Abstract
The exact origin of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) and source of introduction into humans has not been established yet, though it might be originated from animals. Therefore, we conducted a study to understand the putative reservoirs, transmission dynamics, and susceptibility patterns of SARS‐CoV‐2 in animals. Rhinolophus bats are presumed to be natural progenitors of SARS‐CoV‐2‐related viruses. Initially, pangolin was thought to be the source of spillover to humans, but they might be infected by human or other animal species. So, the virus spillover pathways to humans remain unknown. Human‐to‐animal transmission has been testified in pet, farmed, zoo and free‐ranging wild animals. Infected animals can transmit the virus to other animals in natural settings like mink‐to‐mink and mink‐to‐cat transmission. Animal‐to‐human transmission is not a persistent pathway, while mink‐to‐human transmission continues to be illuminated. Multiple companions and captive wild animals were infected by an emerging alpha variant of concern (B.1.1.7 lineage) whereas Asiatic lions were infected by delta variant, (B.1.617.2). To date, multiple animal species – cat, ferrets, non‐human primates, hamsters and bats – showed high susceptibility to SARS‐CoV‐2 in the experimental condition, while swine, poultry, cattle showed no susceptibility. The founding of SARS‐CoV‐2 in wild animal reservoirs can confront the control of the virus in humans and might carry a risk to the welfare and conservation of wildlife as well. We suggest vaccinating pets and captive animals to stop spillovers and spillback events. We recommend sustainable One Health surveillance at the animal–human–environmental interface to detect and prevent future epidemics and pandemics by Disease X.
Keywords: alpha variant, COVID‐19, delta variant, horseshoe bat, mink, pangolin, Rhinolophus bats
1. INTRODUCTION
Several human cases of pneumonia were reported in Wuhan, China during December 2019 and later the disease was named as novel coronavirus disease 19 (COVID‐19) (Chan et al., 2020a). The causal agent was detected as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) and the outbreak was linked to the seafood and live animal market in Wuhan (Zhu et al., 2020). The animal market is involved in trading poultry, snakes, hedgehogs and other wildlife (Wu et al., 2020). Meat and carcasses were traded in that market at the time of the outbreak (Ashour et al., 2020). Initially, bats are thought to be the reservoir of the SARS‐CoV‐2 (Mallapaty, 2020). Bats are reservoirs of several other emerging zoonotic pathogens like Nipah, Hendra, Ebola, rabies, Rotavirus and CoVs (Brook & Dobson, 2015; Islam et al., 2020). Specifically, the Indian Flying Fox, Pteropus medius, harbours more than 50 viruses, based on which researchers estimated that at least 320,000 mammalian viruses from nine viral families are still undiscovered (Anthony et al., 2013). Besides, more than 200 novel CoVs have been detected in bats throughout the world (Chen et al., 2014). It is assumed that Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) and SARS‐CoV originated in bats and transmitted through dromedary camels and civets, respectively (El‐Sayed & Kamel, 2021). Not only SARS or MERS, other human CoVs (HCoV) like HCoV‐NL63 and HCoV‐229E have been also originated from bats (Lu et al., 2015; Skirmuntt et al., 2020).
Furthermore, cats and dogs in contact with SARS‐CoV‐2‐infected humans have been identified as SARS‐CoV‐2 positive (Islam et al., 2021a). Therefore, there is occasional spillover evidence among human to animal species. Under experimental conditions, the virus can infect and replicate in the respiratory tracts of several animal species like ferrets, cats, hamsters and rhesus macaques. Nevertheless, cattle, sheep and goats are not susceptible to SARS‐CoV‐2 in the experimental condition (OIE, 2021). Experimental studies have shown that animals are not playing a crucial role in the transmission of SARS‐CoV‐2 (Parolin et al., 2021). Rather human‐to‐human transmission helps the persistence of the infection throughout the world.
SARS‐CoV‐2 enters host cells by its spike protein (S‐protein). The receptor‐binding domain (RBD) of S protein binds to angiotensin‐converting enzyme 2 (ACE‐2) for entering host cells. Then a cellular protease (TMPRSS2) acts on the S protein (Lam et al., 2018; Wrapp et al., 2020) and divides it into 2 subunits to merge the virus and the cell membrane (Hoffmann et al., 2020; Stopsack et al., 2020). ACE‐2 receptors are very much preserved in different vertebrate species. So animals that have a receptor for this protein can be a vessel for entry (Lam et al., 2018). SARS‐CoV‐2 can be harboured by any animal that possesses ACE‐2 receptors (Damas et al., 2020; Lam et al., 2020; Lan et al., 2020).
