Abstract
Recently, the coronavirus disease 2019 (COVID‐19) has caused a global pandemic. Several studies indicate that the digestive system can also be affected by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Therefore, patients with digestive symptoms should have a capsule endoscopy (CE). COVID‐19 patients with gastrointestinal (GI) symptoms who underwent CE were recruited from March 2020 to April 2020. We collected patients’ data and performed a prospective follow‐up study for 6 months. All 11 COVID‐19 cases with GI symptoms who underwent CE presented gastritis. Eight cases (72.7%) had intestinal mucosa inflammation. Among them, two cases showed intestinal ulcers or erosions. Moreover, two cases displayed colonic mucositis. One case was lost during follow‐up. At 3–6 months after hospital discharge, five patients underwent CE again, presenting gastrointestinal lesions. Five of the 10 cases had GI symptoms, such as abdominal pain, diarrhea, constipation, and others. Among these five cases, the GI symptoms of three patients disappeared at the last follow‐up and two patients still presented diarrhea symptoms. Overall, we observed damaged digestive tract mucosa that could be caused by SARS‐CoV‐2. Moreover, after discharge, some patients still presented intestinal lesions and GI symptoms.
Keywords: capsule endoscopy, COVID‐19, follow‐up, SARS‐CoV‐2
1. BACKGROUND
The novel coronavirus‐infected pneumonia was first identified in Wuhan, central China. 1 The World Health Organization (WHO) termed this new virus the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The coronavirus disease 2019 (COVID‐19) has caused a global pandemic and become a global public health problem. On August 9, 2021, the number of infections was above 200 million worldwide according to the worldometers data (https://www.worldometers.info/coronavirus/). The major SARS‐CoV‐2 transmission route was spread droplets or direct contact with fomites. 2 COVID‐19 received wide concerns due to viral pneumonia with respiratory manifestations, such as fever and cough. However, some patients present only gastrointestinal (GI) symptoms as initial signs, without fever and cough. 3
The GI symptoms were not very common in COVID‐19 patients: diarrhea (2%–10.1%), and nausea and vomiting (1%–3.6%), for example. 4 , 5 Nevertheless, another study showed that a significant proportion of COVID‐19 patients presented GI symptoms on admission. 6 Additionally, several studies found that feces of COVID‐19 patients are viral RNA positive. 7 , 8 Moreover, a recent study demonstrated active replication of SARS‐CoV‐2 in human intestinal organoids. 9 SARS‐CoV‐2 enters cells mainly through angiotensin‐converting enzyme 2 (ACE2). 10 Importantly, the ACE2 expressing levels in the GI tract are higher than in respiratory organs (lung). 11 , 12 Thus, SARS‐CoV‐2 can enter the GI tract cells through ACE2, then the virus replication can cause GI symptoms, including diarrhea, nausea, vomiting, and others.
Although growing evidence suggests that SARS‐CoV‐2 colonizes the digestive tract and can cause mucosa damage, no direct images showing mucosa lesions in the digestive tract, especially small intestinal, are available. Therefore, we performed capsule endoscopy (CE) on COVID‐19 patients with digestive symptoms, especially those with diarrhea, to observe if damages to the digestive tract mucosa had occurred. Additionally, patients undergoing CE were followed up for 6 months.
2. METHODS
2.1. Study design
This is a prospective observational and follow‐up study approved by the Medical Ethical Review Committee, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, China, and was conducted at the Union Hospital of Tongji Medical College (Wuhan, China). We analyzed clinical, laboratory testing, and CE image characteristics of COVID‐19 patients with only/accompanied with GI symptoms. The CE indications were positive GI symptoms for COVID‐19 patients. Considering the risks of performing CE and patients’ safety, we excluded severe COVID‐19 patients or patients with abdominal pain. Patients were considered with positive GI symptoms if they presented at least one of the following symptoms: anorexia, nausea, vomiting, diarrhea, or others. GI symptoms were recorded on admission, precluding the medical therapy influence or other nonviral factors. The COVID‐19 was diagnosed as positive SARS‐CoV‐2 by real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) assay for nasal or pharyngeal swab specimens. From March 2020 to April 2020, 11 COVID‐19 patients underwent CE (PillCam™ SB3, PillCam™ COLON2, Medtronic, Minneapolis, MN, USA) at the Union Hospital of Tongji Medical College. For cases 1–6, CE was performed with PillCam™ SB3. To evaluate colonic lesions better, PillCam™ COLON2 was used for cases 7–11 and follow‐ups. All participants signed informed consent. At least 6 months of clinical follow‐up time was conducted for all patients. However, one patient was lost during follow‐up. At 3–6 months after hospital discharge, five of the patients underwent CE again. Figure 1 displays patient flow.
