Abstract
Although significant research has been done to find effective drugs against coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), no definite effective drug exists. Thus, research has now shifted towards immunomodulatory agents other than antivirals. In this review, we aim to describe the latest findings on the role of type I interferon (IFN)‐mediated innate antiviral response against SARS‐CoV‐2 and discuss the use of IFNs as a medication for COVID‐19. A growing body of evidence has indicated a promoting active but delayed IFNs response to SARS‐CoV‐2 and Middle East respiratory syndrome coronavirus in infected bronchial epithelial cells. Studies have demonstrated that IFNs' administration before the viral peak and the inflammatory phase of disease could offer a highly protective effect. However, IFNs' treatment during the inflammatory and severe stages of the disease causes immunopathology and long‐lasting harm for patients. Therefore, it is critical to note the best time window for IFNs' administration. Further investigation of the clinical effectiveness of interferon for patients with mild to severe COVID‐19 and its optimal timing and route of administration can be beneficial in finding a safe and effective antiviral therapy for the COVID‐19 disease.
Keywords: COVID‐19, interferon type I, SARS‐CoV‐2
Highlights
1‐IFNs have many antiviral actions including; the activation of cytotoxic T‐cell responses, the inhibition of the viral mRNA translation, the degradation of the viral RNA, RNA editing and modulating the synthesis of Nitric Oxide.
2‐IFNS are two‐edged immunomodulatory agents; as they can provide a protective effect if administered in the early phases of the disease before the viral peak, whereas a harming effect is observed when administered in the inflammatory phase.
3‐More human trials are needed to find the best time window for administrating type I IFN for patients with various COVID‐19 modalities.
1. INTRODUCTION
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was detected in China, and as soon as March 11, 2020, the World Health Organization (WHO) declared it as a pandemic. 1
Our understanding of SARS‐CoV‐2 has significantly improved since its emergence in China due to the approval of various pharmacological managements that treat or relieve the COVID‐19 disease, such as antiviral drugs (e.g., remdesivir, favipiravir, ribavirin, and lopinavir/ritonavir), anti‐SARS‐CoV‐2 monoclonal or polyclonal antibodies (e.g., bamlanivimab/etesevimab, casirivimab/imdevimab), convalescent plasma and immunomodulatory agents (e.g., corticosteroids, interferons, baricitinib, and tocilizumab). 2 , 3
Interferons are cytokines required for the induction of antiviral immune responses. Infected cells release interferons to activate the antiviral state in nearby cells and induce inflammatory cytokine production. 4 Type 1 interferons (IFN‐α/β) are mainly released by plasmacytoid dendritic cells (pDSs), while type 2 IFNs are secreted by natural killer (NK) cells, macrophages, and T cells. 5 This review describes the interaction between SARS‐CoV‐2 and various type 1 IFNs and the related clinical studies based on the available evidence.
2. VIROLOGY OF SARS‐CoV‐2
Coronaviruses are enveloped positive‐sense single‐stranded RNA viruses with crown‐like morphology. 6 SARS‐CoV‐2 belongs to Nidovirales order, Coronaviridae family, and Coronavirinae subfamily. This subfamily is categorized into four genera: α‐, β‐, γ‐, and δ‐CoV. 6 SARS‐CoV‐2 is a β coronavirus that binds to its receptor, human angiotensin‐converting enzyme 2 (ACE2), for cellular entry. 7 Two‐thirds of the SARS‐CoV‐2 genome consists of Open Reading Frame (ORF) 1a and 1b, which encodes replicase polyproteins pp1a and pp1ab, nsp3‐PLpro, and nsp5‐Mpro of SARS‐CoV‐2 cleave these polyproteins into nonstructural proteins (nsps), nsp1‐11, and nsp1‐16, respectively. The remaining genome encodes structural proteins including spike (S), envelope (E), membrane (M), and nucleoprotein (N) as well as accessory proteins (ORF‐3a, −3b, −6, −7a, −7b, −8, −9a, −9b, and 10). 8 , 9 SARS‐CoV‐2 uses a similar mechanism to those employed by prior SARS‐CoV‐1 infection for cell entry. 10 , 11
3. THE LIFE CYCLE OF SARS‐CoV‐2
SARS‐CoV‐2 enters host cells via interlocking its spike protein to the host's ACE2 receptor. To complete cell entry, the S protein priming, also known as the proteolytic separation of S1 and S2 proteins of SARS‐CoV‐2, is needed during the membrane fusion reactions. This phenomenon is facilitated by the host's expression of transmembrane protease serine 2. 7 , 12 , 13 Several publications have demonstrated that IFN‐stimulated genes (ISGs) induce a high expression of ACE2 in host cells and thus enhance virus entry and infection; an article, however, discovered that following SARS‐CoV‐2 infection, IFNs trigger a truncated version of ACE2 called dACE2, not ACE2 thus, it would not promote the viral entry. 14 The life cycle of SARS‐CoV‐2 includes the following five steps: (1) its attachment binding the cellular receptor, (2) its penetration and entrance into host cells through endocytosis or penetration, (3) expressing its RNA polymerases in the host cell cytoplasm and starting replication and biosynthesis of other viral contents, (4) mature new viral products are packed, and (5) released from the host cell. 15 , 16 Several mechanisms have been proposed to cause cell damage following SARS‐CoV‐2 entry to host cells, including direct viral cell damage; dysregulating the renin‐angiotensin‐aldosterone system (RAAS, a type of hormonal system which controls blood pressure, the balance of fluid and electrolyte, vascular permeability, as well as tissue growth), dysregulating the immune system, endothelial cell injury, and thromboinflammation. 12 ACE2 is an essential regulator of the RAAS system; ACE2 converts angiotensin I to inactive angiotensin 1–9 and subsequently converts angiotensin II into angiotensin 1–7. 17 , 18
4. CLINICAL AND IMMUNOLOGICAL CHARACTERISTICS OF COVID‐19 PATIENTS
COVID‐19 disease is classified into three stages: Stage 1 is considered as early infection with the highest degree of host viral response as well as mild symptoms including fever and dry cough, Stage 2 is considered as pulmonary phase, and at last Stage 3 is considered as a hyperinflammation phase, which shows the greatest disease severity as well as presentations of acute respiratory distress syndrome (ARDS). 19 Approximately 20% of patients with COVID‐19 manifest severe infection and progress to Stage 3 of the disease, while nearly 80% of patients exhibit mild symptoms of infection and managed to clear viral particles. 20 COVID‐19 has a wide range of clinical respiratory symptoms (mild fever, dry cough, sore throat, malaise, headache, and muscle pain) and digestive symptoms (nausea, vomiting, abdominal pain, and diarrhea), as well as hepatic, renal, and neurologic diseases. 9 Pathological damage of the pulmonary system includes pulmonary edema, diffuse alveolar damage associated with the hyaline membrane, fibrosis, and multinucleated giant cells. 20
SARS‐CoV‐2 infection induces an enhanced innate immune response; following virus entry, necrosis, or pyroptosis of host cells leading to the production of inflammatory cytokines and chemokines, aka the cytokine storm (CS). Thereupon the migration of monocytes/macrophages and neutrophils to the site of infection resulting in an uncontrolled immune response and severe tissue damage, which consequently contributes to morbidity and mortality. 21 , 22 Supportably, according to evidence, the severity of the COVID‐19 infection is correlated with the level of inflammatory cytokines. Increased expression of pro‐inflammatory cytokines and dysregulated immune response seems to be the leading cause of pathological events and could result in ARDS and organ failure. 23 In patients with the COVID‐19 infection, plasma levels of cytokines and inflammatory factors including interleukin (IL)‐1β, IL‐7, IL‐8, IL‐10, IFN‐γ, monocyte chemoattractant peptide‐1, macrophage inflammatory protein (MIP)‐1A, MIP‐1B, granulocyte colony‐stimulating factor, and tumor necrosis factor‐alpha (TNF‐α) increase. 24 The disease severity is directly proportional to elevated immune cells, IL‐6, TNF‐α, the chemokine (C‐C motif) ligand 7, and the chemokine (C‐X‐C motif) ligand 10 (CXCL10). 25 In addition to cytokines and other inflammatory factors, SARS‐CoV‐2 dysregulates the proper function of immune cells via reducing the total count of B lymphocytes, CD4+ and CD8+ T lymphocytes, and NK cells. Moreover, SARS‐CoV‐2 simultaneously upregulate expression of NKG2A receptor (a family of C‐type lectin receptors on the surface of NK cells), leading to the exhaustion of NK and CD8+ T cells. 26 , 27 In contrast to reduced lymphocytes, the levels of neutrophils, leukocytes, and neutrophil–lymphocyte‐ratio are increased in severe COVID‐19 patients. 23 In conclusion, several organs of the COVID‐19 patients undergo prominent immune response dysregulation, hardening the treatment process. 28 Therefore, this review firstly focuses on the role of an innate immune response, especially interferons, in COVID‐19 infection, then describes the interaction between SARS‐CoV‐2 and IFNs based on related clinical studies to pave the way towards finding an effective immunomodulatory agent against the COVID‐19 infection.
5. INNATE IMMUNE RESPONSE
Innate immune response serves as the first defensive line against pathogens and plays a vital role in antiviral immunity. The first step of innate immunity is initiated by engaging pattern recognition receptors (PRRs). 29 The viral‐derived pattern‐associated molecular patterns (PAMPs) such as viral single‐stranded RNA (ssRNA) and double‐stranded RNA (dsRNA) are sensed through the cytosolic retinoic acid‐inducible gene‐1‐like receptor (RIG‐1 like receptors, RLRs) and melanoma differentiation‐associated protein (MDA5). 30 Moreover, PAMPs are sensed through the endosomal Toll‐like receptor (TLR)3, 7, 8, and 9, anchored on the endosomal membrane TLR‐4 deficiency predisposes mice models to SARS‐CoV‐2 infection. Adaptor protein molecules such as MyD88 (for TLR4, TLR7, and TLR8) and TRIF (for TLR3 and TLR4) contribute to the protective effect of innate immunity. 31 MyD88 and TRIF are two adaptor protein‐dependent signaling pathways known to stimulate inflammatory cytokines and chemokines released after activating specific TLRs, leading to upregulation of immune cell expression. In MyD88‐deficient mice, the production of inflammatory cytokines is suppressed, leading to uncontrolled viral replication, severe lung pathology, and more weight loss than wild‐type mice during SARS‐CoV‐1 infection. 31 , 32 Similarly, TRIF knockout mice showed decreased inflammatory cytokines in the first two days after coronavirus infection, followed by increased production of IFN‐β and inflammatory cytokines on the fourth day of infection. 33 Following activation of MyD88 and TRIF, NF‐κB (an essential modulator known as IKKγ) forms a complex with IKKα and IKKβ and phosphorylates NF‐κB. On the other hand, activated MDA5 and RIG‐1 collaborate with mitochondrial antiviral signaling protein (MAVS). Following the interaction of MDA5 and RIG‐1 with MAVS, IKK‐related kinase, including TBK1/IKKi is activated, activating IFN regulatory factor (IRF) 3 and 7. 34 Induction of IRF3 and seven increase expression of T1IFN, while nuclear factor‐κB (NF‐κb) activation triggers the increased production of pro‐inflammatory cytokines, including IL‐1, IL‐6, and TNF‐α 34 (Figure 1)
Figure 1.
