Abstract
Introduction
COVID‐19 pandemic and associated lockdown measures have deeply modified the natural course of seasonal viral infections, such as respiratory syncytial virus (RSV).
Methods
We analyzed French national data from three networks: emergency departments (ED) of French hospitals, general practitioners (GP), and hospital laboratories. We compared the number of ED or GP visits for bronchiolitis in children <2 years of age, and the percentage of RSV positive tests in the 2020 to 2021 season with those of the two previous seasons (2018–2019 and 2019–2020). We used time series of the previous 5 years to calculate epidemic thresholds.
Results
During the 2020–2021 season, the epidemic begun in February (Week 05) in the Ile de France (Paris and suburbs) region, 12 weeks later compared with the previous seasons and progressively spread across all the French metropolitan regions. The highest number of bronchiolitis cases in 2021 (Week 12) occurred 10–12 weeks after the previous seasonal peaks of previous seasons, but the number of cases remained lower than in the previous seasonal peaks.
Conclusion
We identified a delayed RSV epidemic in the period that usually corresponds at the end of the epidemic season, raising concerns for the burden of RSV in the already strained healthcare systems during the COVID‐19 pandemic.
Keywords: bronchiolitis, covid‐19, epidemic, respiratory syncytial virus
1. INTRODUCTION
SARS‐CoV‐2 is responsible for an ongoing global pandemic and France was among the main countries affected in Europe, with more than 32 million cases and nearly 713,000 deaths to date. Nonetheless, several studies suggest both a lower rate of infection in children than in adults and a very high proportion of mild forms in infected children. 1 , 2 In France as elsewhere, social distancing currently remains one of the main tools for preventing the transmission of the SARS‐CoV‐2 virus. These nonmedical measures implemented during the COVID‐19 pandemic have deeply modified the natural epidemic course of common childhood respiratory infections, including those caused by respiratory syncytial virus (RSV) in several European and Southern Hemisphere countries. 3 , 4 , 5 RSV is considered the major pathogen causing severe lower respiratory tract infections, mainly acute bronchiolitis, in infants and young children, 6 leading to seasonal epidemics with one to two epidemics each year. 7 , 8 It causes substantial morbidity and hospitalization for acute bronchiolitis in children younger than 5 years of age and especially in the first year of life 9 , 10 with a mortality estimated between 66,000 and 199,000 deaths worldwide every year. 8
In March 2020, the spread of SRAS‐CoV‐2 infection increased dramatically in France, overloading health systems. A first stringent lockdown was ordered on March 17 to limit indoor and outdoor social interactions. All nonessential public places, including, restaurants, cafés, cinemas, nightclubs together with schools and children day care centers were closed. The lockdown was extended to May 11, while physical distancing, wearing a mask in public places, and hand hygiene continued to be recommended. SARS‐CoV‐2 transmission substantially decreased and was limited for 14 weeks during the summer (Weeks 20–34). Following a further increased incidence of SARS‐CoV‐2 infections, France implemented a second nationwide but less stringent lockdown from October 30 to December 15, including the closure of all nonessential businesses, but schools and day care centers remained open, and activities for children younger than 11 years of age were not restricted.
In this context, we aimed to assess the course of RSV infections in France during 2020–2021 season and compare them with the two previous RSV seasons.
2. METHODS
We used data collated by Santé publique France from three networks. First, the Oscour® (“Organisation de la Surveillance Coordonnée des Urgences,” Coordinated Health Surveillance of Emergency Departments [ED]) network includes approximately 650 EDs, representing 92% of all ED visits in France. The network collates syndromic surveillance data, including bronchiolitis cases, from computerized medical files collected during ED visits and hospitalizations. Second, the SOS Medecins, a network of more than 1300 general practitioners (GPs) and pediatricians, including 61 of the 62 medical associations working in the various local and regional units all over France. 11 They provide 70% of private practice in urban and semiurban areas, 24 h a day (60% of the consultations are performed at night, on weekends, and on public holidays). They all provide data regularly. Third, the RENAL (Réseau national des laboratoires hospitaliers) network of French hospital laboratories includes 24 University or Regional Hospitals in Northern France, and 14 in Southern France. Surveillance is carried out on a weekly basis until April and then monthly from May to September. The network provides virological data, including RSV test results (by nucleic acid amplification).