Figure 1.
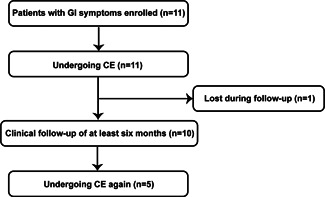
Patient flow. CE, capsule endoscopy; GI, gastrointestinal
2.2. Collection of patients’ information
Information including clinical and laboratory data was retrieved from patients’ medical records and was verified by doctors. Clinical data include age, gender, time from onset of symptoms to diagnosis, respiratory symptoms, gastrointestinal symptoms, medication, and hospital stay length. Laboratory assessments consisted of white blood cell (WBC) count, neutrophil ratio (N%), lymphocyte ratio (L%), total bilirubin, direct bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine, and potassium. For missing information, direct communications with attending doctors or patients were performed.
2.3. CE image review
All CE images were reviewed by one experienced endoscopist. For all patients, CE images were evaluated for the following characteristics: (a) presence of gastric mucosa lesions; (b) presence of small intestinal mucosa lesions; and (c) presence of colonic mucosa lesions.
2.4. Statistical analysis
Data are reported as mean ± standard deviation (SD), maximums, minimums, number, and percentages, when appropriate.
3. RESULTS
3.1. Clinical characteristics
Four men and seven women were included in our study with an age range of 27–73 years. Nasal or pharyngeal swab samples were collected and SARS‐CoV‐2 nucleic acid tests results were positive 1–45 days after disease onset. Nine patients presented respiratory symptoms and two (18.2%) did not experience clear respiratory symptoms. All patients presented GI symptoms and eight experienced diarrhea. Diarrhea lasted from 5 to 40 days, with an average duration of 14.0 ± 12.4 days. The daily average frequency was 3–18 bowel movements per day. One patient received systematic glucocorticoid, antivirus, and nonsteroidal anti‐inflammatory drugs (NSAIDs), and three received antivirus and NSAIDs. Four patients received only one of these three drugs and no drugs were used in three cases. The hospital stay ranged between 9 and 41 days. Clinical characteristics are summarized in Table 1.
Table 1.
General information of COVID‐19 patients (n = 11)
| Parameters | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 55 | 73 | 69 | 30 | 27 | 38 | 28 | 41 | 37 | 35 | 32 |
| Gender | Male | Male | Female | Female | Female | Male | Female | Female | Male | Female | Female |
| Time from onset of symptoms to diagnosis (days) | 20 | 45 | 7 | 39 | 32 | 19 | 1 | 3 | 8 | 2 | 3 |
| Respiratory symptoms | |||||||||||
| Fever | No | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Cough/expectoration | Yes | No | No | Yes | Yes | Yes | No | No | No | No | Yes |
| Othersa | Yes | No | No | No | No | No | Yes | No | Yes | Yes | No |
| Gastrointestinal symptoms | |||||||||||
| Anorexia | Yes | No | No | No | No | Yes | No | No | No | No | No |
| Nausea | No | Yes | Yes | No | No | No | No | No | No | No | No |
| Vomiting | No | Yes | No | No | No | No | No | No | No | No | No |
| Diarrhea | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Othersb | No | No | No | No | No | No | No | No | No | No | No |
| Medication | |||||||||||
| Glucocorticoid | Yes | No | No | No | No | No | No | No | Yes | No | No |
| Antiviral drugs | No | No | Yes | No | No | Yes | Yes | No | Yes | No | Yes |
| NSAIDs | No | No | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| Hospital stay length (days) | 38 | 19 | 37 | 9 | 38 | 38 | 20 | 41 | 20 | 17 | 14 |
Abbreviations: COVID‐19, coronavirus disease 2019; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Others included chest distress, pharyngodynia, breath shortness, stuffiness, and runny nose.
Others included abdominal pain, constipation, and abdominal distension.
3.2. Laboratory tests
WBC counts and N% were high in Case 1 and WBC counts were low in Case 3. L% levels decreased in Cases 1 and 2. Total and direct bilirubin did not increase in all cases. Case 6, 7, and 9 showed abnormal levels of AST or ALT. Creatine only increased in Case 2. Potassium levels were mildly low only in Case 6. These results are shown in Table 2.