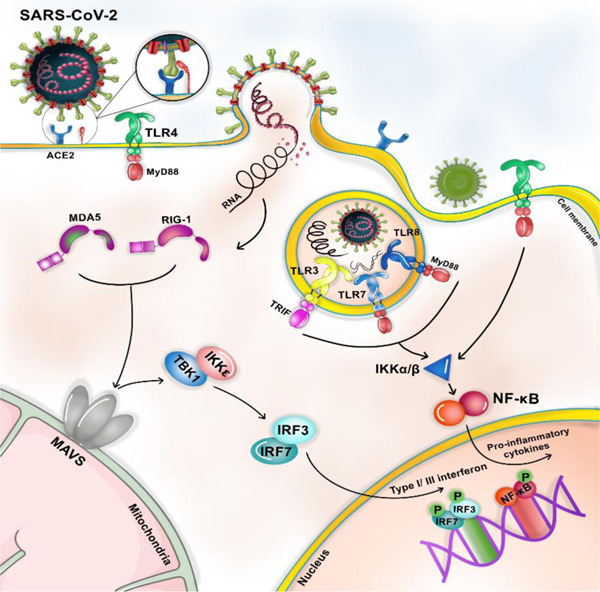
Innate immune response In the first step, infectious viral particles (PAMPs) were sensed through PRRs. SARS‐CoV‐2 is detected through endosomal PRRs including TLR 3, 7, 8, and 9 and/or through cytoplasmic PRRs such as MDA5 and RIG‐1. Moreover, the virus can be sensed through TLR4, which is localized in the cell membrane. Following TLRs activation, NF‐'kB's transcription factor induces inflammatory cytokines, whereas activated MDA5 and RIG‐1 recruit IRF3 and IRF7 for interferon production. When interferon binds its receptor, IFRAR1/2, an IFN‐induced signaling pathway is initiated, resulting in the recruitment of JAK‐1 and TYK. JAK‐1 and TYK activation trigger the phosphorylation of the signal transducer and activator of transcription (STAT)1 and 2, respectively. Subsequently, an IFN‐stimulated gene factor 3 (ISGF3) complex is formed, containing STAT1, STAT2, and IRF‐9. This complex translocated to the nucleus and increases the expression of IFN‐stimulated genes (ISGs). ISGs translation and posttranslational modification trigger antiviral action interferons, including Inhibition of mRNA inhibition, RNA degradation, RNA editing, and the initiation of T cell response, and NO synthesis. IFN, interferon; IRF, IFN regulatory factor; MDA, melanoma differentiation‐associated protein; mRNA, messenger RNA; PRRs, pattern recognition receptors; RIG, retinoic acid‐inducible gene‐1‐like receptor; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TLR, Toll‐like receptor
SARS‐CoV‐2 implements different strategies to block PRRs signaling pathways and escape from the innate immune response. It can lead to the degradation of RLRs (RIG‐1 and MDA5), essential for recognizing virus dsRNA. 35 It has been shown that hereditary mutations of TLR3‐ and IRF7‐dependent type I IFN signaling cause lethal pneumonia in patients with no underlying diseases. 34 These antiviral agents can promote an adaptive immune response, and interferons can potentially limit the COVID‐19 infection.
In addition, SARS‐CoV‐2 impairs the proper function of the dendritic cells (DCs), which play an essential role in controlling viral “particles” spread as they act as antigen‐presenting cells and deliver viral antigens to naïve T cells. DCs present viral antigens through Class II and Class I major histocompatibility complex (MHC) molecules for CD4+ and CD8+ T lymphocytes, respectively. 36 SARS‐CoV‐2 inhibits the expression of costimulatory molecules (CD80/86) on the host cell's surface and interferes with the antigen presentation process response. Moreover, it downregulates the human leukocyte antigen Class II (HLA‐II) expression on immature DC cells' surface. As a consequence of this phenomenon, the production of antibodies will be decelerated, accelerating the viral replication. 37 SARS‐CoV‐2 interacts with DCs function and causes impaired DCs maturation, suppressed T‐cell mediated humoral immunity and T cell maturation, and decreased CD4+ and CD8+ levels in the peripheral blood of the COVID‐19 patients. 38 DC cells include subpopulations of myeloid DCs, plasmacytoid DCs (pDC), and monocyte‐derived DCs. 39 DCs, especially pDCs, produce high amounts of IFNs. A study conducted on pDCs isolated from the peripheral blood of mice models of coronavirus infection demonstrated that these subpopulations of DCs are probably the major source of IFNs expression in response to coronavirus infection. 40 This result suggests that IFNs production by pDCs is critical for the Control and reduction of the replication and spreading of the highly pathogenic coronavirus infection. A recent publication showed that circulating pDCs decreased in the peripheral blood of SARS‐CoV‐2 infected patients compared to the controls, which can be due to the recruitment of these cells to the infection site. 41
6. IFN‐I SIGNALING PATHWAY
The IFN‐I signaling pathway is initiated through binding to a unique heterodimeric receptor, interferon‐alpha receptor (IFNAR). This receptor is expressed in most tissues and compromises two subunits: IFNAR1 and IFNAR2. 42 Following IFNs binding to their receptors, several signaling cascades will occur, ending in transcriptional regulation of ISG. IFNAR1 activates tyrosine kinase 2 (TYK2) in the first signaling pathway, while IFNAR2 seems to be associated with the Janus activated kinase (JAK1). TYK2 and JAK1 phosphorylate conserved tyrosine residues in the cytoplasmic tails of the IFNAR. These phosphorylated residues recruit src‐homology 2–containing signaling molecules, including STAT1 and STAT2. STAT1 and STA2 are then phosphorylated and dimerized. In the next step, the IFN‐regulatory factor 9 (IRF9) binds to them and forms the ISG factor 3 (ISGF3) transcription factor complex, which then moves to the nucleus and binds to the IFN‐stimulated response elements (ISRE) 43 (Figure 2).
Figure 2.
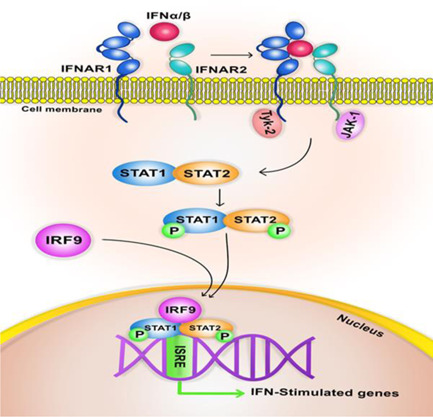
Interferon signaling pathway. IFN, interferon; IRF, IFN regulatory factor; IFNAR, interferon‐alpha receptor; ISRE, IFN‐stimulated response elements; JAK, Janus activated kinase; TYK, tyrosine kinase
Several proteins of SARS‐CoV‐2 interfere with the signaling pathways of NF‐κB and IRF3/7. ORF6, ORF8, and nucleocapsid proteins of SARS‐CoV‐2 strongly repress type I interferon (IFN‐β) signaling pathway and NF‐κB activation. 44 Further studies demonstrated that Orf9b of SARS‐CoV‐2 inhibits type I interferon responses. 45 Moreover, ORF3b proteins of SARS‐CoV‐2 have a similar effect on the IFN signaling pathway. 46 Similarly, ORF9b of SARS‐CoV‐2 antagonizes IFN expression through interaction with multiple components of RIG‐I/MDA‐5‐MAVS and TLR3. 47 As another viral strategy, the S protein of SARS‐CoV‐2 induces the expression of SOCS3/1 (suppressor of cytokine signaling) in infected cells. The induction of SOCS1/3 represses IFNs production and increase SARS‐CoV‐2 replication in infected cells.
7. ANTIVIRAL ACTIONS OF IFNs
Antiviral actions of IFNs can be classified into six classes (Figure 3). First, protein kinase‐R is activated upon viral infection through an autophosphorylation process induced by IFNs signaling. 48 Moreover, dsRNA mediates the activation of PKR. PKR activation catalyzes the phosphorylation of the α subunit of initiation factor 2 (eIF2α, also known as an inhibitor of transcription factor IkB), which decreases mRNA translation and subsequent inhibition of viral protein synthesis. 49 Activated PKR induces the activation of transcription factor NF‐κB through the phosphorylation of its inhibitory subunit, IkB. Activated NF‐κB promotes the expression of inflammatory cytokines and prohibits the virus from spreading through the host cells. 50 It is not well understood whether the novel coronavirus modulates the PKR pathway, but accessory protein 4a (p4a) of Middle East respiratory syndrome coronavirus (MERS‐CoV) blocks the PKR pathway and increases viral replication. 51 Further studies are needed to discover the interplay between SARS‐CoV‐2 and PKR pathways.
Figure 3.
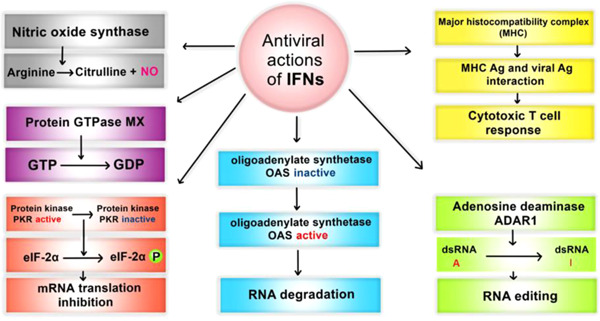
Antiviral actions of interferons. ADAR, adenosine deaminase acting on RNA; dsRNA, double‐stranded RNA; IFNs. interferons; mRNA, messenger RNA; OAS, 2ʹ−5ʹ oligoadenylate synthesis
Second, IFNs induce the activation of 2ʹ−5ʹ oligoadenylate synthesis (OAS), which phosphorylates 2ʹ−5ʹ oligoadenylate (2,5A). Subsequently, 2,5A activates latent RNase that results in viral RNA degradation. 52
Third, IFNs activate nitric oxide synthase (NOS); NOS catalyzes the oxidation of arginine to produce citrulline and nitric oxide (NO). The latter exhibits cytotoxic effects that result in the death of the infected cells.
