Abstract
Objectives
To assess the effectiveness and safety of tocilizumab, a humanised anti‐interleukin‐6 receptor antibody, in the treatment of critical or severe coronavirus disease 2019 (COVID‐19) patients.
Methods
This was a retrospective cohort study of severe or critical COVID‐19 patients (≥18 years) admitted to one hospital in Kuwait. Fifty‐one patients received intravenous tocilizumab, while 78 patients received the standard of care at the same hospital. Both groups were compared for clinical improvement and in‐hospital mortality.
Results
The tocilizumab (TCZ) group had a significantly lower 28‐day in‐hospital mortality rate than the standard‐of care‐group (21.6% vs. 42.3% respectively; p = 0.015). Fifty‐five per cent of patients in the TCZ group clinically improved vs. 11.5% in the standard‐of‐care group (p < 0.001). Using Cox‐proportional regression analysis, TCZ treatment was associated with a reduced risk of mortality (adjusted hazard ratio 0.25; 95% CI: 0.11–0.61) and increased likelihood of clinical improvement (adjusted hazard ratio 4.94; 95% CI: 2.03–12.0), compared to the standard of care. The median C‐reactive protein, D‐dimer, procalcitonin, lactate dehydrogenase and ferritin levels in the tocilizumab group decreased significantly over the 14 days of follow‐up. Secondary infections occurred in 19.6% of the TCZ group, and in 20.5% of the standard‐of‐care group, with no statistical significance (p = 0.900).
Conclusion
Tocilizumab was significantly associated with better survival and greater clinical improvement in severe or critical COVID‐19 patients.
Keywords: Covid‐19, Kuwait, mortality, survival, tocilizumab
INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic, caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), has resulted in a global health crisis with more than 214 million cases and 4.47 million deaths worldwide, as reported by WHO in August 2021 [1, 2].
COVID‐19 shows a heterogeneous clinical presentation, ranging from mild symptoms to generalised vascular disease with multiple organ damage [3, 4]. SARS‐CoV‐2 infection is associated with a dysregulated inflammatory response explained by several pathogenesis pathways [5]. Early reports of COVID‐19 highlighted the development of a cytokine storm, described as an excessive production of pro‐inflammatory cytokines, particularly IL‐6, IL‐1β and TNF‐α, which are correlated with the clinical deterioration and other inflammatory biomarker in patients with severe or critical COVID‐19 [6]. However, the levels of many pro‐inflammatory cytokines in COVID‐19 were lower than those seen in other critical illnesses, such as cytokine release syndrome, sepsis and acute respiratory distress syndrome, which questions the role of the cytokine storm in COVID‐19‐induced organ dysfunction [5, 7]. Furthermore, hyperferritinaemia, lymphopaenia with a higher neutrophil‐to‐lymphocyte ratio (NLR), high lactate dehydrogenase (LDH) levels and high D‐dimer levels were also recorded in COVID‐19 patients [6, 8].
IL‐6 is an important inflammatory cytokine released by endothelial cells, fibroblasts, T‐cells, monocytes, keratinocytes and macrophages during the early phase of infectious inflammations [6]. Excessive IL‐6 production plays an important role in the pathogenesis of the extensive lung injury seen in severe or critical COVID‐19 [8]. IL‐6 activity predisposes to thrombotic and microangiopathic vasculopathy through endothelial activation and precipitation of pulmonary immune‐mediated thrombosis. It is also a strong inducer of acute‐phase reactive proteins that induce hepatocytes to synthesise amyloid A and C‐reactive protein (CRP) [8]. High plasma IL‐6 levels were associated with increased admission to the intensive care units (ICU), and increased mortality among patients with severe or critical COVID‐19 [7, 9, 10]. However, recent evidence showed that multiple other mechanisms and pathways were involved in the pathogenesis of COVID‐19‐related lung injury, such as microangiopathic vasculopathy and B‐cell secretion of specific sarsCov‐2 antibodies [5, 7].
Many interventions are being evaluated for benefit to COVID‐19 patients [11, 12], and much has changed since the initial findings were announced. Given the association between the host immune dysregulation and the level of harm associated with COVID‐19, a particular focus is directed towards the beneficial effects of immunomodulatory therapies in mitigating this host‐mediated damage [5, 10, 11]. Accordingly, two landmark trials – RECOVERY and REMAP‐CAP – have highlighted the benefit of corticosteroids and tocilizumab, a recombinant humanised monoclonal antibody against IL‐6 receptors in reducing mortality and morbidity among severely ill patients with COVID‐19 [13, 14, 15]. Although early observational studies demonstrated the potential benefit of tocilizumab [16, 17, 18, 19, 20, 21, 22], data from patients with severe or critical illness and COVID‐19 are lacking. Therefore, the purpose of this study was to assess the effectiveness and safety of tocilizumab use in patients with critical or severe COVID‐19.
METHODS
This is a retrospective cohort, single‐centre study on adult patients (≥18 years) admitted to Jaber Al‐Ahmad Hospital in Kuwait with a confirmed diagnosis of SARS‐Cov‐2 infection between March 30th and May 10th, 2020, who fulfilled the criteria of severe or critical COVID‐19. According to the Chinese guidelines for COVID‐19 management, severe COVID‐19 was defined as the presence of radiological evidence of more than 50% lung infiltrate plus one of the following: (1) respiratory rate ≥30 breaths/min; (2) oxygen saturation (SaO2) ≤93% while breathing ambient air at rest or (3) ARDS, defined as the ratio of arterial oxygen partial pressure (PaO2) to the fraction of inspired oxygen (FiO2) (PaO2:FiO2) ≤300 mmHg. In addition, critical COVID‐19 was defined as respiratory failure requiring ventilator support, either invasive or none; septic shock and/or any organ dysfunction requiring supportive treatment in the ICU [23]. The diagnosis of SARS‐Cov‐2 infection was confirmed by real‐time reverse transcription–polymerase chain reaction (rRT‐PCR) assays of nasopharyngeal swabs.