Table 2.
Laboratory findings in patients
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Normal range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC count (×109/L) | 10.25↑ | 7.05 | 2.69↓ | 5.25 | 7.51 | 4.74 | 6.24 | 3.62 | 5.25 | 4.55 | 4.83 | 3.5–9.5 |
| N% | 77.20↑ | 73.90 | 57.80 | 63.45 | 61.20 | 53.80 | 61.70 | 52.20 | 51.10 | 52.90 | 63.0 | 40–75 |
| L% | 14.2↓ | 14.5↓ | 29.4 | 27.1 | 30.6 | 35.2 | 28.8 | 37.0 | 36.0 | 35.2 | 28.4 | 20–50 |
| Total bilirubin (μmol/L) | 10.9 | 17.0 | 4.7 | 13.5 | 11.7 | 8.3 | 6.1 | 7.2 | 4.8 | 8.5 | 3.5 | 3.0–20 |
| Direct bilirubin (μmol/L) | 3.1 | 5.4 | 1.9 | 3.0 | 2.8 | 3.6 | 1.8 | 2.1 | 1.2↓ | 3.5 | 0.8↓ | 1.7–6.8 |
| ALT (U/L) | 24 | 20 | 12 | 20 | 16 | 26 | 100↑ | 30 | 90↑ | 12 | 26 | 5–40 |
| AST (U/L) | 16 | 28 | 15 | 23 | 15 | 43↑ | 69↑ | 18 | 30 | 16 | 24 | 8–40 |
| Creatine (μmol/L) | 52.0↓ | 176.8↑ | 59.6 | 65.0 | 65.5 | 94.8 | 52.9↓ | 53.7↓ | 68.6 | 44.1↓ | 59.2 | 57–111 |
| Potassium (mmol/L) | 4.47 | 3.66 | 4.18 | 4.10 | 4.65 | 3.48↓ | 3.94 | 4.54 | 4.38 | 4.52 | 4.59 | 3.5–5.3 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; L%, lymphocyte ratio; N%, neutrophil ratio; WBC, white blood cell.
3.3. Imaging characteristics of CE
In this study, all cases presented gastritis. Additionally, only Case 2 showed gastric ulcer. Eight (72.7%) patients had intestinal mucosa inflammation. Among these eight patients, Case 1 and 5 presented intestinal erosions or ulcers. Case 5 and 8 showed colonic mucositis. However, the CE imaging for colonic mucosa was not available in Case 2. These details are shown in Table 3 and representative images are shown in Figure 2.
Table 3.
Inflammation and ulcer findings at CE imaging in 11 patients
| Variables | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Superficial gastritis | No | No | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Erosive gastritis | Yes | Yes | No | Yes | No | Yes | No | No | Yes | No | No |
| Atrophic gastritis | No | No | No | No | No | Yes | No | No | No | No | No |
| Gastric ulcer | No | Yes | No | No | No | No | No | No | No | No | No |
| Intestinal mucositis | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No |
| Intestinal erosions | No | No | No | No | Yes | No | No | No | No | No | No |
| Intestinal ulcer | Yes | No | No | No | Yes | No | No | No | No | No | No |
| Colonic mucositis | No | NA | No | No | Yes | No | No | Yes | No | No | No |
| Melanosis coli | No | NA | Yes | No | No | No | No | No | No | No | No |
Abbreviations: CE, capsule endoscopy; NA, not available.
Figure 2.