Fourth, adenosine deaminase acting on RNA (ADAR) is triggered by IFNs and catalyzes the deamination of adenosine to inosine (A‐to‐I editing) that leads to RNA editing. 49
Fifth, the expression of Mx genes is mediated by type I and III interferons; Mx proteins inhibit the early stage of the replication cycle of a wide range of viruses, especially negative‐stranded RNA viruses, through their GTPase activity. 53 , 54
Lastly, it has been shown that following viral infection, interferons can increase the level of MHC class I and II to better inhibit viral replication. IFN‐treated human cells have elevated MHC antigen levels. Viral infection activates MHC Class I‐restricted CD8+ T cells and MHC class II‐restricted CD4+ T cells, presenting antigen peptides to cytotoxic T cells. 55 , 56
In the early stage of the disease, IFNs exert a highly potential antiviral activity by stimulating antigen‐presenting cells (APC) such as natural killer cells and dendritic cells. 57 Similarly, IFNs show an impressive capacity for enhancing and regulating key immune cells, such as localization and differentiation of virus‐specific T cells, the turnover of memory T cells, and the recruitment of T and B lymphocytes to the infection site. 57 IFNs expression upregulates the level of transmembrane protein (IFITM3), which is involved in the fusion of the pathogenic virus and endocytic vesicles and combines it with the lysosomes. In this way, the host cell prevents viral “particles” release into the cytoplasm and controls viral spread. 58 It has been suggested that type 1 interferon impairment in severe and critical cases of COVID‐19 is associated with deteriorated inflammatory response and higher viral load in the blood. NF‐κB is considered as the main driving factor in exacerbating the disease, indicating the vitality of the deficiency or the impairment of the IFN‐I signaling pathway in the COVID‐19 infection. 59 Briefly, IFN is suggested to be the first defensive line against viral infection and its downregulation or delayed action, as discussed later, can lead to a more severe form of the disease and viral spread throughout the host body.
8. DELAYED/IMPAIRED TYPE I IFNs DURING INFECTION
During the initial phase of the COVID‐19 infection, when viral load and disease severity are low, IFN‐I expression plays a pivotal role against virus replication. Studies have reported that SARS‐CoV‐2 induces dysregulated IFN‐I production in host cells. 60 According to several studies, mild to moderate COVID‐19 infected patients show increased IFN‐I in the infection site and the peripheral blood. In contrast, COVID‐19 patients in the severe stages of the disease and patients with a higher viral load, such as patients with comorbidities and elderly cases, seem to have suppressed expression of IFN‐I and increased tissue inflammation and pathology. 60 Reduced levels of IFN‐I in the peripheral blood of infected patients could provide a hallmark of disease severity and a better combination of therapeutic approaches for physicians. In patients with severe COVID‐19 infection, impaired expression of IFN‐I contributes to high viral loads in peripheral blood and exacerbated inflammatory and pathological responses. 60
Clinical studies have reported that SARS‐CoV‐1 induces impaired IFN expression following infection. 61 , 62 Other studies also suggest that IFNs expression can be delayed during SARS‐CoV‐1 pathogenesis. 63 A growing body of evidence indicated that human bronchial epithelial cells, rather than complete absence, promote active but delayed IFNs response to SARS‐CoV‐2 and MERS‐CoV infection. 63 , 64 As COVID‐19 triggers a robust production of inflammatory cytokines and induces impaired/delayed IFNs expression, it is assumed that infected patients show the accumulation of pathogenic monocyte/macrophage (IMMs), increased lung pathology, and dysregulated viral‐specific T cell response. 65 Depending on the onset of IFNs production in SARS‐CoV‐2 infection, disease severity can be categorized into three types: (1) early response of type 1 IFNs leads to decreased viral titers, regulated inflammatory response, and mild‐clinical features; (2) delayed response causes dysregulated IMM response, lung damage, and severe pneumonia; (3) and lastly, the absence of type 1 IFNs signaling pathways results in invasive ventilation (delivering positive pressure through an endotracheal tube), poorer outcomes, higher viral load, and longer intensive care unit admission. 66 Inconsistently, the increased expression of IFNs contributes to disease severity and viremia in COVID‐19 patients. 62 The peripheral blood mononuclear cells (PBMC) of the COVID‐19 patients demonstrated that the viral load is associated with enhanced type 1 IFNs and disease severity. 41 Furthermore, increased IFNs at the later stages of the severe COVID‐19 cases worsen the pathophysiology and is accompanied by pyroptosis (a highly inflammatory programmed cell death that occurs in infected cells). 67 , 68 Similarly, in a cohort of COVID‐19 patients, high viremia, as well as disease severity, was associated with IFN‐α and ISGs levels. 41
Moreover, IFN signaling is pivotal for recruiting inflammatory cells to the infection site, but it cannot clear viral particles and control viral replication. 69 As aforementioned, it can be due to late IFN expression or since SARS‐CoV‐2 dampens IFN responses and ISG induction through nonstructural protein (nsp) 1, nsp 6, nsp 13, ORF3a, M, ORF7a, ORF7b, and ORF6. 70 To explain these paradoxical effects of IFNs administration and achieve a beneficial effect and maximal protection of IFNs in the treatment of SARS‐CoV‐2, it is critical to notice the best time window for IFNs administration. IFNs administration before the viral peak and inflammatory phase of disease could offer a highly protective effect, while IFN treatment during the inflammatory and severe stages of the disease would rather cause immunopathology and long‐lasting harm. 62 , 71 Early exogenous administration of IFN‐β showed a full protective effect against virus replication and expression of inflammatory cytokines in mice models of MERS‐CoV infection. In contrast, delayed Treatment with IFN‐β resulted in the high production of type 1 interferon, inflammatory cytokines, and ISGs. 72
The same concepts of the critical role of timing of IFNs administration were also concluded in the clinical trials. 73 It is worth mentioning that COVID‐19 treatment guidelines recommend against the therapeutic effectiveness of IFNs for severe or critical SARS‐CoV‐2‐infected patients. In addition to the influential role of IFNs therapy in infected patients, it has been demonstrated that IFNs treatment may offer a defensive effect against viral invasion in asymptomatic people who had been exposed to infected patients. Hence, this result shows that pretreatment with IFNs can stop SARS‐CoV‐2 infection before its occurrence. 71
Besides the essential role of the early administration of IFNs for COVID‐19 patients, studies also showed homozygosity for the C allele of rs12252 in the interferon‐induced transmembrane protein 3 (IFITM3) gene, supporting the severity of disease in an age‐dependent manner. This explains why the minority of infected patients develop progressive disease and show severe and lethal infection, while others manifest mild or moderate symptoms. 74 However, a German cohort reported that polymorphism in the IFITM3 gene, including rs12252 and rs34481144 variants, do not influence the disease severity or risk of infection. 75 A worldwide epidemiological study demonstrated that a minor allele of rs12252 polymorphism has a beneficial effect against SARS‐CoV‐2 infection, but a minor allele for rs34481144 can increase the SARS‐CoV‐2 infection and mortality. 76 Further studies are needed to determine the exact association between the population's genetic variants and the risk of SARS‐CoV‐2 severity. In addition to disease severity and the COVID‐19 infection risk, studies also demonstrated a strong correlation between the rate of case fatality in COVID‐19 infection and the allele frequency in the IFITM3 gene. As the first report, Kim et al. 77 revealed the positive correlation between fatality rate and rs6598045 single‐nucleotide polymorphism in the IFITM3 gene. 77 Thus, appropriate and timely IFN treatment of this group of patients may protect them from lethal and pathogenic infection.
9. THERAPEUTIC EFFECTS OF IFN‐I
9.1. In vitro and animal implications of IFN‐I
A limited number of animal and clinical studies evaluate the therapeutic effects of IFN‐I alone on SARS‐CoV‐2 infection. Additionally, most studies have relied on previous trials of SARS‐CoV‐1 and MERS viruses to design the studies on the novel member of this family as they share many similarities in structure and function. Herein, we review the recent in vitro and animal studies and move on to the existing clinical studies of IFN‐I therapy alone or in combination with other drugs.
