To the Editor,
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Delta variant (B.1.617.2) has drastically altered the landscape of the coronavirus disease 2019 (COVID‐19) pandemic. 1 Despite a reported two‐to‐three‐fold reduction in neutralizing antibody titers against the Delta variant in vaccinees, 2 , 3 breakthrough infections are frequently noticed, 4 raising the possibility that the Delta variant has evolved mechanisms other than escaping from vaccine‐ or natural infection‐induced immunity to become the dominant variant around the globe. 5 In consistence, experimental data do not predict the mutations found in the Delta variant spike protein to confer the highest affinity toward its receptor, 6 the human angiotensin‐converting enzyme 2 (ACE2). Several studies of the Delta variant spike suggest that the P681R mutation at the furin cleavage site may enhance the proteolytic processing of the protein and hence renders unique features to the variant during entry. 7 , 8 , 9
Here, we conducted a study to investigate the relationship between ACE2 expression levels on human airway way cells and the susceptibilities of these cells to pseudoviruses bearing the spike protein of several SARS‐CoV‐2 variants. Under a protocol approved by the Qiqihar Medical University Institutional Review Board, primary human bronchial epithelial cells (HBEpC) were collected by brushing the epithelium of healthy nonsmokers during bronchoscopy and cultured in bronchial epithelial cell growth medium (BEGM; Lonza). Deidentified HBEp cells were immediately analyzed for the surface expression of ACE2 by flow cytometric analysis using an R&D Systems ACE2 antibody (FAB9332G). As these primary cells are composed of several cell types, 10 including the basal cell, secretory cell, Clara cell, and ciliated cell, and because we are unable to sort individual cell populations due to rapid senescence, only the mean fluorescence intensity (MFI) of ACE2 was quantified in total cell populations from 12 donors. These cells were subsequently infected by lentiviral pseudo particles bearing the Spike protein of the initial Wuhan‐Hu‐1 isolate (MN908947.3), the Alpha (B.1.1.7), Beta (B1.351), Gamma (P.1), and the Delta variant (B.1.617.2) (human codon‐optimized and synthesized by Genscript). Pseudoviruses were produced by cotransfection of HEK 293T cells with pCMV‐dR8.2 dvpr, pTRIP‐luc, and SARS‐CoV‐2 Spike expressing plasmid using Lipofectamine 2000. The supernatants were harvested at 72 h posttransfection and filtered through 0.45‐mm membranes before use. As the spike protein of each variant differs in pseudotyping efficiency, we, therefore, determined viral titer using an endpoint titration assay; infection unit/ml is used to represent the titer of pseudoviruses. 105 infectious units of pseudovirus bearing each variant Spike protein were added to 105 HBEpC. Forty‐eight hours later cells were lysed for firefly luciferase activity measurement. The infectivity of pseudovirus bearing each variant Spike protein is then plotted against the ACE2 level of HBEpC from each donor using GraphPad Prism 8. As shown in Figure 1, the infectivity of pseudoviruses bearing Spike protein of all variants in general positively correlated with the ACE2 levels of HBEpC. Strikingly, in ACE2low HBEpC, the pseudovirus bearing the Delta variant spike protein displayed infectivity that is three‐fold higher than that of the alpha variant and then five times higher than that of the Beta (B1.351) and Gamma (P.1) variants. The difference is statistically significant. When the MFI of ACE2 is higher than 100, there is no longer statistical significance among pseudoviruses bearing spikes of different SARS‐CoV‐2 variants.
Figure 1.