The standard of care, issued by the Kuwaiti Ministry of Health, included oxygen therapy, anticoagulants, antivirals (hydroxychloroquine (HCQ), lopinavir/ritonavir, azithromycin), corticosteroids, vitamins, statins, angiotensinogen‐converting enzyme (ACE) inhibitors and/or angiotensin II receptor blockers (ARBs), and was offered to COVID‐19 patients based on the existence of contraindications, probable medication interactions and toxicities. In April 2020, the Kuwaiti Ministry of Health issued the guideline for TCZ administration to adults with severe or critical COVID‐19 who are suspected to have cytokine release syndrome, as shown by high CRP (≥100 mg/L) or ferritin (≥1000 ng/ml) levels, in addition to high LDH (≥200 U/L) levels, elevated D‐dimer (>250 ng/ml) and need for ICU supportive care with mechanical ventilation for ≤48 h. Exclusion criteria included a concomitant active bacterial infection, neutropaenia <1000 × 109 cells/L, baseline elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels by more than five‐fold the upper limit of the normal range and positive testing for hepatitis B virus, hepatitis C virus or tuberculosis.
Upon approval and due to a shortage of tocilizumab in Kuwait, only 51 patients, admitted between April 15th and May 10th 2020, fulfilled the eligibility criteria for tocilizumab therapy. Among patients admitted to the hospital before the date of tocilizumab availability, only 78 patients who received the standard of care had retrospectively fulfilled the eligibility criteria for tocilizumab treatment, and served as a comparison group (Figure 1).
FIGURE 1.
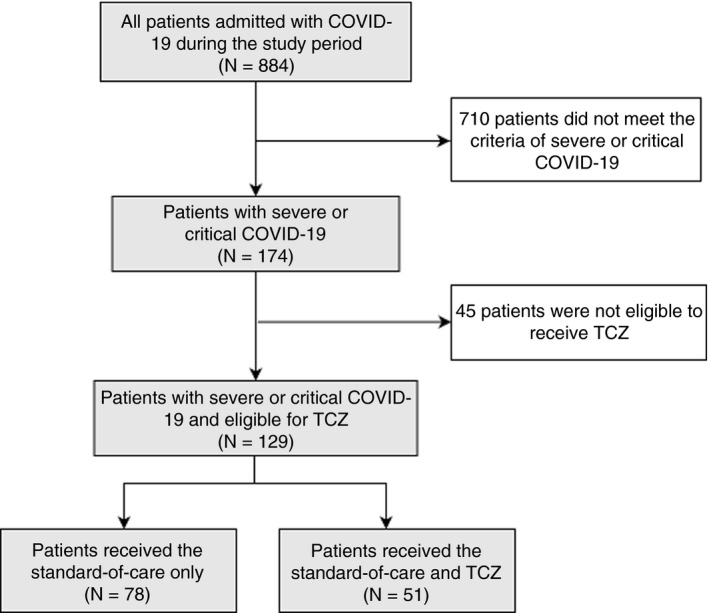
Flow chart of the study population
The size of the study groups (i.e. 51 vs. 78) was large enough to detect at least 30% reduction in the in‐hospital mortality after administration of TCZ, at a 5% level of confidence and achieved a power of 93.5% (G*Power version 3.1.9.6, Kiel University, Germany). TCZ (Actemra®, Roche Holding AG, Basel) was reconstituted with 100 ml of 0.9% sodium chloride and injected intravenously at a dose of 4–8 mg/kg over 60 min with a maximum 800 mg per dose. Doses were rounded to the nearest available full vial (80 mg, 200 mg, 400 mg vials). A dose of 4 mg/kg was given to patients with mild baseline elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >1 to 3 × ULN, mild neutropaenia (1500–1000 cell/mm3) and baseline thrombocytopaenia (<100,000/mm3). A second dose was injected after 24 h to patients who showed no improvement in oxygen requirements.
All patients were followed up for up to 28 days of hospitalisation; from the date of TCZ eligibility in the control group and the injection date in the TCZ group. In‐hospital mortality and failure of clinical improvement were censored at the last day of follow‐up or 28 days, whichever came first. All clinical and laboratory data were retrieved from the hospital's records at baseline and on days 3, 5, 7, 10 and 14. The primary outcome was in‐hospital 28‐day mortality. Secondary outcomes were observed 28‐day clinical improvement, changes in inflammatory markers by day 14 and 28‐day secondary infections confirmed by positive cultures. Clinical improvement was defined as: discharged alive without worsening or at least a 2‐point decrease of the WHO disease severity score during the 28 days or maximum follow‐up, whichever came first. The WHO severity score is a 4‐point ordinal scale defined as: (1) Invasive ventilation defined as patients requiring mechanical ventilation or extracorporeal membrane oxygenation (ECMO); (2) non‐invasive ventilation defined as patients requiring bilevel positive airway pressure (BiPAP), continuous positive airway pressure (CPAP), high‐flow oxygen (O2) or mid‐flow O2; (3) low‐flow O2 defined as patients requiring nasal cannula or a non‐rebreather mask and (4) ambient air defined as patients requiring room air at the time of tocilizumab administration [24]. Furthermore, TCZ‐treated patients were monitored for any adverse effects, incidence of neutropaenia (defined as neutrophils <1500 × 109 cells/L) and secondary infections confirmed by positive cultures.
The Cerrahpaşa‐PREDICT score proposed by Eşkazan et al (2021) was used to predict 28‐day mortality following tocilizumab treatment in COVID‐19 patients. It identified five parameters (platelet count, procalcitonin, SO2‐Room air, D‐dimer and time from symptom onset to tocilizumab use) with specific points assigned to each patient if the parameter was unfavourable. A total score for each patient could be calculated with a cut‐off value of 63 or higher, as recommended by the author [25].