As mentioned before, SARS‐CoV‐2 interferes with the interferon I‐mediated immune response. 78 In vitro implication of type I IFN inhibits the replication of SARS‐CoV‐2, SARS‐CoV‐1, and MERS‐CoV in cultural cell lines and animal models of viral infection. 79 An in vitro experiment comparing IFN‐I sensitivity between SARS‐CoV‐1 and the novel SARS‐CoV‐2, using Vero E6 and calu3 cells, has firmly proposed that SARS‐CoV‐2 is significantly more sensitive to IFN‐I treatment either pre‐or postinfection. The study has attributed higher sensitivity of SARS‐CoV‐2 to the lack of an equivalent ORF3b and some genetic differences against ORF6 in SARS‐CoV‐2, which are both known as IFN antagonizing factors. Moreover, pretreatment of SARS‐CoV‐2 and influenza A infected cells with IFN‐I showed similar results, with SARS‐CoV‐2 infected cells being unable to stimulate IFN‐I release before treatment and unsuccessful to counteract the phosphorylation of STAT‐I when pretreated with IFN‐I. 80 The combination of IFN‐α and IFN‐β exhibits dramatic antiviral effects in Vero cells infected with SARS‐CoV‐2 which were lacking IFN‐α and IFN‐β genes; the study predicted that IFN‐β administration could be more effective on IFN‐competent cells, as IFN‐β upregulates the expression of other IFN‐I subtypes and reinforces the IFN dependent immune response. 81 IFN‐β1b has also shown a striking antiviral activity in IFN‐deficient cells. 82 In line with this finding, one publication has proposed that treating Vero cells with recombinant IFN‐I is associated with a 30–40 folds reduction in viral titers. 83 Interestingly, one manuscript comparing transcriptional status in juvenile and old rhesus macaques following SARS‐CoV‐2 infection found that IFN‐I expression was more upregulated in the lungs of juvenile macaques than the old ones might justify the age risk factor in COVID‐19 infection. 84 This publication suggested that the induction of type I interferon signaling pathway in juvenile macaques infers its protective role rather than a deteriorative effect on disease progression. 85 Investigating bats' cell lines, some protective signaling pathways were found that might explain the viral tolerance of bats' immune system against SARS‐CoV‐2, with one of them being perpetual IFN‐α expression as was seen in the cells of Pteropus Alecto bats. 86 Previous studies have shown that PEGylated IFN‐α protected type I pneumocytes reduced the pulmonary damage in SARS infected macaques. 87 It has been demonstrated that IFN‐α shows a better in vitro inhibitory effect against SARS‐CoV‐1 virus growth than ribavirin. 88 Comparatively, IFN‐β 1a showed a potential inhibitory effect against SARS virus replication in vitro. 89 Moreover, IFN‐α/β, combined with IFN‐γ, inhibits viral RNA replication and DNA replication. 90 Some in vitro studies suggested that IFN‐I produces a potential antiviral activity against SARS‐CoV‐1 rather than IFN‐γ, 80 , 91 , 92 but other studies have demonstrated that combined administration of IFN‐I and IFN‐γ synergistically inhibits viral replication in vitro. 93 In addition to IFN therapy alone, studies showed that IFN‐β, combined with antiviral drugs, Ribavirin, would produce antiviral activity synergistically in Vero cells. 94 A similar result was achieved through the combination of IFN‐α2b with ribavirin in infected cultural cells of SARS‐CoV‐1. 95 The matter of timing in IFNs administration is a highly critical issue in animal models. IFN‐I administration before the viral peak results in complete protection and regulates the lethal replication of the virus in ifnar1−/− BALB/c mice. However, after the viral peak, the late delivery of IFNs failed to establish a protective effect and caused severe infection of SARS‐CoV‐1. 65 In another manuscript, early administration of IFN‐β before the viral peak led to protective effect, while its late delivery caused inflammation and severe pneumonia. 72
9.2. The clinical implication of IFN‐I in COVID‐19
Since the outbreak of COVID‐19, massive efforts have been devoted to finding the optimal therapeutic and preventive approach to break the contagion chain. Numerous studies, some of which we reviewed here, have investigated interferon therapy to assess its effectiveness in alleviating COVID‐19 disease. The efficacy of IFN in COVID‐19 Treatment comes from the fact that coronaviruses have developed intelligent strategies to escape the innate immune induction and, in turn, IFN response 96 ; so IFN administration might resolve some adverse outcomes of COVID‐19. Compared with other respiratory RNA viruses, the IFN response is much weaker and more delayed in SARS‐CoV‐2 infection, leading to adverse pathological events such as infiltration of inflammatory cells in the lungs and developing what is happening as a CS. 96
In previous studies on mice models, early treatment by IFN rescued SARS‐CoV‐1, or MERS infected mice, while late treatment was followed by an exacerbated immune response. 97 The results from two in vitro studies have shown that SARS‐CoV‐2 is much more sensitive to IFN‐I administration than other SARS‐CoVs; these findings suggest that IFN‐α or IFN‐β administration is associated with a significant reduction in viral loads and that IFN administration can be used as a prophylactic factor or early treatment for this family of viruses. 62 IFN‐α is known to reduce viral shedding and inflammatory markers, while IFN‐β is associated chiefly with improved viral clearance. 98 In addition to that, it has been firmly suggested that IFN‐β is more effective than IFN‐α on SARS‐CoV‐2 99 ; this has been the basis of many studies considering IFN‐β subtypes as a key component of IFN‐I containing regimens.
We concluded that IFNs effectiveness is still debatable because most of the studies evaluating the efficacy of IFNs on SARS‐CoV‐2 infection have investigated its effect in combination with other drugs. Among few studies investigating the efficacy and safety of type I IFN therapy alone, a randomized clinical trial is underway to explore IFNβ‐Ia therapeutic effects on mild to moderate COVID‐19 patients when added to standard care in the absence of any other antiviral drug. Clinical implications of PEGylated subtypes of IFN such as IFNβ‐Ia, IFNα−2b, or IFN lambda for COVID‐19 patients have also gained attention recently. PEG polymer is attached to the drug in terms of the pegylation process and improves its safety and efficacy. However, most of the studies are not completed yet and are in recruiting status. A considerable number of similar trials on IFN‐I therapeutic efficacy are on their way to be published soon, with some of them investigating its effect in combination with other antivirals and others, alone mostly in a nebulized form. One example is a Phase 3 clinical trial that aims to evaluate the efficacy of nebulized IFN by comparing recovery time in hospitalized patients who receive SNG001 nebulizer versus placebo receiving group. A Phase 2 randomized clinical trial has recently addressed the same comparison by using nebulized interferon beta‐1a (SNG001) as a treatment against the placebo; the findings indicate that receiving SNG001 is associated with sooner recovery based on the WHO Ordinal Scale for Clinical Improvement (OSCI) and lower incidence of adverse events. 100 Similarly, one randomized clinical trial has recently shown that using IFNβ−1a nebulizers is associated with clinical improvement in hospitalized COVID‐19 patients, based on OSCI; whereas, placebo and treatment groups did not differ in hospital discharge rate by Day 28. 101
The effect of IFNs on mortality rates differed in several studies. A randomized controlled trial evaluating the efficacy of IFN‐β1a in patients with severe COVID‐19 showed a significantly lower 28‐day mortality rate in the IFN‐received group (receiving IFN in addition to hydroxychloroquine, lopinavir/ritonavir, or atazanavir/ritonavir). 102 In contrast, a relatively similar publication using IFN‐β1b (combined with the same drugs just mentioned above) showed no significant change in the 28‐day mortality outcomes. 103 The efficacy of the intramuscular form of INF‐β1b (betaferon) has been evaluated in a retrospective cohort study; it was indicated that this treatment had no significant impact on in‐hospital survival. Both study groups in this article received other effective drugs such as hydroxychloroquine, antivirals (liponavir/ritonavir), and/or anti‐inflammatory drugs (steroids and/or tocilizumab), making the comparison difficult. 104 A recent randomized clinical trial compared clinical outcomes in two groups of COVID‐19 patients with moderate to severe pneumonia, with one of them receiving favipiravir and interferon beta‐1b, and the other one receiving hydroxychloroquine; the length of hospital stay, intensive care unit (ICU) admissions, discharge, and mortality rates, oxygen saturation at discharge, and changes in the inflammatory biomarkers at the time of discharge were not significantly different between the two groups. 105
Moreover, a more extensive cohort study on the therapeutic effectiveness of intramuscular administration of IFN‐α2b in combination with oral antivirals such as lopinavir/ritonavir plus chloroquine (as recommended in Cuban protocol of COVID‐19) suggested that the patients receiving Heberon Alpha R (IFN‐α2b, liquid formulation) showed improved recovery rates as well as lower fatalities. 106 The WHO SOLIDARITY trial results from a vast number of hospitalized COVID‐19 patients revealed that IFN regimens did not significantly affect the mortality rate nor on the initiation of ventilation or the duration of hospitalization. 107 Speaking of the hospitalization period, two separate studies evaluating the effectiveness of IFN‐β1b on hospitalized COVID‐19 patients reported different opposing results, with one of them showing a significant reduction in the duration of hospital stay in the combination group (receiving IFN in addition to antivirals such as lopinavir/ritonavir and ribavirin) 78 and the other one showing no significant change in patients receiving combination therapy (including IFN‐β1b plus lopinavir/ritonavir or atazanavir/ritonavir plus hydroxychloroquine). 103 However, the latter publication showed positive outcomes in severe cases of COVID‐19, such as a significant increase in discharge rate at Day 14, a decreased need for ICU admission but not the length of ICU stay, and shortened time to reach clinical response. 103
A recent meta‐analysis study has concluded that interferon‐1 therapy is associated with a higher discharge and lower deaths than standard care groups. Simultaneously, the need for ICU transforms, mechanical ventilation, or severe or critical disease development did not differ between them. 108 Another meta‐analysis study has recently investigated clinical trials on the therapeutic effects of IFN‐β; the results support the effectiveness of IFN‐β on improving discharge rate, duration of hospital stays, and in some cases, mortality. The study has suggested that administration of IFN‐β with other antivirals in the early days of viral shedding improves viral clearance and antiviral response, especially in patients with autoantibodies against IFN‐I. 99 Noteworthily, another manuscript on the efficacy of IFN‐β1a in the treatment of severe COVID‐19 patients showed the same results in terms of improvement in the discharge rate at Day 14, yet suggesting no significant change in the time to reach clinical response following IFN addition to the national protocol medications (lopinavir/ritonavir or atazanavir/ritonavir plus hydroxychloroquine). 102 Concerning therapeutic effectiveness, more controversy is observed between different studies; two extensive cohort studies (one retrospective and the other one prospective) supported the effective role of Heberon Alpha R (in combination with other drugs included in the Cuban COVID‐19 protocol) in improving the recovery 106 and survival rates 109 in severe cases of COVID‐19. At the same time, a separate clinical trial indicated no significant difference among the three IFN containing regiments included (ribavirin + IFN‐α or lopinavir/ritonavir + IFN‐α or ribavirin + liponavir/ritonavir + IFN‐α), in terms of antiviral effectiveness (median time to polymerase chain reaction [PCR] negative conversion, days of hospital stay, days of noticeable computed tomography (CT) improvement, and fever clearance time) in patients with mild to moderate COVID‐19. 110