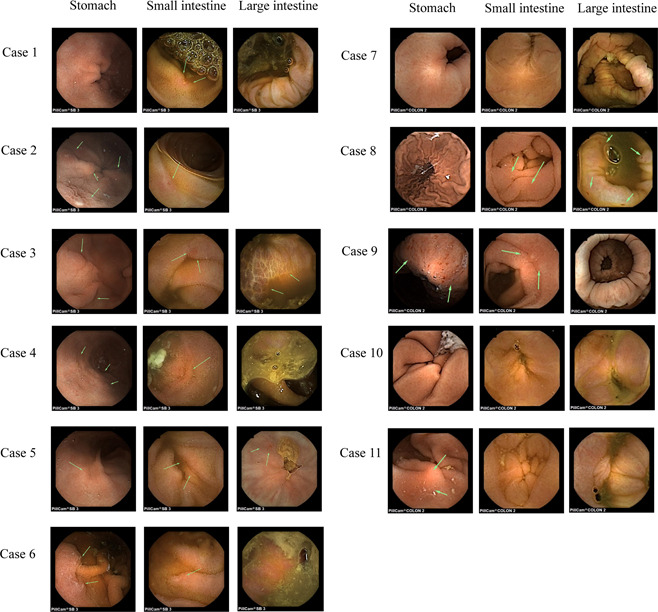
Representative images of CE during hospitalization. CE, capsule endoscopy
3.4. Follow‐up
Case 2 was lost during follow‐up. At 3–6 months after hospital discharge, five patients (Case 1, 5, 6, 7, and 8) underwent CE again. Five patients still presented gastrointestinal lesions. These results are shown in Table 4 and the representative images are shown in Figure 3. Five cases (Case 5, 7, 8, 9, and 10) had GI symptoms, such as abdominal pain, diarrhea, constipation, and others. Among these five patients, the GI symptoms of three cases (Case 5, 7, and 10) disappeared at the last follow‐up. Cases 8 and 9 still showed diarrhea symptoms.
Table 4.
CE imaging findings in six patients during follow‐up
| Variables | Case 1 | Case 5 | Case 6 | Case 7 | Case 8 |
|---|---|---|---|---|---|
| Superficial gastritis | No | Yes | No | Yes | Yes |
| Erosive gastritis | Yes | Yes | Yes | No | No |
| Atrophic gastritis | No | No | Yes | No | No |
| Gastric ulcer | No | No | No | No | No |
| Intestinal mucositis | No | Yes | No | Yes | Yes |
| Intestinal erosions | Yes | No | No | No | No |
| Intestinal ulcer | Yes | No | No | No | No |
| Colonic mucositis | No | No | Yes | No | No |
Abbreviation: CE, capsule endoscopy.
Figure 3.
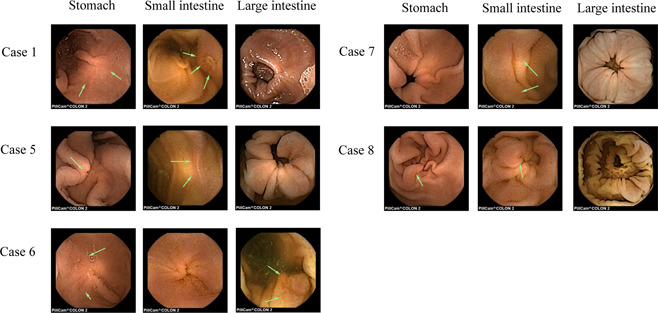
Representative images of CE during follow‐up. CE, capsule endoscopy
4. DISCUSSION
In the past, severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome, both caused by coronaviruses, represented two large‐scale pandemics. 13 , 14 Now SARS‐CoV‐2 caused a global outbreak and has become an enormous threat to human beings. Increasing evidence showed that COVID‐19 can cause digestive system damages. However, most of the researchers have focused on common COVID‐19 symptoms, such as fever, cough, and breath shortness. This focus increases the risk of ignoring patients showing GI symptoms on admission. Our results indicated that SARS‐CoV‐2 can actively infect and affect the GI tract. Also, after discharge, five patients had GI symptoms, such as abdominal pain, diarrhea, constipation, and others.
Two studies have found that SARS‐CoV‐2 RNA is detectable in feces. 7 , 8 However, there is no clear evidence that digestive tract mucosa damage happens in COVID‐19 patients. Therefore, it is necessary to perform CE on patients with digestive symptoms. Performing GI endoscopy in COVID‐19 patients can be challenging. The contact at proximity increased medical staff exposure and infection risk during GI endoscopy procedures, with further transmission to other staff members and patients, causing an outbreak in the hospital. 15 CE does not have the disadvantage and can be used to observe the total digestive tract. Therefore, in our current study, we used CE to observe digestive tract lesions in COVID‐19 patients. To our knowledge, this is the first report that describes CE imaging of COVID‐19 patients with GI symptoms.
We found that all eleven COVID‐19 patients with GI symptoms had gastritis. Eight (72.7%) patients showed intestinal mucosa inflammation and two had colonic mucositis. To preclude the influence of medical therapy or other nonviral factors, GI symptoms were recorded on admission. However, it is still challenging to exclude nonviral factors affecting GI lesions, such as antiviral drugs, NSAIDs, glucocorticoid, and others. However, our findings regarding CE on COVID‐19 patients with GI symptoms can still be helpful for disease evaluation.