The exact precursor for coronavirus is not established yet and has the potential for wildlife origin. Phylogenetic analysis of SARS‐CoV‐2 sequences from different animals, for example, dog, cat, tiger, lion, mink and gorilla, proved their relation to human sequences from the same region during the same time period (Barrs et al., 2020; Hamer et al., 2020; McAloose et al., 2020; Pagani et al., 2021; Sit et al., 2020). The SARS‐CoV‐2 strains detected in animals showed various mutations in their nucleotide sequences, and some resultant mutates have given origin to new variants, harmful for the human population or other species of animals. Therefore, we thoroughly reviewed the available literature to understand the comprehensive transmission dynamics and susceptibility patterns of SARS‐CoV‐2 and its related viruses in domestic, farmed and wild animal species, and to highlights the significance of sustainable One health surveillance at the animal–human–environment interface as an early warning tool to detect novel virus (disease X) to prevent future epidemics and pandemics globally.
2. MATERIAL AND METHOD
We conducted detailed literature search in the databases namely, Scopus (https://www.scopus.com/home.uri), PubMed (https://www.ncbi.nlm.nih.gov/pubmed/), Web of Science (http://login.webofknowledge.com/), Google Scholar (https://scholar.google.com/) and preprint servers, using several keywords (Table 1). We also searched publicly available information from the World Organization for Animal Health (OIE), the Global Initiative on Sharing All Influenza Data (GISAID) (https://www.gisaid.org/; accessed on July 10, 2021) and the United States Department of Agriculture (USDA) (https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/sa_one_health/sars‐cov‐2‐animals‐us). Then we selected the literature based on reporting of the natural and experimental infections in different animal species.
TABLE 1.
Keywords for searching published literature in different databases
| Term | Keywords |
|---|---|
| Descriptive terms | Prevalence OR Incidence OR Frequency OR Occurrence OR Infection OR Detection OR Identification OR Isolation OR Characterization OR Investigation OR Survey OR Rate |
| Outcome term | COVID‐19 OR SARS‐CoV‐2 |
| Population terms | Bat OR Pangolin OR Dog OR Cat OR Domestic animals OR Pig OR Poultry OR Avian OR Turkey OR Chicken OR Goose OR Cattle OR Camel OR Bovine OR Equine OR Horse OR Wild animals OR Zoo animals OR Tiger OR Lion OR Canine OR Feline OR Mink OR Mammals OR Non‐human primates OR Monkey OR Macaque OR Rodents OR Mice OR Rat OR Ferret OR Guinea pig OR Masked Civet |
We then collected mink and pangolin population density worldwide from the IUCN red‐listed threatened species database (IUCN, 2021). Besides, we collected the fur production status and country‐specific number of confirmed SARS‐CoV‐2 reported mink farms from the published GLEWS+ risk assessment literature WHO (2021). We produced the choropleth map for mink population density, country‐specific numbers of SARS‐CoV‐2 reported mink farms, and geospatial distribution of different pangolin species in ArcGIS 10.4 software using standard procedures described by Islam et al. (2021d) and Sayeed et al. (2020).
A total of 450 complete genome sequences of SARS‐CoV‐2 and SARS‐CoV‐2 like CoVs from animals (Aonyx cinereus, Gorilla gorilla gorilla, Canis lupus familiaris, Panthera leo, Prionailurus bengalensis euptilurus, Panthera uncia, Panthera tigris, Mustela putorius furo, Rhinolophus affinis, Rhinolophus malayanus, Rhinolophus malayanu, Rhinolophus stheno, Rhinolophus sinicus, Felis catus, Manis javanica, P. tigris jacksoni, Neovison vison) and humans were submitted in the GISAID, between 18 April 2020 and 07 July 2021. We retrieved all 450 sequences initially in this study. The reference SARS‐CoV‐2 Wuhan genome (NC_045512) was utilized. For phylogenetic analysis, later 112 out of 450 genome successions were chosen (Supplementary Table) because of the great grouping quality as verified in the GISAID data set for additional investigation, while genome arrangements having >5% NNNs as well as <29,000 nt were avoided considering bad quality successions. Succession DAtaset developer (SEDA; https://www.sing‐group.org/seda/) was utilized to eliminate all interior stop codon‐containing arrangements. Moreover, numerous grouping arrangement program (MAFFT) order line (https://mafft.cbrc.jp/arrangement/programming/) (Katoh & Standley, 2013) was utilized to adjust all recovered genome successions over the reference succession. Uber 7 apparatus was utilised for the phylogenetic examination as portrayed by Islam et al. (2021e). The neighbour‐joining technique (Kumar et al., 2016) and the Kimura–Nei strategy (Saitou & Nei, 1987) were considered for all the detailed developmental relationship examinations where the bootstrap test (1000 reproduces) is displayed close to the branches. During the basic decision communication, we have pondered declaring time, the geographical region close by human–animal interface reports, unpredictable model combination dates, close by and large between every plan whether their uncovered pathogenic power. This phylogenetic tree addresses the developmental relationship of delegates both SARS‐CoV‐2 and SARS‐CoV‐2 like viruses from humans and animals. Its fundamental design was to clarify the human–animal interfacial transformative relationship alongside the transmission dynamic of SARS‐CoV‐2 and SARS‐CoV‐2 like infection from humans and animals.
