptides, Miltenyi Biotech) at 37°C (5% CO2), according to. 7 A superantigen was used as positive control. Cultured plasma was harvested after 16–20 h of stimulation and stored at −80°C. Th1 cytokine production of interferon‐γ (IFN‐γ) and interleukin‐2 (IL‐2) were quantified in plasma using an automatic ELISA (ELLA, Protein Simple). Detection limits of these assays were 0.17 pg/ml and 0.54 pg/ml for IFN‐γ and IL‐2, respectively. Positive response was defined as >10 pg/ml for IFN‐γ and >25 pg/ml for IL‐2. 11
2.4. Clinical features
LTRs were evaluated for obesity, diabetes mellitus, chronic renal disease with estimated glomerular filtration rate (eGFR). Immunosuppressive regimens were defined as containing calcineurin inhibitors (CNIs), MMF or steroids.
2.5. Statistical analysis
Continuous variables including anti‐spike, IFN‐γ and IL‐2 levels were reported as median and interquartile range (IQR). Comparisons of medians across groups were evaluated using Kruskal–Wallis analysis with the Mann–Whitney U‐test with Bonferroni correction for pairwise comparisons. Categorical variables including dichotomous anti‐spike, N‐Ab, IFN‐γ and IL‐2 response were summarized as counts and percentages and compared with Chi‐square test or Fisher's Exact test. Correlations between assays were assessed by non‐parametric Spearman's rank tests. To identify significant variables that could contribute to the anti‐spike, N‐Ab and IFN‐γ response, a multivariate regression analysis model was constructed including gender, age, years since transplant, immunosuppression and comorbidities. Analyses were performed using R software (version 4.0.3). A two‐sided P value <.05 was considered to be statistically significant.
3. RESULTS
Sixty‐one LTRs (70% males, median age 59 years [IQR 56‐61, range 27‐76] and 51 HCs (25.5% males, median age 43 years [IQR 36‐53, range 26‐64] were enrolled. LTRs were significantly older and male gender was more prevalent compared with HCs (P < .001 and P < .0001, respectively). All subjects completed two vaccination doses.
In LTRs, median time from transplant was 6 years (IQR 3‐10, range 1‐26). CNIs were used as immunosuppressive regimen backbone in 59 (96%) and 29 (47.5%) received MMF in combination with CNIs. Among LTRs, diabetes was present in 15 patients (24.6%) and obesity defined as Body Mass Index>30 in 14 (23%). Only 9 patients (14.5%) showed eGFR<51 ml/min. Specific T‐Cell cytokine production at T0 did not differ between the two groups.
3.1. Humoral response
Median anti‐spike titre in LTRs increased significantly between doses: 1.7 BAU/ml (IQR 0.47‐9.12) at T1 vs. 82.5 BAU/ml (IQR 6.15‐419.20) at T2 (P < .008).
Median anti‐spike IgG titre in HCs increased significantly between doses: 100.2 BAU/ml (IQR 54.70–231.60) at T1 vs. 1991 BAU/ml (IQR 1164.0–4451.0) at T2 (P < .0001).
Anti‐spike response at T2 was significantly lower in LTRs compared with HCs (77.0% vs. 100%, P = .001). Median anti‐spike IgG titre was significantly lower in LTRs compared with HCs at both time points (P < .0001). The kinetics of anti‐spike titres is shown in Figure 1A.
FIGURE 1.