Conversely, the combination of IFNβ1 with lopinavir/ritonavir was associated with higher survival and discharge rates and improvement in oxygen supply in hospitalized COVID‐19 patients. 111 Another retrospective multicenter cohort study also supported improvement in the clinical findings of COVID‐19 patients with pneumonia. The results from this publication showed that patients who received Arbidol/IFN‐α2b treatment experienced CT improvement sooner than nucleic acid clearance; they also benefited from the treatment in terms of reducing their incidence of lung inflammation without invasive ventilation techniques compared with those who only received IFN‐α2b, suggesting a synergistic effect that Arbidol/IFN‐α2b combination can bring about; the RNA clearance and hospital stay days were not changed, though. 112 Additionally, the incidence rates of common and serious adverse events in severe cases of COVID‐19 who received IFN‐β1b were less than the control group. 103 A more recent publication, which has assessed different medications in treating COVID‐19 in two Chinese case cohorts, supported the therapeutic effect of IFNβ−1b in terms of earlier discharge. The study further demonstrated that coadministration of IFNβ−1b with ribavirin brings better clinical outcomes, including higher survival rate, lower mechanical ventilation, and intensive care requirements, and shorter length of stay, especially when administered in the early phases of the disease. 113
The efficacy of PEGylated IFNα−2b in addition to the standard care was studied against the standard care only group; the findings supported the effectiveness of PEG IFN‐α2b in rapid viral clearance and improving clinical status on Day 15 as patients who received PEG IFN‐α2b required oxygen supplementation for a shorter period than those who only received standard care treatments. 114 A recent publication has reported the beneficial impact of adding PEGylated interferon to ruxolitinib treatment by describing the case of an elderly woman with primary myelofibrosis, who was persistently tested positive for COVID‐19, as ruxolitinib is known to have an immunosuppressive impact on innate immunity signaling and, most importantly interferon receptors. 115
Despite the controversial opinions about the effect of IFN on clinical findings in COVID‐19 patients, its effectiveness on serological and molecular findings has been commonly supported by a considerable number of studies. Viral load and the duration of reverse‐transcription‐PCR‐positivity were significantly reduced in the patients receiving a triple combination of IFN‐β1b, lopinavir/ritonavir, and ribavirin; the virus shedding was notably suppressed in those patients as well. 78 Likewise, another cohort study 8 mentioned that the duration of virus‐positive detection in the upper respiratory tracts and the length of viral shedding period were both shortened in patients treated by nebulized IFN‐α2b either with or without Arbidol. The same manuscript also demonstrated that the markers of acute inflammation, such as C‐reactive protein and IL‐6, decreased in the treatment course. 8 The authors found more promising findings in their subsequent publication that included extended analysis on the same study groups; the analysis indicated that interferon treatment reduces CT scores and prevents exacerbation of lung abnormalities by restricting the effect of SARS‐CoV‐2 infection on reducing CD8+ T cells and increasing IL‐6 and TNF‐α levels. 116 Interestingly, the findings of a randomized controlled trial comparing the effectiveness of INF‐α2b/IFN gamma (HeberFERON) treatment with IFN‐α2b alone (Heberon Alpha R) indicated that HeberFERON is a safe treatment and more effective in shortening the time of virus elimination with more than 95% of patients becoming negative for SARS‐CoV‐2 within 5 days of treatment. 117
Recently, a three‐armed randomized controlled trial has compared IFNβ1a and IFNβ1b together and with a control group; time to clinical improvement was similar between IFN groups and slightly lower than the control group; moreover, the number of deaths was also lower in treatment groups while there was no significant difference in the occurrence of adverse events between the three arms. 118
Besides the discussions around the efficacy of IFN therapy, its administration's timing is proposed to be of utmost importance. The results from a randomized controlled trial demonstrated that patients who received a combination of IFN‐β1a and lopinavir/ritonavir or atazanavir/ritonavir plus hydroxychloroquine in the early phases of the disease experienced significantly more benefits from the treatment. 102 Additionally, a cohort study also supported an association between early administrations of IFN‐α2b with a significant reduction in mortality rate, whereas late administration was correlated with increased deaths and delayed recovery. 119
Generally speaking, the optimal time for IFN administration appears to be the early stages of the disease to maximally achieve its biological activities and prevent the progression to the severe forms of COVID‐19. 57 The article has proposed the best therapeutic window for IFN‐α or ‐β administration to be in the first 10 days from COVID‐19 diagnosis; if IFN administration is carried out around the viral peak when the virus mediates stimulation of alveolar macrophages and epithelial cells of lungs to secrete significant amounts of inflammatory cytokines (i.e., CS), the likelihood of adverse events occurring in patients will be significantly increased. Another beneficial effect of early administration of IFNs is seen among old patients deficient in proper IFN signaling activity and have a lower innate and adaptive immune response. 57 A recent propensity‐score analysis study suggested that administering IFNα−2b beyond 7 days after symptoms onset can turn the antiviral effects into pro‐inflammatory ones. 113
The IFN administration route might be another determinant factor in evaluating its efficacy; some studies have suggested IFN aerosols or nebulizers as a possible solution since they can be directly and noninvasively delivered to the lungs and reach a higher local concentration than the intramuscular form. 96 A recent clinical trial was carried out on Chinese medical staff exposed to COVID‐19 at low‐ or high‐risk levels, they were both receiving IFN‐α nasal drops and first‐level protection, but the high‐risk group was also supplied by second‐ or third‐ protection level and thymosin‐α1; both studied groups benefited from the preventive effects of the interventions for 28 days. 120 Another clinical trial in the UK evaluated the efficacy of nebulized IFN‐β1a (SNG001) to treat COVID‐19 patients. 'Patients' follow‐up for 28 days revealed that the patients treated by SNG001 showed accelerated recovery and general improvement compared with the placebo group. 100 Nasal administration of IFN‐α and IFN‐β is proposed to be a functional and relatively safe strategy, especially for prophylactic uses in those at a high exposure to infected patients such as medical staff or house caregivers. 57 The sublingual administration route has recently gained much attention due to its prophylactic effect in healthy individuals and lack of hepatic and neurotoxicity concerns; sublingual IFN‐I administration can exert its antiviral effects through a local interaction between the given interferon and the cytokines and specific immune cells such as intraepithelial T cells which are known to mediate IFN function. 57
Chuan et al. 97 conducted a randomized clinical trial to evaluate the effectiveness of the traditional interferon‐alpha in comparison with recombinant super‐compound interferon (rSIFN‐co) (a new genetically engineered interferon but is not commercialized yet). rSIFN‐co resulted in significantly shorter clinical improvement compared with interferon‐alpha in COVID‐19 cases. 117 More potent during preclinical therapy, rSIFN‐co has more substantial antiviral effects, and fewer side effects than traditional interferon‐alpha. 13 and 18 patients in the rSIFN‐co group interferon‐alpha group showed adverse events (AEs), respectively. Most of the AEs categorized as grade or two, and no severe AEs were reported. 117 More detailed information on study design, intervention, and outcomes of mentioned clinical studies is displayed in Table 1.
Table 1.
Clinical implication of interferon‐1 in the context of COVID‐19
| Title | First author | Date of publication | Country | Type of study | Sample number | Mean age of patients | Intervention | Outcomes |
|---|---|---|---|---|---|---|---|---|
| The triple combination of interferon beta‐1b, lopinavir‐ritonavir, and Ribavirin in the Treatment of patients admitted to hospital with COVID‐19: an open‐label, randomized, phase 2 trial 81 | Ivan Fan‐Ngai Hung | May 8, 2020 | China | Clinical Trial |
Combination group: 86 Control group: 41 |
Combination Group: 51 Control Group:52 |
Combination group: lopinavir, ritonavir, ribavirin and interferon beta‐1b Control group: lopinavir and ritonavir |
(1) Combination therapy suppressed the shedding of SARS‐CoV‐2 in all clinical specimens (by day 8) and reduced the duration of in‐hospital stay (2) Combination therapy significantly reduced the duration of positive RT‐PCR and viral load |
| A Randomized Clinical Trial of the Efficacy and Safety of Interferon β−1a in Treatment of Severe COVID‐19 105 | Effat Davoudi‐Monfared | August 20, 2020 | Iran | Randomized Controlled Trial |
Interferon group: 42 Control group: 39 |
Interferon group: 56.50 Control group: 61.00 |
Interferon group: Interferon β−1a and national protocol (hydroxychloroquine plus lopinavir‐ritonavir or atazanavir‐ritonavir) Control group: national protocol |
(1) Adding IFN to the national protocol medications did not change the time of clinical improvement. (2) IFN significantly improved the discharge rate by day 14. (3) The 28‐day mortality was significantly lower in the IFN group. (4) Patients who received IFN in the early phase of the disease experienced significantly more benefits from the Treatment. |
| Interferon‐α2b Treatment for COVID‐19 8 | Qiong Zhou | May 15, 2020 | China | Uncontrolled, exploratory cohort study |
Interferon group: 7 Arbidol group: 24 Combination group: 46 |
Interferon group: 41.3 Arbidol group: 64.5 Combination group: 40.4 |
Interferon group: Nebulized IFN‐α2b Arbidol group: arbidol hydrochloride Combination group: IFN‐α2b and Arbidol |
IFNα2b therapy, with or without Arbidol, appears to: (1) Reduce the duration of detectable virus in the upper respiratory tract (2) Shorten the duration of viral shedding (3) Decrease the markers of acute inflammation such as CRP and IL‐6. |
| Interferon β−1b in Treatment of severe COVID‐19: A randomized clinical trial 105 | Hamid Rahmani | August 24, 2020 | Iran | Randomized Controlled Trial |
Interferon group: 33 Control group: 33 |
Interferon group: 60 Control group: 61 |
Interferon group: Interferon β−1b Control group: lopinavir/ritonavir or atazanavir/ritonavir plus hydroxychloroquine |
(1) Interferon decreased time to clinical improvement, increased the discharge rate at Day 14, and reduced the need for ICU admission. (2) There was no difference in duration of hospitalization, intubation rate, length of ICU stay, and all‐cause 28‐day mortality (3) Incidence rates of common and serious adverse events were higher in the control group compared with the IFN group. |