Ethical approval
The study protocol was approved by the ethical committee for coordination of medical research, Kuwait Ministry of Health (2020/1422), in compliance with the latest version of the Declaration of Helsinki [26].
Statistical analysis
All data manipulation and analyses were performed using SPSS® Statistics version 24 (IBM Corporation, Armonk, NY, USA). Data normality was tested using the Kolmogorov–Smirnov test. All laboratory variables, body temperature, PaO2:FiO2 ratio, ICU stay and time to hospital admission, which were not normally distributed, were summarised as median (25th–75th percentiles). Patients’ age and BMI variables were normally distributed and were summarised as the mean ±standard deviation (SD). Categorical variables were described as frequencies and percentages (%). Associations between categorical variables were tested for statistical significance using Chi‐square test or Fisher's exact test as appropriate, while Mann–Whitney test or Kruskal–Wallis test was used for continuous variables. Survival time was tested for normality by Kolmogorov–Smirnov test, which yielded non‐significant p‐value denoting that the survival time was not normally distributed. Accordingly, non‐parametric survival analysis was performed using Kaplan–Meier and Cox proportional hazards regression methods. The distributions of survival function were plotted using Kaplan–Meier curve, where the between‐group differences were tested for statistical significance using the log‐rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using multivariable Cox proportional hazards models for the 28‐day in‐hospital mortality and clinical improvement variables. Potential confounders were identified through a series of bivariate analyses of baseline variables where variables with p‐values <0.10 were included in the Cox models. All assumptions of the Cox proportional regression were met. A p‐value of <0.05 was considered statistically significant.
RESULTS
Mean patients’ age in the TCZ and control groups was 54.1 and 57.2 years respectively. Male patients constituted 64.7% and 64.1% of all patients in TCZ and control groups respectively. No statistically significant differences existed between the study groups regarding the demographic or the baseline clinical and laboratory data as summarised in Table 1. Median time from hospital admission to the first TCZ dose was 3 days. The mean TCZ dose was 630.3 ± 126.5 mg (6.43 ± 1.84 mg/kg), and only three patients required a second TCZ dose.
TABLE 1.
Baseline characteristics of studied COVID‐19 patients treated with tocilizumab (TCZ) versus the standard of care
| Parameters | TCZ (n = 51) | Standard of care (n = 78) | p‐value |
|---|---|---|---|
| Age (years), Mean ± SD (range) | 54.1 ± 14.7 (19–88.5) | 57.2 ± 13.2 (21–84.0) | 0.291 |
| Gender, no. (%) | |||
| Male | 33 (64.7%) | 50 (64.1%) | 0.944 |
| Female | 18 (35.3%) | 28 (35.9%) | |
| Nationality, No. (%) | |||
| Kuwaiti | 30 (58.8%) | 39 (50%) | |
| Non‐Kuwaiti | 21 (41.2%) | 39 (50%) | 0.326 |
| BMI (kg/m2), Mean ± SD | 29.1 ± 3.2 | 29.0 ± 4.3 | 0.505 |
| BMI Class, No. (%) | |||
| Normal | 4 (7.8%) | 9 (11.5%) | 0.731 |
| Overweight | 29 (56.9%) | 40 (51.3%) | |
| Obese | 18 (35.3%) | 29 (37.2%) | |
| Comorbidities No. (%) | |||
| None | 19 (37.3%) | 22 (28.2%) | 0.280 |
| DM | 23 (45.1%) | 36 (46.2%) | 0.906 |
| HTN | 19 (37.3%) | 42 (53.8%) | 0.065 |
| IHD | 4 (7.8%) | 7 (9.0%) | 1.000 |
| CKD | 3 (5.9%) | 5 (6.4%) | 1.000 |
| COPD | 3 (5.9%) | 0 | 0.060 |
| BA | 2 (3.9%) | 5 (6.4%) | 0.703 |
| Others | 4 7.8%) | 10 (12.8%) | 0.374 |
| Charlson's Comorbidity Score, median (IQR) | 2 (1–3) | 2 (1–4) | 0.216 |
| Days from the onset of symptoms to hospital admission | 5 (3–7) | 6 (4–7) | 0.109 |
| Body temperature (°C) | 37.7 (37–38) | 37.5 (37–37.8) | 0.069 |
| Respiratory rate (breaths/min) | 33.4 (31 – 38) | 34.1 (32 – 37) | 0.368 |
| PaO2:FiO2 ratio | 136.4 (93 – 182) | 119.7 (76.0 – 200.0) | 0.505 |
| qSOFA | 1 (1–2) | 1 (1–2) | 0.934 |
| Laboratory tests, median (IQR) | |||
| Haemoglobin (g/L) | 115.0 (107.0–128.0) | 115.0 (104.0–123.0) | 0.760 |
| WBCs (109/L) | 10.1 (7.2–13.3) | 8.6 (6.0–11.5) | 0.134 |
| Lymphocyte count | 0.8 (0.6–0.8) | 0.8 (0.6–1.0) | 0.130 |
| Neutrophils | 9.0 (5.6–11.2) | 7.2 (4.8–10.1) | 0.080 |
| Platelets (×103) | 301 (203 – 383) | 262 (178 – 337) | 0.076 |
| Ferritin (ng/ml) | 1758 (1300–2413) | 1666 (1405–2156) | 0.897 |
| CRP (mg/L) | 246 (192–392) | 204 (157–383) | 0.109 |
| LDH (IU/L) | 645 (513–815) | 605 (479–750) | 0.189 |
| D‐Dimer (ng/ml) | 1775 (1024–3218) | 1920 (1475–2895) | 0.615 |
| PCT (ng/mL) | 0.2 (0.1–0.4) | 0.2 (0.1–0.7) | 0.840 |
| Serum AST (IU/L) | 38.0 (31.0–51.0) | 44.0 (29.0–61.0) | 0.180 |
| Serum ALT (IU/L) | 35.0 (24.0–47.0) | 29.5 (21.0–50.0) | 0.571 |
| Serum creatinine (μmol/L) | 73.0 (56.0–87.0) | 78 (67.0–92.0) | 0.098 |
| Radiological lung involvement | |||
| Unilateral | 6 (11.8%) | 8 (10.3%) | 0.788 |
| Bilateral | 45 (88.2%) | 70 (89.7%) | |
| Co‐interventions, No. (%) | |||
| Antibiotics | 49 (96.1%) | 74 (94.8%) | 0.750 |
| Antiviral (Lopinavir/ritonavir, HCQ) | 29 (56.9%) | 42 (53.8%) | 0.736 |
| Azithromycin | 2 (3.9%) | 3 (3.85%) | 1.000 |
| Glucocorticoids | 38 (74.5%) | 68 (87.2%) | 0.066 |
| Vitamin D | 21 (41.2%) | 43 (55.1%) | 0.121 |
| Therapeutic anticoagulants | 51 (100%) | 73 (93.6%) | 0.156 |
| Statin | 9 (17.6%) | 21 (26.9%) | 0.223 |
| ARB/ACE | 11 (21.6%) | 20 (25.6%) | 0.597 |
| Need for ventilatory support, No. (%) | 26 (51.0%) | 44 (56.4%) | 0.545 |
| ICU admission, No. (%) | 43 (84.3%) | 63 (80.8%) | 0.607 |
Numerical variables – median (interquartile range); Categorical variables – frequency (%).