In hospitals of the Oscour network, surveillance was based on tracking emergency room visits and hospital admissions for bronchiolitis, coded as acute bronchiolitis corresponding to children <2 years of age with a clinical medical diagnosis of acute bronchiolitis according to the International Classification of Diseases, Tenth Revision (J21, J21.0, J21.8, J21.9). For SOS Médecins associations, a clinical definition of bronchiolitis based on 2019 consensus conference 12 included the following criteria: age ≤24 months; occurring immediately after rhinopharyngitis, associating cough, and obstructive dyspnea; accompanied by wheezing and/or sibilant and crackling rales on auscultation. For RENAL, we included RSV laboratory‐confirmed cases.
All records are transmitted daily and anonymously to Santé publique France in line with national patient confidentiality rules. 13 We compared the number of ED visits, GP visits, and hospitalizations for bronchiolitis in children <2 years of age, as well as the percentage of RSV positive tests in the 2020–2021 season with those of the two previous seasons (2018–2019 and 2019–2020). The methods for calculating the epidemic threshold were described elsewhere. 14 Briefly, to calculate the baseline levels and prediction intervals, time series was used fitting three different models (periodic regression, robust periodic regression, and Markov models) on the weekly numbers of bronchiolitis cases reported via Oscour or SOS Medecins during the previous 5 years (6 models). An alarm was generated when the number of cases exceeded the 95% prediction limit. An epidemic phase was declared when at least one alarm was generated for both Oscour and SOS Médecins data or at least two alarms for Oscour data, and a preepidemic when one alarm was only detected. 15
3. RESULTS
During the 2018–2019 and 2019‐2020 seasons, acute bronchiolitis epidemics in metropolitan France began in October (Weeks 43 and 44), reached a peak at the beginning of December (Weeks 49 and 50), and ended at the beginning of March (Weeks 9 and 10), concomitantly with the accelerated spread of SARS‐CoV‐2 infection in France in 2020 (Figure 1). However, during the 2020–2021 season, the number of acute bronchiolitis cases in children <2 years of age remained low until the beginning of February (Week 05), when the epidemic threshold was exceeded in the Ile de France (Paris and suburbs) region (Figure 1), 12 weeks later compared with the previous seasons. The epidemic progressively spread across all the French metropolitan regions. By the end of March 2021, the percentage of RSV positive tests rose from 3% in Week 4 (125/4734) to 11% in Week 13 (612/5600), higher than that observed in Week 13 in 2019 and 2020 (1% and 0%, respectively), but lower than that during the peak weeks of the previous two seasons (21% in Week 49 in 2019 and 27% in Week 50 in 2018) (Figure 2). The highest number of bronchiolitis cases in 2021 (Week 13) occurred 11–13 weeks after the expected peak during the previous seasons. As of Week 13 in 2021, all regions of metropolitan France were in epidemic phase. However, 2436 acute bronchiolitis cases in children <2 years of age presented at ED during the peak week of 2021 (Week 13) compared with 4943 case in the peak week of 2019–2020 (Week 52) and 5478 cases in the peak week of 2018–2019 (Week 51) (mean = 5211 cases). The epidemic began to decline in Week 15 and ended in Week 18 at the end of the third lockdown. In Week 19, 3% (52/1993) of specimen were positive for RSV, similar to the two previous seasons at the end of the epidemic, 2% (Week 10) in 2019–2020, and 3% (Week 9) in 2018–2019.
Figure 1.