| Arbidol/IFN‐α2b therapy for patients with coronavirus disease 2019 a retrospective multicenter cohort study 113 | Ping Xu | May 20, 2020 | China | Retrospective Cohort Study |
Combination group: 71 Interferon group: 70 |
Combination group: 50.9 Interferon group: 53.2 |
Combination group: Arbidol and IFN‐ α2b Interferon group: IFN‐ α2b |
(1) The median days of CT improvement in the combination group were less than that of the IFN‐a2b, the only group. (2) Combination group has potential effects on inhibiting COVID‐19 lung inflammation in mild cases without invasive ventilation, but no effects on RNA clearance and hospitalization days. |
| Retrospective Multicenter Cohort Study Shows Early Interferon Therapy Is Associated with Favorable Clinical Responses in COVID‐19 Patients 120 | Nan Wang | September 9, 2020 | China | Retrospective Cohort Study |
Early IFN group: 216 No IFN group: 204 Late IFN group: 26 |
Early IFN group: 50 No IFN group: 49 Late IFN group: 51.5 |
Early IFN group: 83 patients received IFN + lopinavir/ritonavir (LPV/r), 94 patients received IFN + umifenovir (UFV), 39 patients received IFN alone No IFN group: 122 patients received LPV/r, 82 patients received UFV Late IFN group: 26 |
(1) Patients in the early IFN‐a2b administration group showed a lower mortality rate than those with no IFN‐a2b admission. (2) Patients who received late administration of IFN‐a2b experienced increased mortality. (3) Late IFN‐a2b administration was associated with delayed recovery among survivors. (4) Early IFN‐a2b and Umifenovir alone or in combination were associated with reduced mortality and a faster recovery in comparison with Treatment with lopinavir/ritonavir (LPV/r) alone. |
| No Statistically Apparent Difference in Antiviral Effectiveness Observed Among Ribavirin Plus Interferon‐Alpha, Lopinavir/Ritonavir Plus Interferon‐Alpha, and Ribavirin Plus Lopinavir/Ritonavir Plus Interferon‐Alpha in Patients With Mild to Moderate Coronavirus Disease 2019: Results of a Randomized, Open‐Labeled Prospective Study 97 | Yin‐Qiu Huang | July 14, 2020 | China | Clinical Trial |
Ribavirin (RBV) and interferon α group: 33 Lopinavir/ritonavir (LPV/r) and IFN α group: 36 RBV, LPV/r and IFN α group: 32 |
Ribavirin (RBV) and interferon α group: 40.3 Lopinavir/ritonavir (LPV/r) and IFN α group: 43.3 RBV, LPV/r and IFN α group: 43.8 |
First group: ribavirin (RBV) and interferon α Second group: Lopinavir/ritonavir (LPV/r) and IFN α Third group: RBV, LPV/r and IFN α |
There were no significant differences among the three regimens regarding antiviral effectiveness in patients with mild to moderate COVID‐19. |
| Therapeutic effectiveness of interferon‐alpha 2b against COVID‐19: the Cuban experience 108 | Ricardo Pereda | June 9, 2020 | Cuba | Prospective cohort study |
Interferon group: 761 Control group: 53 |
Interferon group: 42.9 Control group: 66.9 |
Interferon group: Heberon® Alpha R (IFN‐α2b, liquid formulation) and Cuban COVID protocol (lopinavir/ritonavir and chloroquine) Control group: Cuban COVID protocol |
The use of Heberon® Alpha R may contribute to recovery from COVID‐19 and improving both the rates of recovery and case fatalities. |
| Therapeutic effectiveness of interferon‐alpha 2b treatment for COVID‐19 patient recovery 111 | Ricardo Pereda | August 4, 2020 | Cuba | Prospective cohort study |
Interferon group: 2165 Control group: 130 |
Interferon group: 44 Control group: 68 |
Interferon group: IFN‐α2b and Cuban COVID protocol (lopinavir/ritonavir and chloroquine) Control group: Cuban COVID protocol |
Interferon‐alpha 2b is effective for critical patient survival and decreases disease progression to severe stages. |
| Effect of combination of interferon alpha‐2b and interferon‐gamma or interferon alpha2b alone for the elimination of SARS‐CoV‐2 viral RNA. Preliminary results of a randomized controlled clinical trial. 118 | Idelsis Esquivel‐Moynelo | August 4, 2020 | Cuba | Randomized controlled clinical trial |
Combination group: 30 Control group:33 |
Combination group: 42 Control group: 31 |
Combination group: IFN‐α2b and IFN‐γ (HeberFERON), lopinavir‐ritonavir and chloroquine Control group: IFN‐α2b (Heberon Alpha R), lopinavir‐ritonavir and chloroquine |
HeberFERON was a safe treatment, superior to Heberon Alpha R in shortening the time to SARS‐CoV‐2 viral RNA elimination, with more than 95% of patients negative for SARS‐CoV‐2 within five days of Treatment. |
| Clinical evaluation of IFNbeta1b in COVID‐19 pneumonia: a retrospective study 106 | Miriam Estébanez | May 21, 2020 | Spain | Retrospective cohort study |
Interferon group: 106 Control group: 150 |
Interferon group: 61.9 Control group: 64.83 |
Interferon group: beta1b (Betaferon) and antivirals (lopinavir/ritonavir, and/or hydroxychloroquine or chloroquine), and/or anti‐inflammatory drugs (steroids and/or tocilizumab) Control group: antivirals (lopinavir/ritonavir, and/or hydroxychloroquine or chloroquine), and/or anti‐inflammatory drugs (steroids and/or tocilizumab) |
The interferon beta1b treatment had no significant effect on in‐hospital survival. Because most patients in both treatment groups received other potential drugs such as hydroxychloroquine or Azithromycin, it was difficult to evaluate the treatment's effectiveness individually. |
| An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area 121 | Zhongji Meng | May 7, 2020 | China | Clinical trial |
Low‐risk group: 2415 High risk group: 529 |
Low‐risk group: 35.13 High risk group: 32.87 |
Low‐risk group: rhIFN‐α (recombinant human interferon alpha) nasal drops with first‐level protection High‐risk group: rhIFN‐α nasal drops combined with thymosin‐α1 along with secondary‐level or third‐level protection |
If standard first‐ and second‐level protections are strictly implemented, the IFN‐α nasal drops could significantly prevent medical staff at low exposure level from COVID‐19 pneumonia. Besides, IFN‐α nasal drops combined with weekly thymosin‐α1 could prevent medical staff at high exposure levels from developing the disease over a period of 28 days. |
| Repurposed antiviral drugs for COVID‐19 –interim WHO SOLIDARITY trial results 109 | Hongchao Pan | October 15, 2020 | Multi National (30 countries) | WHO Solidarity trial consortium |
Remdesivir group: 2743 Hydroxychloroquine group: 947 Lopinavir‐ritonavir group: 1399 Interferon‐β1a group: 2050 Control group: 4088 |
Remdesivir group Hydroxychloroquine group Lopinavir‐ritonavir group Interferon‐β1a group Control group: no study drug |
The main outcomes of mortality, initiation of ventilation, and hospitalization duration were not clearly decreased by any type of study drugs (Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon). | |
| Safety and efficacy of inhaled nebulized interferon beta‐1a (SNG001) for Treatment of SARS‐CoV‐2 infection: a randomized, double‐blind, placebo‐controlled, phase 2 trial 103 | Phillip D Monk | November 12, 2020 | United Kingdom | Randomized, controlled, phase 2 trial |
Interferon group: 48 Control group: 50 |
Interferon group: 57.8 Control group: 56.5 |
Interferon group: Inhaled nebulized interferon beta‐1a (SNG001) Control group: placebo |
(1) Greater improvement in the SNG001 group (2) More rapid recovery in the SNG001 group (3) Greater improvement in breathlessness and total BCSS over the treatment period in the SNG001 group (4) No significant differences in time to hospital discharge between treatment groups |
|
Engineered interferon‐alpha effectively improves clinical outcomes of COVID‐19 patients124 |
Chuan Li | China | Randomized controlled trial |
rSIFN‐co group: 46 Interferon‐alpha group: 48 |
rSIFN‐co group: 51 Interferon‐alpha group: 56 |
rSIFN‐co group: Recombinant super‐compound interferon combined with baseline antiviral agents (lopinavir–ritonavir or umifenovir) interferon‐alpha combined with baseline antiviral agents (lopinavir–ritonavir or umifenovir) |
(1) rSIFN‐co combined with antiviral agents significantly improved the recovery in moderate‐to‐severe COVID‐19 patients compared to combination of traditional interferon‐alpha and antiviral agents. (2) rSIFN‐co‐lead to a shorter time to clinical improvement, radiological improvement, and virus nucleic acid negative conversion (3) Higher clinical improvement rate on day 28 in rSIFN‐co group |
|
|
Combination therapy of IFNβ1 with lopinavir–ritonavir, increases oxygenation, survival and discharging of sever COVID‐19 infected inpatients 112 |
P. Baghaei | December 26, 2020 | Iran | Retrospective cohort study |
IFN group: 152 Control group: 304 |
IFN group: 56 Control group: 56 |
IFN group: IFN‐β1‐a, lopinavir and ritonavir Control group: lopinavir and ritonavir |
Combination therapy resulted in reduced risk of mortality. IFN‐β1‐a can lower mortality rate, invasive ventilation, comorbidity, and improve requirement of oxygen. |
|
Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta‐1b in hospitalized patients with moderate to severe COVID‐19 pneumonia 107 |
F. Khamis | November 4, 2020 | Oman | Randomized, open label controlled trial |
Combination group: 44 Standard group: 45 |
Combination group: 54 Standard group: 56 |
Combination group: Favipiravir with interferon beta‐1b Standard group:Hydroxychloroquine |
Neither clinical outcomes nor inflammatory markers were different between 2 groups. |
|
Role of Interferon Therapy in Severe COVID‐19: The COVIFERON Randomized Controlled Trial 119 |
Darazam, I.A. | April 13, 2021 | Iran | Randomized controlled trial |
Intervention group 1: 20 Intervention group 2: 20 Control group: 20 |
Intervention group 1: 71.5 Intervention group 2: 65 Control group: 76 |
Intervention group 1: IFNβ1a + Lopinavir/Ritonavir and Hydroxychloroquine Intervention group 2: IFNβ1b + Lopinavir/Ritonavir and Hydroxychloroquine Control group: Lopinavir/Ritonavir and Hydroxychloroquine |
Time to clinical improvement was significantly lower in the IFNβ1a group relative to the control group. |
|
Clinical outcomes of different therapeutic options for COVID‐19 in two Chinese case cohorts: A propensity‐score analysis 114 |
C.K.H. Wong | February 13, 2021 | China |
Propensity‐score analysis |
Lopinavir‐ritonavir: 994 Ribavirin: 377 Umifenovir: 217 Corticosteroids: 1044 Interferon‐alpha‐2b: 495 Antibiotics: 2179 Chinese medicines: 565 Interferon‐beta‐1b: 161 Interferon‐beta‐1b and ribavirin: 634 Interferon‐beta‐1b and ribavirin and lopinavir‐ritonavir: 408 Interferon‐beta‐1b and lopinavir‐ritonavir: 752 |
Lopinavir‐ritonavir Ribavirin Umifenovir Corticosteroids Interferon‐alpha‐2b Antibiotics Chinese medicines Interferon‐beta‐1b Interferon‐beta‐1b and ribavirin Interferon‐beta‐1b and ribavirin and lopinavir‐ritonavir Interferon‐beta‐1b and lopinavir‐ritonavir |
Among all types of medications, interferon‐beta‐1b monotherapy along with interferon‐beta‐1b combined with oral ribavirin showed clinical improvement. Interferon‐beta‐1b combined with oral ribavirin considered to be more beneficial regarding clinical outcomes and hospitalization time. |
|
|
Interferon‐α2b Treatment for COVID‐19 Is Associated with Improvements in Lung Abnormalities 117 |
Zhou, Q | December 30, 2020 | China | Uncontrolled, exploratory cohort study |
Interferon group: 7 Arbidol group: 24 Combination group: 46 |
Interferon group: 41.3 Arbidol group: 64.5 Combination group: 40.4 |
Interferon group: Nebulized IFN‐α2b Arbidol group: arbidol hydrochloride Combination group: IFN‐α2b and Arbidol |
IFN therapy lead to reduced lung abnormalities in COVID‐19 patients. IFN increased CD8 + cells level and lowered TNF‐α and IL‐6. |
The table summarizes valuable information on study design, types of interventions, and most importantly, outcomes from cited articles. Most of the studies shown here have compared the efficacy of IFN‐I presence or absence in specific antiviral regimens, while a few have explored its pure effectiveness either in nebulized or injective form. Utilizing nebulization is gaining lots of attention, with a considerable number of studies are yet to be carried out and published on this specific topic.