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ALT, alanine aminotransferase; ARBs, angiotensin II receptor blockers; AST, aspartate aminotransferase; BA, Bronchial Asthma; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DM, diabetes mellitus; FiO2, fraction of inspired oxygen; GIT, gastrointestinal tract; HCQ, hydroxychloroquine; HTN, hypertension; ICU, intensive care unit;IHD, ischaemic heart disease; LDH, lactate dehydrogenase; PaO2, arterial oxygen partial pressure; PCT, procalcitonin; qSOFA, quick sequential organ failure assessment; TCZ, tocilizumab; WBC, white blood cell.
The median duration of treatment on ICUs in the TCZ group was significantly shorter than in the standard‐of‐care group (18 vs. 21 days, respectively, p = 0.026, Table 2). The mean survival time in TCZ group was significantly longer than in the standard‐of‐care group (24.5 days, 95% CI: 22.2–26.3 vs. 20.0 days; 95% CI: 17.7–22.3; Log‐rank test p‐value = 0.046, Figure 2). The unadjusted in‐hospital 28‐day mortality rate in the TCZ group was significantly lower than in the standard‐of‐care group: 11 cases (21.6%) vs. 33 cases (42.3%) respectively (p = 0.015, Table 2). Further adjustment of potential confounders using Cox proportional regression revealed that tocilizumab therapy was associated with a significantly lower adjusted in‐hospital 28‐day mortality than the standard of care. Patients who received tocilizumab were 75% less likely to die in hospital within 28 days than patients in the standard‐of‐care group (HR: 0.25; 95% CI: 0.11–0.61; p = 0.002), adjusted for potential confounders listed in Table 3.
TABLE 2.
Outcome data among studied COVID‐19 patients treated with tocilizumab versus the standard of care
| Parameters |
TCZ (n = 51) |
Standard of care (n = 78) |
p‐value |
|---|---|---|---|
| Length of ICU stay (days) | 18.0 (12 – 22) | 21 (14 – 28) | 0.026* |
| In‐hospital 28‐day mortality | 11 (21.6%) | 33 (42.3%) | 0.015* |
| 28‐day clinical improvement | 28 (54.9%) | 9 (11.5%) | 0.000* |
| Need for mechanical ventilation (by day 14) | 10 (19.6%) | 46 (59.0%) | 0.000* |
| 28‐day secondary infections | 10 (19.6%) | 16 (20.5%) | 0.900 |
Numerical variables – median (interquartile range). Categorical variables – frequency (%).
Abbreviations: ICU, intensive care unit; TCZ, tocilizumab.
Statistically significant test (p < 0.05).
FIGURE 2.
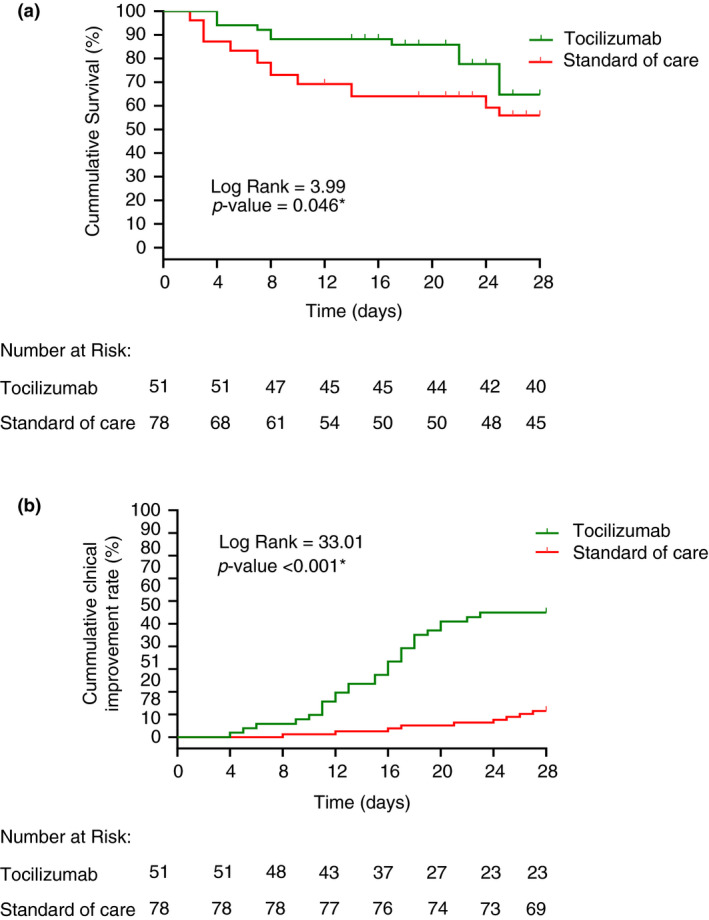
Kaplan–Meier curves of equality of survival distributions between Tocilizumab‐ and the standard‐of‐care‐treated groups during the 28‐day observational period; (a) In‐hospital mortality, (b) Clinical improvement
TABLE 3.