Also, other studies support our findings. Electron microscopy on biopsy and autopsy specimens showed active SARS‐CoV replications in both small and large intestines. 16 Genome sequences showed that SARS‐CoV‐2 shared 79.6% sequence identity to SARS‐CoV. Moreover, both can bind to the entry receptor ACE2 and enter human cells. 10 , 17 , 18 SARS‐CoV‐2 gastrointestinal tract infection mechanism might be associated with ACE2. SARS‐CoV‐2 uses ACE2 , as SARS‐CoV, and does not use other coronavirus receptors. 10 Also, ACE2 is expressed in gastrointestinal epithelial cells. 19 Moreover, ACE2 amino acid transport function was linked to gastrointestinal tract microbial ecology and altered gut microbial composition. 20 Therefore, we speculate that COVID‐19 would affect the gut microbiota through ACE2, leading to gastrointestinal lesions and frequent occurrence of GI symptoms in COVID‐19 patients. Further studies explaining the underlining mechanisms for these GI symptoms are urgently needed. Moreover, after discharge, five patients had GI symptoms. This was a observational study about COVID‐19 after‐effects for 6 months. This may also help understand the characteristics of the virus.
Our study also has limitations. First, the GI symptoms and lesions in these COVID‐19 patients could be caused by other reasons. For example, antiviral drugs clinically applied, such as abidol, lopinavir, ritonavir, may cause diarrhea, nausea, and other digestive system adverse reactions. Moreover, coexisting stress ulcers in COVID‐19 patients can also lead to GI symptoms.
5. CONCLUSIONS
Overall, the gastrointestinal involvement of COVID‐19 and its after‐effects requires considering several clinical policies, such as diagnosis, management, and infection prevention. These considerations are crucial in the battle against COVID‐19. Finally, medical staff should pay more attention to those untypical patients with initial presentations of GI symptoms.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHORS CONTRIBUTIONS
Xiao‐Ping Xie, Li‐Ping Sheng, and Chao‐Qun Han collected medical records data, analyzed the data, drafted the manuscript, and equally contributed to this article. Xiao‐Ping Xie helped operating capsule endoscopy. Yu Jin and Tao Bai helped with the data statistics. Xiao‐Hua Hou contributed with manuscript revisions and important intellectual content. Zhen Ding and Rong Lin designed, supervised the study, and revised the manuscript as the corresponding authors. All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
This study was supported by a novel coronavirus pneumonia emergency science and technology project from the Science and Technology Department of Hubei Province, Wuhan, China (No. 2020FCA014). Also, it was partly supported by the National Natural Science Foundation of China (No. 81720108006, 81800467, 81770637).
Xie X‐P, Sheng L‐P, Han C‐Q, et al. Features of capsule endoscopy in COVID‐19 patients with a six‐month follow‐up: A prospective observational study. J Med Virol. 2021;94:246‐252. 10.1002/jmv.27308
Xiao‐Ping Xie, Li‐Ping Sheng, and Chao‐Qun Han contributed equally to this study.
Contributor Information
Rong Lin, Email: selinalin35@hotmail.com.
Zhen Ding, Email: docd720@126.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin X, Lian J‐S, Hu J‐H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut. 2020;69:1002‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen C, Gao G, Xu Y, et al. SARS‐CoV‐2‐positive sputum and feces after conversion of pharyngeal samples in patients with COVID‐19. Ann Intern Med. 2020;172:832‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang T, Cui X, Zhao X, et al. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J Med Virol. 2020;92:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS‐CoV‐2. Nat Med. 2020;26:1077‐1083. [DOI] [PubMed] [Google Scholar]
- 10. Zhou P, Yang X‐L, Wang X‐G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lau YL, Peiris JSM. Pathogenesis of severe acute respiratory syndrome. Curr Opin Immunol. 2005;17(4):404‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drosten C, Günther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967‐1976. [DOI] [PubMed] [Google Scholar]
- 14. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus ADME, Fouchier RAM. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814‐1820. [DOI] [PubMed] [Google Scholar]
- 15. Ang TL. Gastrointestinal endoscopy during COVID‐19 pandemic. J Gastroenterol Hepatol. 2020;35:701‐702. [DOI] [PubMed] [Google Scholar]
- 16. Leung WK, To K‐F, Chan PK, et al. Enteric involvement of severe acute respiratory syndrome‐associated coronavirus infection. Gastroenterology. 2003;125(4):1011‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020;158:1831‐1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487(7408):477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