10. CONCLUSION
There is a controversy about the effectiveness of IFN therapy alone or in combination with other antiviral drugs, thus making it more challenging for us to integrate all the results into one unique conclusion. Based on this review, Interferon therapy, in general, effectively improves some clinical aspects of COVID‐19 and reduces the mortality rate if utilized properly and in the proper combination. However, its effect is not definite in all study groups and might depend on the patient's polymorphisms or the disease phase. 62 It has been firmly suggested that IFN administration before the viral peak exerts the maximum protective effect without adverse pathological consequences. 62 Administrating IFNs in the later stages of the infection, on the other hand, exacerbates the disease severity due to excessive inflammation and direct tissue damage. 96 Further investigation on the clinical effectiveness of interferons for patients with mild to severe COVID‐19 and its optimal timing and route of administration can help find a safe and functional antiviral therapy for COVID‐19 disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Fatemeh Sodeifian conceptualized the study, then Mahsa Nikfarjam and Naghmeh Kian conducted the database search. Each one of Fatemeh Sodeifian, Mahsa Nikfarjam, and Mahsa Nikfarjam narratively screened the databases then extracted their data and prepared the initial draft of the manuscript. Kawthar Mohamed prepared the final draft of the manuscript and Nima Rezaei supervised the project and critically assessed the manuscript.
ACKNOWLEDGMENT
There is no funding for the current study.
Sodeifian F, Nikfarjam M, Kian N, Mohamed K, Rezaei N. The role of type I interferon in the treatment of COVID‐19. J Med Virol. 2021;94:63‐81. 10.1002/jmv.27317
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Habas K, Nganwuchu C, Shahzad F, et al. Resolution of coronavirus disease 2019 (COVID‐19). Expert Rev Anti Infect Ther. 2020;18(12):1201‐1211. [DOI] [PubMed] [Google Scholar]
- 2. Jean S‐S, Lee P‐I, Hsueh P‐R. Treatment options for COVID‐19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cascella M, Rajnik M, Aleem A, Dulebohn SC & Napoli RD Features, evaluation, and treatment of coronavirus (COVID‐19). Accessed April 20, 2021. https://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed]
- 4. Walz L, Cohen AJ, Rebaza AP, et al. JAK‐inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID‐19 patients: a systematic review and meta‐analysis. BMC Infect Dis. 2021;21(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruetsch C, Brglez V, Crémoni M, et al. Functional exhaustion of Type I and II interferons production in severe COVID‐19 patients. Front Med. 2020;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cui J, Li F, Shi Z‐L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neerukonda SN, Katneni UJP. A review on SARS‐CoV‐2 virology: pathophysiology animal models, and anti‐viral interventions. Pathogens. 2020;9(6):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS‐CoV‐2. Nature. 2020;581(7807):221‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lotfi M, Rezaei N. SARS‐CoV‐2: s comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol. 2020;92:1864‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID‐19. Nat Med. 2020;27(7):1017‐1032. [DOI] [PubMed] [Google Scholar]
- 13. Seyran M, Takayama K, Uversky VN, et al. The structural basis of accelerated host cell entry by SARS‐CoV‐2. FEBS J. Published online December 02, 2020. [DOI] [PMC free article] [PubMed]
- 14. Onabajo OO, Banday AR, Stanifer ML, et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full‐length SARS‐CoV‐2 receptor. Nature Genet. 2020;52(12):1283‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lotfi M, Rezaei N. SARS‐CoV‐2: A comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol. 2020;92(10):1864‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crescioli G, Lombardi N, Bettiol A, et al. Adverse events following cannabis for medical use in Tuscany: an analysis of the Italian Phytovigilance database. Br J Clin Pharmacol. 2020;86(1):106‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuba K, Imai Y, Ohto‐Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin–angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siddiqi HK, Mehra MR. COVID‐19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao Q, Bao L, Mao H, et al. Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science. 2020;369(6499):77‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang W, Zhao Y, Zhang F, et al. The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): the perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rokni M, Hamblin MR, Rezaei N. Cytokines and COVID‐19: friends or foes? Hum Vaccines Immunother. 2020;16(10):2363‐2365. https://pubmed.ncbi.nlm.nih.gov/32841579/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tufan A, Güler AA, Matucci‐Cerinic M. COVID‐19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI‐1):620‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vaninov N. In the eye of the COVID‐19 cytokine storm. Nat Rev Immunol. 2020;20(5):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17(5):533‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taghiloo S, Aliyali M, Abedi S, et al. Apoptosis and immunophenotyping of peripheral blood lymphocytes in Iranian COVID‐19 patients: Clinical and laboratory characteristics. J Med Virol. 2021;93(3):1589‐1598. [DOI] [PubMed] [Google Scholar]
- 28. Mohamed K, Yazdanpanah N, Saghazadeh A, Rezaei N. Computational drug discovery and repurposing for the treatment of COVID‐19: a systematic review. Bioorg Chem. 2021;106:104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. 2008;43(3):336‐341. [DOI] [PubMed] [Google Scholar]
- 30. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity. 2020;52:910‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheahan T, Morrison TM, Funkhouser W. MyD88 is required for protection from lethal infection with a mouse‐adapted SARS‐CoV. PLoS Pathog. 2008;4(12):e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khanolkar A, Hartwig SM, Haag BA, Meyerholz DK, Harty JT, Varga SM. Toll‐like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice. J Virol. 2009;83(17):8946‐8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Totura AL, Whitmore A, Agnihothram S, et al. Toll‐like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015;6(3):e00638‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science. 2020;370(6515):eabd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loo Y‐M, Fornek J, Crochet N, et al. Distinct RIG‐I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82(1):335‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Bon A, Etchart N, Rossmann C, et al. Cross‐priming of CD8+ T cells stimulated by virus‐induced type I interferon. Nature Immunol. 2003;4(10):1009‐1015. https://www.nature.com/articles/ni978 [DOI] [PubMed] [Google Scholar]
- 37. Bouayad AJRimv. Innate immune evasion by SARS‐CoV‐2. Comparison with SARS‐CoV. 2020:e2135. [DOI] [PubMed] [Google Scholar]
- 38. Zhou R, To KK, Wong YC, et al. Acute SARS‐CoV‐2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Portela C, Brites C. Immune response in SARS‐CoV‐2 infection: the role of interferons type I and type III. Braz J Infect Dis. 2020;24:428‐433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cervantes‐Barragan L, Züst R, Weber F, et al. Control of coronavirus infection through plasmacytoid dendritic‐cell–derived type I interferon. Blood. 2007;109(3):1131‐1137. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8254533/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe COVID‐19 patients. MedRxiv. Published online April 23, 2020. [DOI] [PMC free article] [PubMed]
- 42. De Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors biochemistry and biological functions. J Biol Chem. 2007;282(28):20053‐20057. [DOI] [PubMed] [Google Scholar]
- 43. Hervas‐Stubbs S, Perez‐Gracia JL, Rouzaut A, Sanmamed MF, Bon AL, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17(9):2619‐2627. [DOI] [PubMed] [Google Scholar]
- 44. Li J‐Y, Liao C‐H, Wang Q, et al. The ORF6, ORF8 and nucleocapsid proteins of SARS‐CoV‐2 inhibit type I interferon signaling pathway. Virus Res. 2020;286:198074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang H‐W, Zhang H‐N, Meng Q‐F, et al. SARS‐CoV‐2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell Mol Immunol. 2020;17(9):998‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Konno Y, Kimura I, Uriu K, et al. SARS‐CoV‐2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32(12):108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han L, Zhuang M‐W, Deng J, et al. SARS‐CoV‐2 ORF9b antagonizes type I and III interferons by targeting multiple components of RIG‐I/MDA‐5‐MAVS, TLR3‐TRIF, and cGAS‐STING signaling pathways. J Med Virol. 2020;93(9):5376‐5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Toth AM, Zhang P, Das S, George CX, Samuel CE. Interferon Action and the Double‐Stranded RNA‐Dependent Enzymes ADAR1 Adenosine Deaminase and PKR Protein Kinase. Prog Nucleic Acid Res Mol Biol. 2006;81:369‐434. [DOI] [PubMed] [Google Scholar]
- 49. Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14(4):778‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pfeffer LM. The role of nuclear factor κB in the interferon response. J Interferon Cytokine Res. 2011;31(7):553‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rabouw HH, Langereis MA, Knaap RC, et al. Middle East respiratory coronavirus accessory protein 4a inhibits PKR‐mediated antiviral stress responses. Plos Pathog. 2016;12(10):e1005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sadler AJ, Williams BRJ. Interferon‐inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Haller O, Staeheli P, Kochs G. Interferon‐induced Mx proteins in antiviral host defense. Biochimie. 2007;89(6‐7):812‐818. [DOI] [PubMed] [Google Scholar]
- 54. Lamers MM, Van den Hoogen BG, Haagmans BL. ADAR1: “Editor‐in‐Chief” of cytoplasmic innate immunity. Front Immunol. 2019;10:1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hewitt EW. The MHC class I antigen presentation pathway: strategies for viral immune evasion. Immunology. 2003;110(2):163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hegde NR, Chevalier MS, Johnson DC. Viral inhibition of MHC class II antigen presentation. Trends Immunol. 2003;24(5):278‐285. [DOI] [PubMed] [Google Scholar]
- 57. Aricò E, Bracci L, Castiello L, Gessani S, Belardelli F. Are we fully exploiting type I Interferons in today's fight against COVID‐19 pandemic? Cytokine Growth Factor Rev. 2020;54:43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hachim MY, Al Heialy S, Hachim IY, et al. Interferon‐induced transmembrane protein (IFITM3) is upregulated explicitly in SARS‐CoV‐2 infected lung epithelial cells. Front Immunol. 2020;11:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science. 2020;369(6504):718‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sette A, Crotty S. Adaptive immunity to SARS‐CoV‐2 and COVID‐19. Cell. 2021;184:861‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen J, Subbarao K. The immunobiology of SARS. Annu Rev Immunol. 2007;25:443‐472. [DOI] [PubMed] [Google Scholar]