Cox proportional hazards model for 28‐day In‐hospital mortality among severe/critical COVID‐19 following Tocilizumab versus standard‐of‐care treatments
| Unadjusted HR (95% CI) | p‐value | |
|---|---|---|
| Tocilizumab (vs. standard of care) | 0.508 (0.256–1.010) | 0.054 |
| Adjusted HR (95% CI) | p‐value | |
|---|---|---|
| Tocilizumab (vs. standard of care) | 0.253 (0.105–0.613) | 0.002* |
| Age (years) | 1.008 (0.967–1.050) | 0.719 |
| Charlson's comorbidity score | 1.088 (0.852–1.389) | 0.498 |
| Time to hospital admission (days) | 1.239 (1.028–1.492) | 0.024* |
| qSOFA | 1.748 (0.834–3.664) | 0.139 |
| Need for invasive ventilation (yes vs. no) | 1.416 (0.486–4.122) | 0.523 |
| Antiviral medications (yes vs. no) | 0.597 (0.298–1.194) | 0.144 |
| Glucocorticoids therapy (Yes/No) | 1.220 (0.465–3.197) | 0.686 |
| PaO2:FiO2 ratio | 0.990 (0.982–0.999) | 0.025* |
| Body Temperature (°C) | 2.304 (1.320–4.019) | 0.003* |
| CRP (mg/L) | 1.001 (0.999–1.004) | 0.281 |
| Serum Creatinine (umol/L) | 0.993 (0.98–1.007) | 0.308 |
| AST (IU/L) | 1.013 (0.997–1.031) | 0.121 |
Abbreviations: AST, aspartate aminotransferase; CI, confidence interval; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; HR, hazard ratio; LDH, lactate dehydrogenase; qSOFA, quick sequential organ failure assessment.
*Statistically significant HR (p < 0.05).
−2 Log likelihood =344.02; Chi‐square (df = 15) = 58.5; p < 0.001.
The mean time to 28‐day clinical improvement in the TCZ group was significantly shorter than in the standard‐of‐care group (20.5 days; 95% CI: 18.5–22.6 vs. 27.0 days; 95% CI: 26.3–27.8; log‐rank test p‐value <0.001, Figure 2). Twenty‐eight patients (54.9%) in the TCZ group were clinically improved vs. 9 patients (11.5%) in the standard‐of‐care group (unadjusted p < 0.001, Table 2). Further adjustment of potential confounders using Cox proportional regression revealed that the TCZ group had a significantly higher 28‐day clinical improvement rate than the standard‐of‐care group. Patients receiving tocilizumab were five times more likely to clinically improve within 28 days than those treated according to the standard of care (HR: 4.94; 95% CI: 2.03–12.0; p < 0.001), adjusted for potential confounders listed in Table 4.
TABLE 4.
Cox proportional hazards model for 28‐day clinical improvement among severe or critical COVID‐19 following Tocilizumab versus standard‐of‐care treatments
| Unadjusted HR (95% CI) | p‐value | |
|---|---|---|
| Tocilizumab (vs. standard of care) | 6.739 (3.168–14.33) | <0.001* |
| Adjusted HR (95% CI) | p‐value | |
|---|---|---|
| Tocilizumab (vs. standard of care) | 4.936 (2.033–12.0) | <0.001* |
| Age (years) | 0.99 (0.956–1.032) | 0.726 |
| Charlson's comorbidity score | 0.65 (0.428–0.988) | 0.044* |
| Time to hospital admission (days) | 0.75 (0.613–0.927) | 0.007* |
| Need for invasive ventilation (yes vs. no) | 0.38 (0.157–0.905) | 0.029* |
| Glucocorticoids therapy (yes vs. no) | 0.54 (0.200–1.476) | 0.232 |
| PaO2:FiO2 ratio | 1.02 (1.005– 1.031) | 0.009* |
| CRP (mg/L) | 1.00 (0.997–1.004) | 0.817 |
| D‐Dimer (ng/ml) | 1.00 (1.000–1.000) | 0.077 |
| AST (IU/L) | 0.99 (0.961–1.010) | 0.234 |
| Platelets (×103) | 0.99 (0.996–1.002) | 0.554 |
| Haemoglobin (g/L) | 0.99 (0.973–1.020) | 0.760 |
Abbreviations: AST, aspartate aminotransferase; CI, confidence interval; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; HR, hazard ratio.
*Statistically significant HR (p < 0.05).
−2 Log likelihood = 275.7; Chi‐square (df = 13) = 67.1; p < 0.001.
In the TCZ group, the percentage of patients who needed invasive ventilatory support significantly decreased from 51% on day 0 to 19% on day 14 (p < 0.001) compared to the standard‐of‐care group (Table 2 and Figure 3).
FIGURE 3.
