CONFLICT OF INTERESTS
None to declare.
AUTHOR CONTRIBUTIONS
Kobkan Thongprasom: Conceptualization; investigations; writing‐review and editing, supervision. Nawaporn Pengpis: Data curation;writing‐original draft. Ekarat Phattarataratip: investigations; writing‐review and editing. Lakshman Samaranayake: writing‐review and editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.14034.
To the Editor:
The coronavirus disease 2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, is a major global public health issue (Lai et al., 2020). Numerous efficacious and safe vaccines against COVID‐19 have been developed (Mazur et al., 2021; Samaranayake et al., 2021), one of which ChAdOx1 nCoV‐19 (Astra Zeneca; AZD1222) viral vector vaccine is widely used in Thailand. We present here a case of oral pemphigus lesion in a Thai female that was associated with the administration of the first dose of AZD1222 vaccine. To our knowledge, this particular side effect of the latter vaccine has not been reported elsewhere.
A 38‐year‐old Thai woman was referred to the Oral Medicine Clinic at the Faculty of Dentistry, Chulalongkorn University, Bangkok, with a chief complaint of painful oral lesions lasting over 8 weeks. Her past history revealed that the lesions appeared 1 week following the administration of the first dose of AZD1222 vaccine. Although she attended a medical clinic for the condition approximately one month prior to the dental visit, a specific diagnosis was not made by the physician at the time. Furthermore, she had anemia and hypothyroidism, and was on iron supplements, vitamin C, and an anti‐anxiety drug, clorazepate. She had no known history of allergic conditions.
Her extraoral examination was unremarkable. The intraoral examination revealed generalized desquamative epithelium, erythematous areas along the marginal gingivae and alveolar mucosa. Pseudomembranes, and erosions and ulceration on the buccal gingiva subjacent to the maxillary and mandibular teeth, and the right lingual dorsum could be seen. Furthermore, generalized desquamative epithelium of the alveolar mucosa of the anterior mandibular teeth (Figure 1) as well as the right mandibular and left maxillary posterior molars extending to the mucobuccal folds were present. Erythematous areas also extended from the lingual gingiva of anterior mandibular teeth onto the floor of mouth.
FIGURE 1.
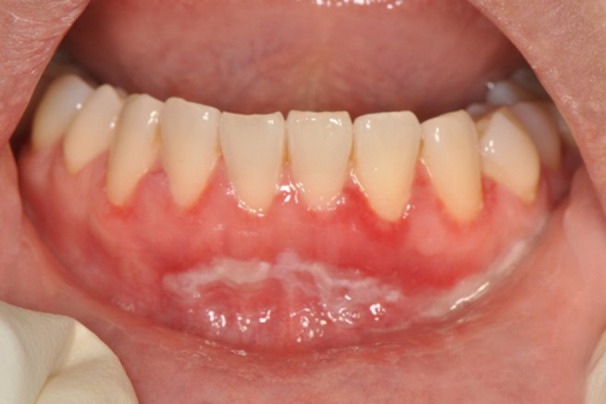
Desquamative epithelium at the anterior mandibular gingiva extending to mucolabial fold
A biopsy of the perilesional tissue at the anterior aspect of the mandibular labial mucosa was taken for histopathologic evaluation. Smear specimens, taken from the base of the lesion for a Tzanck test, showed numerous acantholytic cells. The histopathologic and direct immunofluorescence examination confirmed a diagnosis of pemphigus. After treatment with a potent topical steroid, fluocinolone acetonide 0.05% mouthwash, for one week, her painful oral lesions regressed.
Although some oral adverse reactions have been reported in COVID‐19 vaccinated patients, there is still no definitive evidence supporting a direct link between vaccines and oral manifestations (Azzi et al., 2021; Cirillo, 2021; Kulkarni & Sollecito, 2021; Wan et al., 2021). Other reports of post‐vaccination orofacial adverse effects include swellings of the lips, the face, and tongue associated with anaphylaxis, oral mucositis, and exacerbation of lichen planus lesions (Azzi et al., 2021; Cirillo, 2021; Kulkarni & Sollecito, 2021). Oral healthcare providers should be aware of the possible post‐vaccination, orofacial mucocutaneous adverse effects, and also record these in order to develop a comprehensive database on various COVID‐19 vaccines, that are now delivered globally.
ACKNOWLEDGMENTS
We would like to express our sincere thanks to Assistant Professor Dr. Marisa Pongprutthipan, Head Division of Dermatology, Dr. Nalinee Pitipornchai and Dr. Pawinee Rerknimitr Department of Medicine, Faculty of Medicine, Chulalongkorn University for histopathologic and immunofluorescence studies. We also thank the staff of the Oral Medicine and Oral Pathology Departments, Faculty of Dentistry, Chulalongkorn University, Bangkok, Thailand for their kind support. Our thanks go to Dr. Prasong Kosarussawadee, Dental Division, Saint Louis Hospital, Bangkok, Thailand for providing additional information on the patient.
REFERENCES
- Azzi, L. , Toia, M. , Stevanello, N. , Maggi, F. , & Forlani, G. (2021). An episode of oral mucositis after the first administration of the ChAdOx1 COVID‐19 vaccine. Oral Diseases, 10.1111/odi.13874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo, N. (2021). Reported orofacial adverse effects of COVID‐19 vaccines: The knowns and the unknowns. Journal of Oral Pathology and Medicine, 50(4), 424–427. 10.1111/jop.13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, R. , & Sollecito, T. P. (2021). COVID‐19 vaccination: possible short‐term exacerbations of oral mucosal diseases. International Journal of Dermatology, 60(9), e335–e336. 10.1111/ijd.15779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, C. C. , Shih, T. P. , Ko, W. C. , Tang, H. J. , & Hsueh, P. R. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease‐2019 (COVID‐19): The epidemic and the challenges. International Journal of Antimicrobial Agents, 55(3), 105924. 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur, M. , Duś‐Ilnicka, I. , Jedliński, M. , Ndokaj, A. , Janiszewska‐Olszowska, J. , Ardan, R. , Radwan‐Oczko, M. , Guerra, F. , Luzzi, V. , Vozza, I. , Marasca, R. , Ottolenghi, L. , & Polimeni, A. (2021). Facial and oral manifestations following COVID‐19 vaccination: A survey‐based study and a first perspective. International Journal of Environmental Research and Public Health, 18(9), 4965. 10.3390/ijerph18094965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranayake, L. P. , Seneviratne, C. J. , & Fakhruddin, K. S. (2021). Coronavirus disease 2019 (COVID‐19) vaccines: A concise review. Oral Diseases, 10.1111/odi.13916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, E. Y. F. , Chui, C. S. L. , Lai, F. T. T. , Chan, E. W. Y. , Li, X. , Yan, V. K. C. , Gao, L. E. , Yu, Q. , Lam, I. C. H. , Chun, R. K. C. , Cowling, B. J. , Fong, W. C. , Lau, A. Y. L. , Mok, V. C. T. , Chan, F. L. F. , Lee, C. K. , Chan, L. S. T. , Lo, D. , Lau, K. K. , … Wong, I. C. K. (2021). Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS‐CoV‐2 vaccines: a case series and nested case‐control study. The Lancet Infectious Diseases, 10.1016/s1473-3099(21)00451‐5 [DOI] [PMC free article] [PubMed] [Google Scholar]