- 62. Park A, Iwasaki A. Type I and Type III interferons–induction, signaling, evasion, and application to combat COVID‐19. Cell Host Microbe. 2020;27:870‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoshikawa T, Hill TE, Yoshikawa N, et al. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome‐associated coronavirus infection. PLoS One. 2010;5(1):e8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Menachery VD, Yount BL J.r, Josset L, et al.Attenuation and restoration of severe acute respiratory syndrome coronavirus mutant lacking 2′‐O‐methyltransferase activity. J Virol. 2014;88(8):4251‐4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe. 2016;19(2):181‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Trouillet‐Assant S, Viel S, Gaymard A, et al. Type I IFN immunoprofiling in COVID‐19 patients. J Allergy Clin Immunol. 2020;146(1):206‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gupta R. The double edged interferon riddle in COVID‐19 pathogenesis. Crit Care. 2020;24(1):631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lopez L, Sang PC, Tian Y, Sang Y. Dysregulated interferon response underlying severe COVID‐19. Viruses. 2020;12(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goldman‐Israelow B, Song E, Mao T, et al. Mouse model of SARS‐CoV‐2 reveals inflammatory role of type I interferon signaling. bioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rebendenne A, Chaves Valadão AL, Tauziet M, et al. SARS‐CoV‐2 triggers an MDA‐5‐dependent interferon response which is unable to control replication in lung epithelial cells. J Virol. 2021;95(8):e02415‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wadman M. Can interferons stop COVID‐19 before it takes hold? American Association for the Advancement of Science. 2020;369:125‐126. [DOI] [PubMed] [Google Scholar]
- 72. Channappanavar R, Fehr AR, Zheng J, et al. IFN‐I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129(9):3625‐3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang N, Zhan Y, Zhu L, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID‐19 patients. Cell Host Microbe. 2020;28(3):455‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y, Qin L, Zhao Y, et al. Interferon‐induced transmembrane protein 3 genetic variant rs12252‐C associated with disease severity in coronavirus disease 2019. J Infect Dis. 2020;222(1):34‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schönfelder K, Breuckmann K, Elsner C, et al. The influence of IFITM3 polymorphisms on susceptibility to SARS‐CoV‐2 infection and severity of COVID‐19. Cytokine. 2021;142:155492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pati A, Padhi S, Suvankar S, Panda AK. Minor allele of interferon‐induced transmembrane protein 3 polymorphism (rs12252) is covered against severe acute respiratory syndrome coronavirus 2 infection and mortality: a worldwide epidemiological investigation. J Infect Dis. 2021;223(1):175‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kim Y‐C, Jeong B‐H. Strong correlation between the case fatality rate of COVID‐19 and the rs6598045 single nucleotide polymorphism (SNP) of the interferon‐induced transmembrane protein 3 (IFITM3) gene at the population‐level. Genes. 2021;12(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta‐1b, lopinavir‐ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID‐19: an open‐label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Felgenhauer U, Schoen A, Gad HH, et al. Inhibition of SARS–CoV‐2 by type I and type III interferons. J Biol Chem. 2020;295(41):13958‐13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lokugamage KG, Hage A, De Vries M, et al. Type I interferon susceptibility distinguishes SARS‐CoV‐2 from SARS‐CoV. J Virol. 2020;94(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS‐CoV‐2 infection. Antiviral Res. 2020;179:104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clementi N, Ferrarese R, Criscuolo E, et al. Interferon‐β−1a inhibition of severe acute respiratory syndrome–coronavirus 2 in vitro when administered after virus infection. J Infect Dis. 2020;222(5):722‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Neerukonda SN, Katneni U. A review on SARS‐CoV‐2 virology, pathophysiology, animal models, and antiviral interventions. Pathogens. 2020;9(6):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pandey K, Acharya A, Mohan M, Ng CL, Reid SP, Byrareddy SN. Animal Models for SARS‐CoV‐2 research: a comprehensive literature review. Transbound Emerg Dis. 2020;68:1868‐1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rosa BA, Ahmed M, Singh DK, et al. IFN signaling and neutrophil degranulation transcriptional signatures are induced during SARS‐CoV‐2 infection. Communications Biology. 2021;4(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cleary SJ, Pitchford SC, Amison RT, et al. Animal models of mechanisms of SARS‐CoV‐2 infection and COVID‐19 pathology. Br J Pharmacol. 2020;177(21):4851‐4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Haagmans BL, Kuiken T, Martina BE, et al. Pegylated interferon‐α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10(3):290‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ströher U, DiCaro A, LI Y, et al. Severe acute respiratory syndrome‐related coronavirus is inhibited by interferon‐α. J Infect Dis. 2004;189(7):1164‐1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hensley LE, Fritz EA, Jahrling PB, Karp C, Huggins JW, Geisbert TW. Interferon‐β 1a and SARS coronavirus replication. Emerg Infect Dis. 2004;10(2):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sainz B Jr., Mossel EC, Peters CJ, Garry RF. Interferon‐beta and interferon‐gamma synergistically inhibit the replication of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV). Virology. 2004;329(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zheng B, He ML, Wong KL, et al. Potent inhibition of SARS‐associated coronavirus (SCoV) infection and replication by type I interferons (IFN‐α/β) but not by type II interferon (IFN‐γ). J Interferon Cytokine Res. 2004;24(7):388‐390. [DOI] [PubMed] [Google Scholar]
- 92. Mantlo E, Bukreyeva N, Maruyama J, Paessler S, Huang C. Antiviral activities of type I interferons to SARS‐CoV‐2 infection. Antiviral Res. 2020;179:104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sainz B Jr., Mossel EC, Peters CJ, Garry RF. Interferon‐beta and interferon‐gamma synergistically inhibit the replication of severe acute respiratory syndrome‐associated coronavirus (SARS‐CoV). Virology. 2004;329(1):11‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J. J.r Ribavirin and interferon‐β synergistically inhibit SARS‐associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326(4):905‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon‐α2b and ribavirin. Sci Rep. 2013;3:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS‐CoV‐2 and the type I interferon response. PLoS Pathog. 2020;16(7):e1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Li C, Luo F, Liu C, et al. Engineered interferon alpha effectively improves clinical outcomes of COVID‐19 patients. Res Sq. 2020. [Google Scholar]
- 98. Little C, Cosetti MK. A narrative review of pharmacologic treatments for COVID‐19: safety considerations and ototoxicity. Laryngoscope. 2021;131:1626‐1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nakhlband A, Fakhari A, Azizi H. Interferon‐beta offers promising avenues to COVID‐19 treatment: a systematic review and meta‐analysis of clinical trial studies. Naunyn‐Schmiedeberg's Arch Pharmacol. 2021:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Monk PD, Marsden RJ, Tear VJ, et al. Safety and efficacy of inhaled nebulised interferon beta‐1a (SNG001) for treatment of SARS‐CoV‐2 infection: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Respir Med. 2020;9:196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peiffer‐Smadja N, Yazdanpanah Y. Nebulised interferon beta‐1a for patients with COVID‐19. The Lancet Respir Med. 2021;9(2):122‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Davoudi‐Monfared E, Rahmani H, Khalili H, et al. A randomized clinical trial of the efficacy and safety of interferon β‐1a in treatment of severe COVID‐19. Antimicrob Agents Chemother. 2020;64(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rahmani H, Davoudi‐Monfared E, Nourian A, et al. Interferon β−1b in treatment of severe COVID‐19: a randomized clinical trial. Int Immunopharmacol. 2020;88:106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Estebanez M, Ramírez‐Olivencia G, Mata T, et al. Clinical evaluation of IFN beta1b in COVID‐19 pneumonia: a retrospective study. medRxiv. 2020. [Google Scholar]
- 105. Khamis F, Al Naabi H, Al Lawati A, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta‐1b in hospitalized patients with moderate to severe COVID‐19 pneumonia. Int J Infect Dis. 2021;102:538‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pereda R, González D, Rivero HB, et al. Therapeutic effectiveness of Interferon‐α2b against COVID‐19: the Cuban experience. J Interferon Cytokine Res. 2020;40(9):438‐442. [DOI] [PubMed] [Google Scholar]
- 107. Pan H, Peto R, Henao‐Restrepo A‐M, et al, WHO Solidarity Trial Consortium . Repurposed antiviral drugs for COVID‐19; interim WHO Solidarity Trial results. N Engl J Med. 2021;384(6):497‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Walz L, Cohen AJ, Rebaza AP, et al. JAK‐inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID‐19 patients: a systematic review and meta‐analysis. BMC Infect Dis. 2021;21(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pereda R, González D, Rivero H, et al. Therapeutic effectiveness of interferon alpha 2b treatment for COVID‐19 patient recovery. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 110. Li C, Chen Q, Wang J, et al. Clinical characteristics of chronic liver disease with coronavirus disease 2019 (COVID‐19): a cohort study in Wuhan, China. Aging (Albany NY). 2020;12(16):15938‐15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Baghaei P, Dastan F, Marjani M, et al. Combination therapy of IFNβ1 with lopinavir–ritonavir, increases oxygenation, survival and discharging of sever COVID‐19 infected inpatients. Int Immunopharmacol. 2021;92:107329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xu P, Huang J, Fan Z, et al. Arbidol/IFN‐α2b therapy for patients with coronavirus disease 2019: a retrospective multicenter cohort study. Microbes Infect. 2020;22(4‐5):200‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wong C, Wan E, Luo S, et al. Clinical outcomes of different therapeutic options for COVID‐19 in two Chinese case cohorts: a propensity‐score analysis. EClinicalMedicine. 2021;32:100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pandit A, Bhalani N, Bhushan BLS, et al. Efficacy and safety of pegylated interferon Alfa‐2b in moderate COVID‐19: a phase II, randomized, controlled, open‐label study. Int J Infect Dis. 2021;105:516‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Frankel AE, Reddy R, DeSuza KR, et al. Response to pegylated interferon in a COVID‐19–positive elderly woman with primary myelofibrosis treated with ruxolitinib. Clin Case Rep. 2021;9:2228‐2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhou Q, MacArthur M, He X, et al. Interferon‐α2b treatment for COVID‐19 is associated with improvements in lung abnormalities. Viruses. 2021;13(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Idelsis EMI, Jesus PE, Yaquelin DR, et al. Effect and safety of combination of interferon alpha‐2b and gamma or interferon alpha‐2b for negativization of SARS‐CoV‐2 viral RNA. Preliminary results of a randomized controlled clinical trial. medRxiv. 2020. [Google Scholar]
- 118. Darazam IA, Shokouhi S, Pourhoseingholi MA, et al. Role of interferon therapy in severe COVID‐19: The COVIFERON randomized controlled trial. Sci Rep. 2021;11:8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wang N, Zhan Y, Zhu L, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID‐19 patients. Cell Host Microbe. 2020;28(3):455‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Meng Z, Wang T, Chen L, et al. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. MedRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 121. Huang YQ, Tang SQ, Xu XL, et al. No statistically apparent difference in antiviral effectiveness observed among ribavirin plus interferon‐alpha, lopinavir/ritonavir plus interferon‐alpha, and ribavirin plus lopinavir/ritonavir plus interferon‐alpha in patients with mild to moderate coronavirus disease 2019: results of a randomized, open‐labeled prospective study. Front Pharmacol. 2020;11:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