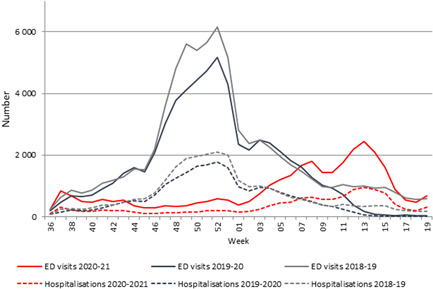
Emergency department visits and hospitalizations for acute bronchiolitis in France, children under 2 years old, 2018–2021
Figure 2.

Percentage of hospital (black) and ambulatory (gray) positive test for respiratory syncytial virus, all ages, Metropolitan France, Weeks 40/2018–Week 19/2021
Concerning rhinovirus, positivity rate was highest at 30% in Week 42 in 2020, followed by a decline at 15% in Weeks 42–52 in 2020, and remained stable during Weeks 01–19 in 2021 with a median of 11% (range 8%–13%).
4. DISCUSSION
We report a delayed start of the bronchiolitis outbreak in France and an ongoing increase in the period that usually corresponds to the end of the epidemic season. During the 2020–2021 winter season, the RSV epidemic, which usually peaks in mid‐December, started in January with a peak shifted by almost 3 months. However, the number of bronchiolitis cases remained much lower than that observed in the previous seasons. Meanwhile, the European center for disease prevention and control (ECDC) surveillance data 16 indicated that this RSV resurgence was almost limited to France. In Europe, RSV circulation remained very low, with only some countries reporting few sporadic cases since the start of the winter season (Belgium, Germany, Spain, Sweden). 16 Iceland reported a peak in the number of cases in Weeks 9 and 10, with over 20% of RSV positive samples. In Australia, the beginning of the COVID‐19 epidemic and the related measures coincided with the usual beginning of the seasonal RSV epidemic, which resulted in absence of RSV activity. 17 However, a marked and unusual resurgence of RSV activity occurred 6 months later during the summer season (2019–2020 season), with the number of cases exceeding the median seasonal peak from 2012 to 2019. 18 This resurgence in Australia may be explained by an expanded cohort of RSV‐naïve patients together with an increased number of older children coupled with waning population immunity. 19 However, this cannot fully apply to France as the end of the bronchiolitis outbreak and the cessation of RSV circulation during the winter of 2019–2020 corresponded to the usual end of the RSV season. The dramatic increase observed in Australia may also be due to a change in testing practices with more systematic tests performed including older children due to the COVID‐19 pandemic, which was not the case in France and may explain the discrepancy between the two countries. 18 Other hypotheses may explain the delayed epidemic in France: first, contrarily to the first lockdown, normal activities of schools and day care centers, with all gatherings, regardless of size, were maintained during the second lockdown. The third lockdown that started in Week 13 at the peak of 2020–2021 epidemic was associated with the closure of all school and day care centers that may have contributed to the following decrease in the number of bronchiolitis cases. Global surveillance data suggest that the stable RSV seasonal pattern (unlike that of influenza) is mainly explained by meteorological factors and facilitated by indoor crowding. For RSV, the generally accepted transmission routes are droplets and contact (either direct or indirect through an intermediate surface) and it spreads in nurseries and schools. Wearing masks has become mandatory in schools for children over 6 years of age at the beginning of November 2020, but these social distancing measures did not apply to infants and young children, the main risk groups for RSV infections. Second, despite applied international travel restrictions, interstate borders remained open during the COVID‐19 epidemic allowing RSV diffusion from other countries including those of the southwestern hemisphere. In Australia, the initial rise in RSV cases preceded the opening of interstate borders, suggesting this pathway was not the primary mechanism. 18 Finally, RSV activity was first observed in Ile de France region (including Paris), with transmission potentially facilitated by increased travel within the state during winter holidays, a decrease in vigilance regarding the risk of contamination and lesser use of protective measures (hydroalcoholic gel), especially within the family or at school. The first cases of RSV occurred after the Christmas holidays, during which the containment measures were relaxed.