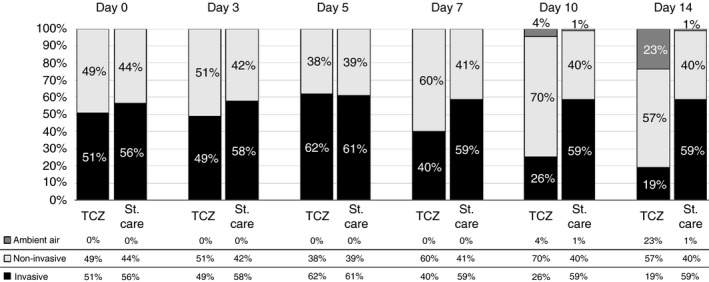
Change in the ventilatory status in the Tocilizumab (TCZ)‐ and the standard‐of‐care (St. care)‐treated groups during the first 14 days of the observation period
The median levels of CRP, Ferritin and LDH in patients treated with TCZ showed significant reduction over the first 14 days, compared to insignificant changes in the standard‐of‐care group: CRP decreased from 246 to 5 mg/L (p < 0.001; Figure 4a); ferritin decreased from 1758 to 371 mg/L (p < 0.001; Figure 4b) and LDH decreased from 645 to 247 IU/L (p < 0.001; Figure 4c). Although PCT and D‐dimer did not show significant changes in the TCZ group, they significantly increased from day 0 to day 14 in the standard‐of‐care group (p < 0.001), with a significant between‐group difference at days 7 and 14 (p < 0.001; Figure 4d,e). Lymphocyte count significantly increased from 0.8 to 1.4 (p < 0.001), which was significantly higher than the corresponding increase in the standard‐of‐care group (p < 0.001; Figure 4f).
FIGURE 4.
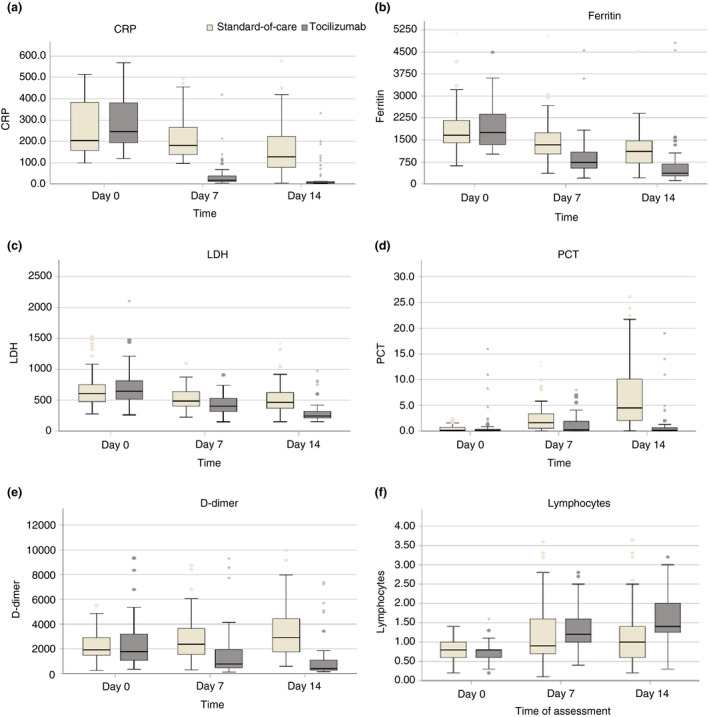
Changes in the inflammatory markers in the Tocilizumab‐ and the standard‐of‐care‐treated groups during the first 14 days of the observation period
Regarding TCZ safety, secondary infections were detected in 10 patients (19.6%) in the TCZ group, and in 16 patients (20.5%) in the standard‐of‐care group, with no statistical significance (p =0.900). Positive cultures included: coagulase‐negative Staphylococcus in six patients (two in TCZ and four in standard of care); Staphylococcus epidermidis in 12 patients (four in TCZ and eight in standard of care); Klebsiella and Acinetobacter in four patients (one in TCZ and three in standard of care); Klebsiella in two patients (one in TCZ and one in standard of care); Enterobacter aerogenes, AMP C producer in one patient treated with TCZ; Candida auris in one patient treated with TCZ and Candida parapsilosis in two patients (one in TCZ and one in standard of care).
Elevated serum ALT or AST levels (more than three times the upper normal values) were reported in nine (17.6%) TCZ patients and in 20 (25.6%) standard‐of‐care patients (p = 0.290). Serum ALT elevation among TCZ patients was observed on days 3 and 5 of TCZ injection. A transitory neutropaenia was noticed in one (1.96%) TCZ patient on day 5 after injection and in one (1.3%) standard‐treatment patient (p = 0.402). No infection occurred in the two patients with transitory neutropaenia. No anaphylactic reaction was observed during TCZ administration.
The Cerrahpaşa‐PREDICT score was calculated with a mean of 35.9 (SD: 31.6; range: 0–107). The distribution of patients with unfavourable parameters by 28‐day mortality is described in Table 5. Of the 44 patients who died during this study, 28 (63.6%) had a total score of 63 or higher (8/11 deaths in TCZ group and 20/33 in the control group). In our study, the total score had an area under the curve of 0.843 (95% Confidence Interval: 0.768–0.901; p‐value < 0.001), and the Youden index criterion exceeded 62 (sensitivity 68.2%; specificity 91.8%; positive predictive value 78.8%; negative predictive value 81.2%).
TABLE 5.