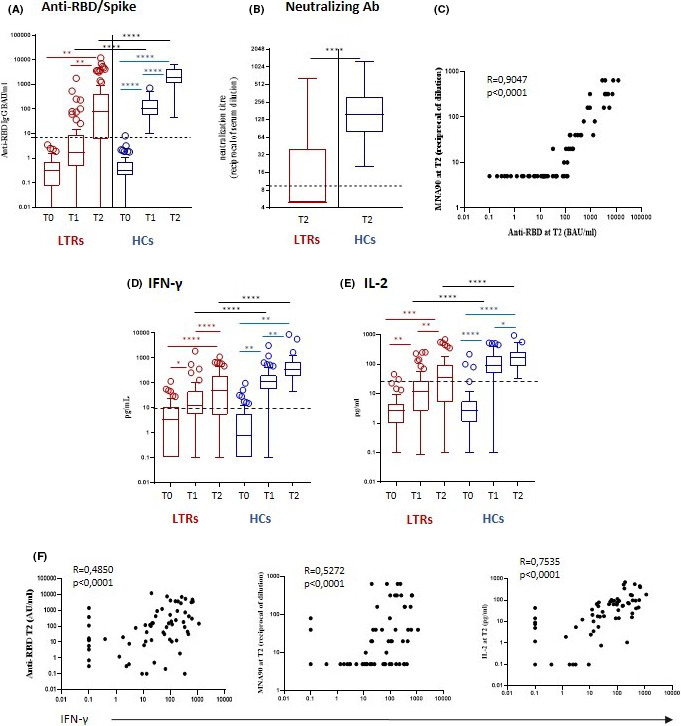
Humoral and cellular responses to anti‐SARS‐CoV2 mRNA vaccination in LTRs and HCs. Panel A: anti‐RBD/spike Ig G titres at T0, T1 and T2. Panel B: Neutralizing Ab titres at T2. Panel C: correlation between anti‐RBD/spike titres anti neutralizing Ab titres at T2. Panel D: IFN‐γ production at T0, T1 and T2. Panel E: IL‐2 production at T0, T1 and T2. Panel F: Correlation of humoral responses and IL‐2 production with IFN‐γ production at T2. *P < .05; **P < .01; ***P < .001; ****P < .0001. T0, before first vaccination dose; T1, before second vaccination dose; T2, two weeks after second vaccination dose; LTRs, liver transplant recipients; HCs, healthy controls; RBD, receptor binding domain; MNA90, 90% micro‐neutralization assay; IFN, interferon; IL‐2, interleukin‐2
N‐Ab response at T2 was observed in 29 LTRs (47.5%) vs. 51 HCs (100%; P < .001). Among responders, median N‐Ab titres at T2 were significantly lower in LTRs compared with HCs: 40 (IQR 20‐160) vs. 160 (IQR 80‐320), P < .0001 (Figure 1B).
A strong correlation between anti‐spike and N‐Ab titres was observed at T2 in LTRs (r = 0.9047, P <.0001, Figure 1C).
3.2. Cellular response
Specific T‐cell response to S‐peptides was measured via Th1 cytokine production of IFN‐γ and IL‐2 released after in vitro stimulation (Figure 1D,E). Before vaccination, 25.4% of LTRs and 17.6% of HCs had detectable levels of IFN‐γ.
In LTRs, median IFN‐γ level was 3.5 pg/ml (IQR 0.1‐10.8) at T0, 9.5 pg/ml (IQR 2.1‐29.9) at T1 and 49.1 pg/ml (IQR 5.3‐189.3) at T2 (T0 vs. T2: P < .0001).
In HCs, median IFN‐γ level was 0.85 pg/ml (IQR 0.1‐5.7) at T0, 112.2 pg/ml (IQR 53.5‐205.0) at T1 and 344.0 pg/ml (IQR 193.6‐699.6) at T2 (T0 vs. T1: P < .0001; T1 vs. T2: P < .0001).
Positive IFN‐γ response at T2 was significantly lower in LTRs compared with HCs (72.1% vs. 100%, P < .0001). Median IFN‐γ levels were significantly lower in LTRs compared with HCs at T1 and T2 (P < .0001, Figure 1D).
In LTRs, median IL‐2 level was 2.6 pg/ml (IQR 0.9‐1.6) at T0, 12.1 pg/ml (IQR 2.6‐27.1) at T1 and 34.2 pg/ml (IQR 5.0‐92.7) at T2 (T0 vs. T1 P < .0001; T1 vs. T2: P < .0001).
In HCs, median IL‐2 level was 2.6 pg/ml (IQR 1.1‐5.5) at T0, 87.1 pg/ml (IQR 48.2‐195.3) at T1 and 166.0 pg/ml (IQR 85.4‐265.0) at T2 (T0 vs. T1: P < .0001; T1 vs. T2: P < .01).
Positive IL‐2 response at T2 was significantly lower in LTRs compared with HCs (50.8% vs. 100%, P < .0001). Median IL‐2 levels were significantly lower in LTRs compared with HCs at T1 and T2 (P < .0001, Figure 1E).
In LTRs the amount of IFN‐γ released by S‐specific T cells correlated with anti‐spike titre (r = 0.4850, P < .0001), N‐Ab titre (r = 0.5272, P < .0001) and IL‐2 levels (r = 0.7535, P < .0001, Figure 1F).
3.3. Multivariate regression analysis
Treatment with MMF was significantly associated with anti‐RBD non‐response [RR 1.60 (1.16‐2.20) P = .0039), while time from transplant >6 years [RR 2.19 (1.15‐4.16); P = .0176], age>55 years [RR 0.72 (0.53‐0.98) P = .0388] were associated with lower nAb and IFN‐ γ production, respectively. At least one comorbidity was not associated with humoral or T‐cell non‐response, although a trend was observed for obesity and GFR<51 ml/min (P = .07). Significant associations are depicted in Table 1.
TABLE 1.