The role of the other viruses is not very clear. Influenza did not circulate at all during the study period. The rhinovirus positivity rate remained stable during Weeks 01–19 in 2021. In contrast, the RSV positivity rate was strongly correlated with the proportion of bronchiolitis cases seen at ambulatory clinics or EDs over time, suggesting that the increase in bronchiolitis may be related to RSV rather than other viruses in circulation. Moreover, data from Southampton cohort (United Kingdom) 20 suggest that current physical distancing measures do not effectively prevent rhinovirus transmission that was only decreased after schools' closure. Of note, schools remained open in France after the end of the first COVID‐19 lockdown in May 2020 and until the beginning of the third lockdown in April 2021.
In conclusion, we observed in France a delayed outbreak in the period that usually corresponds at the end of the epidemic season. At the end of the third lockdown, our findings together with the Australian reports raise concerns for RSV control in the Northern Hemisphere, where a rise in RSV may occur after a shortened winter season increasing the burden in the pediatric structures and the already strained healthcare systems.
AUTHOR CONTRIBUTIONS
Céline Delestrain: conceptualization (equal); formal analysis (equal); methodology (equal); project administration (supporting); validation (equal); writing original draft (lead); writing review and editing (equal). Kostas Danis: formal analysis (equal); investigation (equal); methodology (equal); project administration (supporting); resources (lead); validation (equal); writing original draft (equal); writing review and editing (equal). Isabelle Hau: formal analysis (equal); methodology (equal); validation (equal); writing original draft (supporting); writing review and editing (equal). Sylvie Behillil: data curation (equal); investigation (equal); resources (equal); validation (equal); writing review and editing (equal). Marie‐Noelle Billard: resources (equal); validation (equal); writing review and editing (supporting). Robert Cohen: formal analysis (supporting); methodology (supporting); resources (equal); validation (equal); writing review and editing (equal). Ralph Epaud: conceptualization (equal); methodology (lead); project administration (lead); supervision (lead); validation (lead); writing original draft (supporting); writing review and editing (lead).
ACKNOWLEDGMENTS
We are grateful for the French networks: All GPs, pediatricians, and their patients for providing the samples tested. For the French hospital laboratory network, hospital emergency department staff from the OSCOUR network, RENAL network laboratories: Ile de France, Ambroise Pare, Avicenne, Bichat, Bicêtre, Henri Mondor, Versailles, Necker, Paul Brousse, Pitie Salpetrière, Robert Debré, Saint‐Louis, Cochin, Trousseau‐Saint Antoine‐Tenon, Pontoise, Poissy, and Foch. Other regions: Strasbourg, Dijon, Reims, Nancy, Besançon, Lille, Amiens, Caen, Brest, Rennes, Orléans, Tours, Rouen, Angers, Nantes, Aix‐en‐Provence, Annecy, Bordeaux, Chambéry, Clermont‐Ferrand, Grenoble, Limoges, Lyon, Marseille, Montpellier, Nice, Poitiers, Saint‐Etienne, and Toulouse.