Distribution of patients with unfavourable parameters by the 28‐day mortality
| Unfavourable Parameters | In‐hospital 28‐day mortality (n = 44) | ||
|---|---|---|---|
| Control (n = 33) | TCZ (n = 11) | Total | |
| Platelet count ≤147 × 109/L | 21 (63.6%) | 8 (72.7%) | 29 (65.9%) |
| Procalcitonin ≥0.3555 ng/ml | 20 (60.6%) | 8 (72.7%) | 28 (63.6%) |
| SO2R ≤ 91.5% | 17 (51.5%) | 8 (72.7%) | 25 (56.8%) |
| D‐dimer ≥2.52 mg/L | 29 (87.9%) | 7 (63.6%) | 36 (81.8%) |
| Time from symptom start to TCZ use >12 days | 6 (18.2%) | 4 (36.4%) | 10 (22.7%) |
| Total score ≥63 (high risk for 28‐day mortality) | 20 (60.6%) | 8 (72.7%) | 28 (63.6%) |
DISCUSSION
This study highlights the outcomes of TCZ therapy in severe or critical COVID‐19 patients. Both TCZ and standard‐of‐care groups were matched in terms of age, gender, BMI and disease severity to minimise the risk of selection bias. TCZ administration was associated with a significantly lower in‐hospital 28‐day mortality rate, shorter ICU stay, greater clinical improvement and higher survival time than the standard of care. The percentage of patients on invasive ventilation decreased significantly from 51% to 19% in the TCZ group. These findings reflect a significant improvement and a high survival rate in patients who received TCZ.
An Italian retrospective study by Guaraldi et al. of 544 severe COVID‐19 patients reported a significantly lower death rate in the TCZ group than in the control group (7% vs. 20% respectively) but lower than that reported by our study. Guaraldi et al. also found a significant decrease in the risk of invasive mechanical ventilation or death (adjusted hazard ratio [aHR]: 0.61; 95% CI: 0.40–0.92; p = 0.02) [16]. A study by Biran et al. of 764 COVID‐19 patients in the ICU reported a significant decrease in the mortality rate (HR: 0.71; 95% CI: 0.56–0.89; p = 0.002) and risk of mechanical ventilation (HR: 0.63; 95% CI: 0.46–0.85; p = 0.002) in the TCZ group compared to the control group [27]. Klopfenstein et al. demonstrated insignificant reduction in the mortality rate in the TCZ group compared to the control group (25% vs. 48% respectively; p = 0.066); however, in terms of death and/or ICU admission, the difference was significant (25% vs. 72% respectively; p = 0.002) [28]. Furthermore, in a recently published meta‐analysis, IL‐6 antagonist therapy was associated with an absolute mortality risk of 22% vs. an assumed mortality of 25% in patients treated with the usual care or placebo [10].
In contrast, a recent randomised, double‐blind, placebo‐controlled trial evaluated the efficacy of a single dose of TCZ (8 mg/kg) among moderately ill hospitalised COVID‐19 patients. The authors concluded that TCZ is not effective in preventing intubation or death (HR: 0.83; 95% CI: 0.38–1.81; p = 0.64) [29]. A meta‐analysis of seven retrospective studies showed that there is no statistically significant difference between TCZ and standard of care regarding all‐cause mortality (odds ratio [OR]: 0.62; 95% CI: 0.31–1.22) and ICU admission (relative risk [RR]: 1.51; 95% CI: 0.33–6.78) [30]. These inconsistencies in the findings between studies could be attributed to the variations in sample sizes, the control arm of the studies (i.e. comparator group), the severity of COVID‐19 in the study group and differences in co‐interventions given to the control group as part of the standard of care at the time of the study.
TCZ‐treated patients in our study showed a significant decrease in CRP level by day 14 of the follow‐up period. Some reports have identified an inverse relationship between CRP levels and the overall survival rate in COVID‐19 patients. Biran et al. suggested that TCZ could exert its effects in patients whose COVID‐19 illness is progressing to an inflammatory state (CRP >15 mg/dL) [27]. Toniati et al. reported a rapid and sustained response to TCZ among patients with COVID‐19 pneumonia and hyperinflammatory syndrome [31]. Xu et al. showed that CRP levels normalise in 84.2% of severe COVID‐19 patients after TCZ administration [32].
Several studies have highlighted a correlation of ferritin, D‐dimer and LDH levels with COVID‐19 severity. In this study, among the TCZ group, we observed a significant decrease in D‐dimer, LDH and ferritin levels by day 14, which is consistent with earlier reports by Biran et al [27]. Henry et al. demonstrated an association between elevated LDH levels and worse outcomes in COVID‐19 patients [33]; however, Chen et al. found that even after TCZ administration, D‐dimer levels remained high [34]. The high ferritin levels in such patients might be due to the IL‐6 receptor blockade, increasing serum IL‐6 levels, which contribute directly to ferritin synthesis and accumulation at the injection site, leading to several reactions known as ferritin immunomodulatory effects [35].
Several studies have recommended PCT as an indicator of COVID‐19 severity and prognosis [6, 36, 37, 38]. Hu et al. showed that PCT levels are four times higher in severe COVID‐19 patients than in those with moderate COVID‐19 [39]. The significant decrease in PCT levels in our TCZ group in comparison with the standard‐of‐care group (p < 0.001) indicated the clinical improvement of this population and supported the aforementioned findings.
Secondary infection, neutropaenia and elevated liver enzymes are the most reported adverse events. In a recent randomised controlled trial [29], neutropaenia in the TCZ group was significantly higher than in the placebo group (13.7% vs. 1.2% respectively). However, the authors reported an insignificantly different incidence of elevated liver enzymes between TCZ and placebo groups (8.7% vs. 8.6% respectively). In our study, the occurrence of secondary infections in patients treated with TCZ was comparable to the reported incidence in the standard‐of‐care group. No significant difference in the incidence of elevated serum ALT or AST levels was found between the groups. Furthermore, only one patient from TCZ group had a transient neutropaenia.
This study had several limitations. First, causal association could not be ascertained in this observational study design due to inherent known (e.g. age, pre‐existing comorbidities, co‐interventions for COVID‐19) and unknown confounders. Second, due to the discrepancy between the tocilizumab availability and the number of severe and critical COVID‐19, selection of patients for tocilizumab therapy was subject to indication bias. Third, the study reflects a single‐centre experience in Kuwait with small study groups compared to other studies. Fourth, patients treated earlier in the pandemic may have worse outcomes due to lack of familiarity with the disease and different clinical practices. Fifth, measurements of IL‐6 levels were not available for studied patients. Finally, we could not assess long‐term safety and adverse effects because of the short follow‐up period.