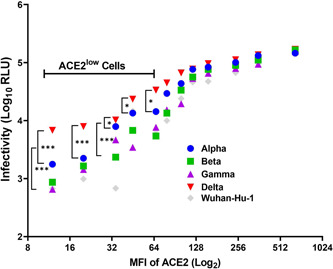
Pseudovirus bearing Delta variant spike protein infects ACE2low HBEpC to a higher level than other variants. HBEpC from 12 different donors were measured for surface expression of ACE2 by flow cytometric analysis and plotted on the x‐axis from low to high. The corresponding infectivities of an equal amount of pseudoviruses bearing the original Wuhan‐Hu‐1, Alpha, Beta, Gamma, and Delta variant spike protein on cells from each donor are plotted on the y‐axis. Statistical analysis was performed using two‐way ANOVA, *p < 0. 05, **p < 0.01, ***p < 0.001
In summary, among the five major SARS‐CoV‐2 variants, including the original Wuhan variant and Alpha, Beta, Gamma, and Delta, the Delta variant spike protein appears to confer greater infectivity on primary human airway cells in which only low levels of ACE2 are found. Our data suggest that the Delta variant virus may be able to infect cells lining the respiratory tract in individuals even when there is very little ACE2. Given that Delta variant spike is not necessarily possessing a higher affinity toward human ACE2, 11 it may have gained the ability to rapidly fuse with the plasma membrane of cells or via an unknown mechanism to gain better entry. Regardless, our findings shed important light on understanding why the Delta variant exhibits greater transmissibility and has become the predominant circulating SARS‐CoV‐2 variant in the world.
AUTHOR CONTRIBUTION STATEMENT
Hongmei Li and Ting Liu conceived and designed the experiments. Liping Wang, Minghui Wang, and Shaoqing Wang performed the experiments. Hongmei Li and Shaoqing Wang analyzed the data. Hongmei Li, Ting Liu, Liping Wang, Minghui Wang, and Shaoqing Wang wrote the paper.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors are grateful to all staff who work at the Department of Respiratory Medicine of The First Hospital of Qiqihar. This study was supported by the Natural Science Foundation of Heilongjiang Province (No. LH2020C108) and the Research Project from Qiqihar Medical University (No. QY2016LX‐02).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dagpunar J. Interim estimates of increased transmissibility, growth rate, and reproduction number of the Covid‐19 B.1.617.2 variant of concern in the United Kingdom. medRxiv. 2021. 10.1101/2021.06.03.21258293 [DOI] [Google Scholar]
- 2. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid‐19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Planas D, Veyer D, Baidaliuk A, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature. 2021;596(7871):276‐280. [DOI] [PubMed] [Google Scholar]
- 4. Musser JM, Christensen PA, Olsen RJ, et al. Delta variants of SARS‐CoV‐2 cause significantly increased vaccine breakthrough COVID‐19 cases in Houston, Texas. medRxiv. 2021. 10.1101/2021.07.19.21260808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kupferschmidt K. New SARS‐CoV‐2 variants have changed the pandemic. What will the virus do next? Science. 2021. https://www.science.org/news/2021/08/new-sars-cov-2-variants-have-changed-pandemic-what-will-virus-do-next [Google Scholar]
- 6. Starr TN, Greaney AJ, Hilton SK, et al. Deep mutational scanning of SARS‐CoV‐2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182(5):1295‐1310.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Liu JY, Johnson BA, et al. Delta spike P681R mutation enhances SARS‐CoV‐2 fitness over Alpha variant. bioRxiv. 2021. 10.1101/2021.08.12.456173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peacock TP, Sheppard CM, Brown JC, et al. The SARS‐CoV‐2 variants associated with infections in India, B.1.617, show enhanced spike cleavage by furin. bioRxiv. 2021. 10.1101/2021.05.28.446163 [DOI] [Google Scholar]
- 9. Saito A, Irie T, Suzuki R, et al. SARS‐CoV‐2 spike P681R mutation, a hallmark of the Delta variant, enhances viral fusogenicity and pathogenicity. bioRxiv. 2021. 10.1101/2021.06.17.448820 [DOI] [Google Scholar]
- 10. Hiemstra PS, McCray PB Jr, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45(4):1150‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim S, Liu Y, Lei ZW, et al. Differential interactions between human ACE2 and spike RBD of SARS‐CoV‐2 variants of concern. bioRxiv. 2021. 10.1101/2021.07.23.453598 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