Multivariate regression analysis: clinical characteristics of liver transplant recipients significantly associated with reduced immune response at T2 included time from transplant<6 years and combined immunosuppression treatment with MMF + CNIs
|
Anti‐spike response N = 61 |
Adjusted multivariable RR* | ||||
|---|---|---|---|---|---|
| Negative | Positive | P value † | RR (95%CI) | P value | |
| N = 14 | N = 47 | ||||
| Immunosuppressive treatment | |||||
| Calcineurine inhibitor + Mycophenolate mofetil | 13 (92.9%) | 17 (36.2%) | .001 | .0039 | |
| Calcineurine inhibitor | 1 (7.14%) | 30 (63.8%) | 1.60 (1.16‐2.20) | ||
|
Neutralizing Ab at T2 N = 61 |
Adjusted ultivariable RR+ | ||||
|---|---|---|---|---|---|
| Negative | Positive | P value † | RR (95%CI) | P value | |
| N = 32 | N = 29 | ||||
| Years since transplant | |||||
| <6 | 24 (75.0%) | 9 (31.0%) | .001 | .0170 | |
| ≥6 | 8 (25.0%) | 20 (69.0%) | 2.19 (1.15‐4.16) | ||
|
IFN‐γ response at T2 N = 61 |
Adjusted multivariable RR+ | ||||
|---|---|---|---|---|---|
| Negative | Positive | P value † | RR (95%CI) | P value | |
| N = 17 | N = 44 | ||||
| Age group | |||||
| 26‐55 years | 2 (11.8%) | 13 (29.5%) | .196 | .0388 | |
| >55 years | 15 (88.2%) | 31 (70.5%) | 0.72 (0.53‐0.98) | ||
Abbreviations: Ab, antbody; IFN, interferon; RR, relative risk.
P values are shown from Chi‐Square test or Fisher's Exact test for categorical variables.
Poisson regression with a robust error variance.
4. DISCUSSION
Our study assessed both humoral and cell‐mediated immune response after the second dose of anti‐SARS‐CoV2 vaccine in LTRs. Our results confirmed, as in previous reports conducted in SOTRs, a significantly lower serological response to the mRNA SARS‐CoV2 vaccine among LTRs compared with HCs, with 77% developing anti‐spike antibodies, and only 47.5% showing positive N‐Ab activity. Among those who developed antibodies, anti‐spike titre was lower than in HCs. 12
Regarding specific T‐cell response, positive IFN‐γ testing after stimulation with S‐peptides was observed at T2 in 77% of LTRs; a single previous report of a small series of 16 SOTRs found a detectable cellular response in 56%, although with a different cut‐off. 10 This difference could be explained by stronger immunosuppression used in non‐liver transplant recipients.
In our study, 25.4% of LTRs and 17.6% of HCs had a positive IFN‐γ test at T0. This could be due to a cross‐reactivity with seasonal coronaviruses.
Furthermore, we tested IL‐2 production, representing a key cytokine in the Th1 response with homeostatic functions. Correlation between IFN‐ γ and IL‐2 produced by S‐specific T cells highlights the ability of anti‐SARS‐CoV2 vaccine to shape a balanced immune response.
The pivotal role of immunosuppression is demonstrated by the negative effect of MMF/CNI combination on both antibody and cytokine production. Treatment with MMF was shown to have a negative effect on influenza vaccine immunogenicity in other SOTRs: in particular, SOTRs receiving MMF ≥2 g/day showed significantly lower mean antibody titres than those receiving <2 g/day, and MMF reduced IL‐4+CD4+ T‐cell frequencies and B‐cell activation. 13 In this setting, a temporary suspension of MMF during the vaccination period could be proposed in selected LTRs.
The use of CNIs alone can be a risk factor in the blunted immune response; however, progressive dose reduction, based on transplant age, may explain the higher immunological response in LTRs with time from transplant≥6 years. In a recent study in LTRs, calcineurin inhibitor monotherapy has been a positive prognostic factor for anti‐SARS‐CoV2 vaccination response compared with other immunosuppressive regimens. 14
Our findings of lower anti‐spike Ab titre, N‐Ab and T‐cell‐specific responses in LTRs after the two‐dose vaccine schedule suggest that part of this group of patients may remain at higher risk for severe COVID‐19.
Almost 30% of immunocompromised patients experience poor vaccination response, as seen for influenza; 15 whether anti‐SARS‐CoV2 vaccination may confer benefit reducing the risk of severe COVID‐19 in immunological responders by decreasing morbidity and mortality remains to be demonstrated with longer follow‐up.
Given the low seroconversion rates after the second dose of vaccine in immunocompromised patients, some recent studies have aimed to assess whether an additional dose could elicit a better immunological response. Werbel 16 described increased antibody responses after a third dose of anti‐SARS‐CoV2 vaccine in SOTRs with a suboptimal anti‐spike titre to a standard two‐dose schedule. However, limits of the study were the lack of N‐Ab and specific T‐cell response assays.