CONCLUSION
Tocilizumab therapy of patients with severe or critical COVID‐19 was significantly associated with better survival and clinical improvement compared to the standard of care. Further well‐designed RCTs are required to validate these findings.
Abdelnaby H, Aboelhassan W, Al‐Jarallah M, Rajan R, Dashti R, Zhanna KD, et al. Outcomes of tocilizumab therapy in severe or critical COVID‐19 patients: A retrospective cohort, single‐centre study. Trop Med Int Health. 2021;26:1689–1699. 10.1111/tmi.13685
Sustainable Development Goal: Good Health and Well‐being
REFERENCES
- 1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Coronavirus (COVID‐19) Dashboard. https://covid19.who.int/. Accessed 4 August, 2021.
- 3. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382(18):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid‐19 pneumonia: pathogenesis and clinical management. BMJ. 2021;10:372. [DOI] [PubMed] [Google Scholar]
- 6. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID‐19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID‐19: a rapid systematic review, meta‐analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–44. 10.1016/S2213-2600(20)30404-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5): 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Domingo P, Mur I, Mateo GM, Gutierrez MM, Pomar V, de Benito N, et al. Association between administration of IL‐6 antagonists and mortality among patients hospitalized for COVID‐19. JAMA. 2021;326(6):499–518. 10.1001/jama.2021.11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis JS, Ferreira D, Denholm JT, Tong SYC. Clinical trials for the prevention and treatment of COVID‐19: current state of play. Med J Aust. 2020; 213(2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fragkou PC, Belhadi D, Peiffer‐Smadja N, Moschopoulos CD, Lescure FX, Janocha H, et al. Review of trials currently testing treatment and prevention of COVID‐19. Clin Microbiol Infect. 2020;26(8):988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid‐19 ‐ preliminary report. N Engl J Med. 2021;384(8):693–704. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angus DC, Berry S, Lewis RJ, Al‐Beidh F, Arabi Y, van Bentum‐Puijk W, et al. The REMAP‐CAP (Randomized Embedded Multifactorial Adaptive Platform for Community‐acquired Pneumonia) study. Rationale and design. Ann Am Thorac Soc. 2020;17:879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angus DC, Derde L, Al‐Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID‐19: the REMAP‐CAP COVID‐19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guaraldi G, Meschiari M, Cozzi‐Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID‐19: a retrospective cohort study. Lancet Rheumatol. 2020;2:e474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamed ML, et al. Association between early treatment with tocilizumab and mortality among critically Ill patients with COVID‐19. JAMA Intern Med. 2021;181:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Recovery Collaborative Group . Tocilizumab in patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. The Lancet. 2021;397:1637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coomes EA, Haghbayan H. Interleukin‐6 in Covid‐19: A systematic review and meta‐analysis. Rev Med Virol. 2020;30(6):1–9. 10.1002/rmv.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Samaee H, Mohsenzadegan M, Ala S, Maroufi SS, Moradimajd P. Tocilizumab for treatment patients with COVID‐19: recommended medication for novel disease. Int Immunopharmacol. 2020;89(Pt A):107018. 10.1016/j.intimp.2020.107018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2385–95. [DOI] [PubMed] [Google Scholar]
- 22. Cortegiani A, Ippolito M, Greco M, Granone V, Protti A, Gregoretti C, et al. Rationale and evidence on the use of tocilizumab in COVID‐19: a systematic review. Pulmonology. 2021;27(1):52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Health Commission . Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin Med J. 2020;133(9):1087–95. 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. World Health Organization . R&D Blueprint: novel coronavirus: COVID‐19 therapeutic trial synopsis. Published February 18, 2020. Accessed August 17, 2020. https://www.who.int/blueprint/priority‐diseases/key‐action/COVID‐19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf.
- 25. Eşkazan AE, Balkan İİ, Demirbaş KC, Ar MC, Karaali R, Sekibağ Y, et al. Tocilizumab in COVID‐19: the Cerrahpaşa‐PREDICT score. J Infect Chemother. 2021;27(9):1329–35. 10.1016/j.jiac.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. JAVA . Declaration of Helsinki World Medical Association Declaration of Helsinki. Bull World Heal Organ. 2013;79(4):373–4. [PMC free article] [PubMed] [Google Scholar]
- 27. Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, et al. Tocilizumab among patients with COVID‐19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2(10):e603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klopfenstein T, Zayet S, Lohse A, Balblanc J‐C, Badie J, Royer P‐Y, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID‐19 patients. Med Mal Infect. 2020;50(5):397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stone JH, Frigault MJ, Serling‐Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with Covid‐19. N Engl J Med. 2020;383(24):2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lan SH, Lai CC, Huang HT, Chang SP, Lu LC, Hsueh PR. Tocilizumab for severe COVID‐19: a systematic review and meta‐analysis. Int J Antimicrob Agents. 2020;56(3):106103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19(7):102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen J, Wang X, Zhang S, Liu B, Wu X, Wang Y, et al. Findings of acute pulmonary embolism in COVID‐19 patients. SSRN Electron J. 2020. 10.2139/ssrn.3548771 [DOI] [Google Scholar]
- 35. Gómez‐Pastora J, Weigand M, Kim J, Wu X, Strayer J, Palmer AF, et al. Hyperferritinemia in critically ill COVID‐19 patients – is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730–41. [DOI] [PubMed] [Google Scholar]
- 37. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bahbah EI, Negida A, Nabet MS. Purposing Saikosaponins for the treatment of COVID‐19. Med Hypo. 2020;140:109782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID‐19 patients. Int J Antimicrob Agents. 2020;56(2):106051. [DOI] [PMC free article] [PubMed] [Google Scholar]