Delestrain C, Danis K, Hau I, et al. Impact of COVID‐19 social distancing on viral infection in France: A delayed outbreak of RSV. Pediatric Pulmonology. 2021;56:3669‐3673. 10.1002/ppul.25644
Céline Delestrain and Kostas Danis contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gaborieau L, Delestrain C, Bensaid P, et al. Epidemiology and clinical presentation of children hospitalized with SARS‐CoV‐2 infection in suburbs of Paris. J Clin Med. 2020;9(7):2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ouldali N, Yang DD, Madhi F, et al. Factors associated with severe SARS‐CoV‐2 infection. Pediatrics. 2020;147(3):e2020023432. [DOI] [PubMed] [Google Scholar]
- 3. Van Brusselen D, De Troeyer K, ter Haar E, et al. Bronchiolitis in COVID‐19 times: a nearly absent disease? Eur J Pediatr. 2021;180:1969‐1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nagakumar P, Chadwick CL, Bush A, Gupta A. Collateral impact of COVID‐19: why should children continue to suffer? Eur J Pediatr. 2021;180:1975‐1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sullivan SG, Carlson S, Cheng AC, et al. Where has all the influenza gone? The impact of COVID‐19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. 2020;25(47):2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354(9181):847‐852. [DOI] [PubMed] [Google Scholar]
- 7. Bloom‐Feshbach K, Alonso WJ, Charu V, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8(2):e54445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haynes AK, Manangan AP, Iwane MK, et al. Respiratory syncytial virus circulation in seven countries with Global Disease Detection Regional Centers. J Infect Dis. 2013;208(Suppl 3):S246‐S254. [DOI] [PubMed] [Google Scholar]
- 9. Heikkinen T, Ojala E. Waris M. Clinical and socioeconomic burden of respiratory syncytial virus infection in children. J Infect Dis. 2017;215(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 10. Stein RT, Bont LJ, Zar H, et al. Respiratory syncytial virus hospitalization and mortality: systematic review and meta‐analysis. Pediatr Pulmonol. 2017;52(4):556‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Flahault A, Blanchon T, Dorleans Y, Toubiana L, Vibert JF, Valleron AJ. Virtual surveillance of communicable diseases: a 20‐year experience in France. Stat Methods Med Res. 2006;15(5):413‐421. [DOI] [PubMed] [Google Scholar]
- 12. Haute Autorité de Santé . Prise en charge du premier épisode de bronchiolite aiguë chez le nourrisson de moins de 12 mois. Recommandation de bonne pratique, texte court. Paris, 14 Novembre 2019. https://www.has-sante.fr/jcms/p_3118176/fr/bronchiolite-aigue-chez-le-nourrisson-recommandations
- 13. Souty C, Guerrisi C, Masse S, et al. Impact of the lockdown on the burden of COVID‐19 in outpatient care in France, spring 2020. Infect Dis (Lond). 2021;53(5):376‐381. [DOI] [PubMed] [Google Scholar]
- 14. Pelat C, Boëlle PY, Cowling BJ, et al. Online detection and quantification of epidemics. BMC Med Inform Decis Mak. 2007;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Belchior E. Équipes de surveillance de la bronchiolite. Surveillance de la bronchiolite en France, saison 2016‐2017. Bull Epidémiol Hebd. 2017;30:650‐657. [Google Scholar]
- 16.European Center for Disease Prevention and Control (ECDC). Surveillance ATLAS for infectious diseases‐Respiratory Syncytial Virus‐RSV detection. 2021; http://atlas.ecdc.europa.eu/public/index.aspx?Dataset=27%26HealthTopic=71%26Indicator=516363%26GeoResolution=1%26TimeResolution=Week%26StartTime=2014-W40%26EndTime=2021-W12%26CurrentTime=2021-W12%26TimeSeries=region%26TimeSeriesRepresentation=T
- 17. Yeoh DK, Foley DA, Minney‐Smith CA, et al. The impact of COVID‐19 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin Infect Dis. 2021;72(12):2199‐2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foley DA, Phuong LK, Peplinsk J, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. Published online August 25, 2021. 10.1136/archdischild-2021-322507. 2021.. [DOI] [PubMed] [Google Scholar]
- 19. Lambert L, Sagfors AM, Openshaw PJ, Culley FJ. Immunity to RSV in early‐life. Front Immunol. 2014;5:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poole S, Brendish NJ, Tanner AR, Clark TW. Physical distancing in schools for SARS‐CoV‐2 and the resurgence of rhinovirus. Lancet Respir Med. 2020;8(12):e92‐e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