Similarly, a French group showed that administration of a third dose of BNT162b2 vaccine to 101 SOTRs (12 LTRs) significantly improved anti‐spike titre with 68% response rate after the third dose. 17 Another series of 48 kidney transplant recipients, with previous non‐response to mRNA vaccination, found humoral and T‐cell response improved after a third dose of vaccination. 18 All the cited studies were conducted in mixed SOTRs patients with very few LTRs. Notably immunosuppressive regimens in non‐liver SOTRs are significantly different in terms of dose, steroids and MMF use. For this reason, clinical trials in LTRs are needed to investigate whether humoral and cellular immunogenicity can be stimulated through strategies such as higher vaccine doses, additional booster doses, use of adjuvants and to assess whether specific vaccines are more effective in LTRs.
The correlation between humoral and T‐cell response may support using only anti‐spike titre, a simple and inexpensive test, as the ideal parameter to assess immunological response in upcoming large‐scale booster dose vaccination studies in LTRs.
In conclusion, despite limitations related to small sample size and brief clinical follow‐up, our study in LTRs demonstrated a blunted but coordinated humoral and T‐cell‐mediated response after two standard doses of mRNA anti‐SARS‐CoV2 vaccine compared with HCs. Longer follow‐up studies are needed to assess durability of immune response in this population.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
All authors contributed substantially to the conception of the work, to the acquisition, analysis, and interpretation of data, and to drafting and critically revising the manuscript. All authors have approved the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Permission to reproduce material from other sources was not required for the present work.
Gianpiero D, Chiara A, Ubaldo V‐C, et al. Coordinated cellular and humoral immune responses after two‐dose SARS‐CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022;42:180–186. doi: 10.1111/liv.15089
Handling Editor: Alejandro Forner
Funding information
Italian Ministry of Health (COVID‐2020 Project −12371675 and Ricerca Corrente – Linea 1) and generous liberal donations for COVID‐19 research from Esselunga S.p.A.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mombelli M, Rettby N, Perreau M, et al. Immunogenicity and safety of double versus standard dose of the seasonal influenza vaccine in solid‐organ transplant recipients: a randomized controlled trial. Vaccine. 2018;36(41):6163‐6169. [DOI] [PubMed] [Google Scholar]
- 2. Kates OS, Haydel BM, Florman SS, et al. COVID‐19 in solid organ transplant: a multi‐center cohort study. Clin Infect Dis. 2020;7:ciaa1097. [Google Scholar]
- 3. Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep. 2020;2(3):100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Caballero‐Marcos A, Salcedo M, Alonso‐Fernandez R, et al. Changes in humoral immune response after SARS‐CoV‐2 infection in liver transplant recipients compared to immunocompetent patients. Am J Transplant. 2021;21(8):2876‐2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody Response to 2‐Dose SARS‐CoV‐2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA. 2021;325(21):2204‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marion O, Del Bello A, Abravanel F, et al. Safety and Immunogenicity of Anti‐SARS‐CoV‐2 Messenger RNA Vaccines in Recipients of Solid Organ Transplants. Ann Intern Med. 2021;174(9):1336‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wadei HM, Gonwa TA, Leoni JC, et al. COVID‐19 infection in solid organ transplant recipients after SARS‐CoV‐2 vaccination. Am J Transplant. 2021;21(10):3496‐3499. doi: 10.1111/ajt.16618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miele M, Busà R, Russelli G, et al. Impaired anti‐SARS‐CoV‐2 humoral and cellular immune response induced by Pfizer‐BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant. 2021;21(8):2919‐2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agrati C, Castilletti C, Goletti D, et al. Coordinated induction of humoral and Spike Specific T‐Cell Reponse in a Cohort of Italian health care workers receiving BNT162b2 mRNA Vaccine. Microrganisms. 2021;9:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75(2):435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Egli A, Humar A, Widmer LA, et al. Effect of Immunosuppression on T‐Helper 2 and B‐Cell Responses to Influenza Vaccination. J Infect Dis. 2015;212(1):137‐146. [DOI] [PubMed] [Google Scholar]
- 14. Ruether DF, Schaub GM, Duengelhoef PM, et al. SARS‐CoV2‐specific humoral and T‐cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2021;9:S1542–3565(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karbasi‐Afshar R, Izadi M, Fazel M, Khedmat H. Response of transplant recipients to influenza vaccination based on type of immunosuppression: a meta‐analysis. Saudi J Kidney Dis Transpl. 2015;26(5):877‐883. [DOI] [PubMed] [Google Scholar]
- 16. Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Del Bello A., Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti‐SARS‐CoV‐2 messenger RNA‐based vaccines in solid organ transplant recipients [published online ahead of print, 2021 Jul 31]. Am J Transplant. 2021. 10.1111/ajt.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stumpf J, Siepmann T, Lindner T, et al. Humoral and cellular immunity to SARS‐CoV‐2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA‐1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021; 9:100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