3. RESULTS AND DISCUSSIONS
The origin of SARS‐CoV‐2 has not been confirmed yet, but it is clear that the virus originated from animal species. Therefore, animal species, which can act as reservoirs, natural hosts and/or intermediate hosts, should be well‐known to health authorities. From this review, we assumed SARS‐CoV‐2 could affect several animal species globally.
3.1. Putative animal reservoirs host dynamics and evolution of SARS‐CoV‐2‐related coronaviruses
3.1.1. SARS‐CoV‐2‐related coronaviruses in bats
Rulli et al. (2020) showed that land‐use change and livestock revolution enhance the zoonotic coronavirus transmission risk from Rhinolophid bats. Asian horseshoe bats usually harbour SARS‐related coronaviruses. They stated that horseshoe bats’ habitat in China has more forest fragmentation and human–livestock density than any other country in the world. As a result, China has become a hotspot for human–livestock–wildlife interactions with the potential for SARS‐related coronavirus spillover from animals to humans.
Soon after the emergence of SARS‐CoV‐2 in humans, researchers found that the virus’ genome has similarities with coronaviruses from Rhinolophus bats (Figure 1) but the route of SARS‐CoV‐2 spread is still unknown (Wong et al., 2020). SARS‐CoV‐2 has a 96% genetic resemblance with the Horseshoe bat (R. affinis) CoV RaTG13, implying bats as reservoir of SARS‐CoV‐2 related viruses (Figure 2) (Lu et al., 2020a; Touati et al., 2020; Zhou et al., 2020). Besides, the SARS‐CoV‐2 genome has an insertion site between the cleavage site of S1 and S2. This insertion has also been seen in R. malaynus. Two bat species, namely R. affinis and R. malaynus, can be the ancestral reservoir of SARS‐CoV‐2. Nevertheless, the direct transmission or spillover of SARS‐CoV‐2 from Horseshoe bats to humans has not been established (Wong et al., 2020). Subsequently, additional SARS‐CoV‐2‐related viral genome sequences from horseshoe bats have been detected in Eastern China (Zhou et al., 2020), Japan (Murakami et al., 2020), Cambodia (Hul et al., 2021) and Thailand (Wacharapluesadee et al., 2021).
FIGURE 1.
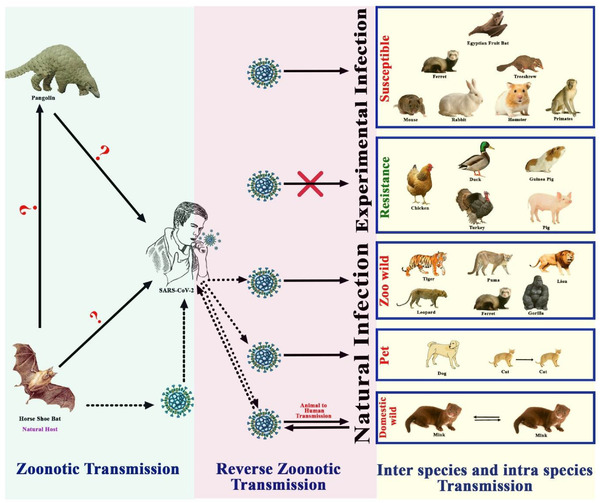
Transmission pathways and susceptibility of SARS‐CoV‐2 to domestic and wild animals
FIGURE 2.
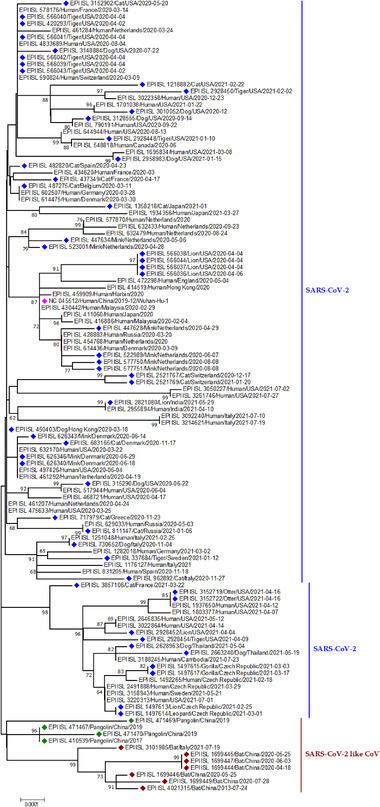
Phylogenetic analysis of SARS‐CoV‐2 and SARS‐CoV‐2 like CoV sequences from animals and humans. Blue dots represent SARS‐CoV‐2 sequences from different animal species; magenta dot refers to a reference sequence from Wuhan; green blocks indicate SARS‐CoV‐2 like CoV sequences from pangolin; mars red blocks indicate SARS‐CoV‐2 like CoV sequences from bats
In Cambodia, coronaviruses with 92.6% genomic similarity with SARS‐CoV‐2 (Figure 2) were detected in two Shamel's horseshoe bats (Rhinolophus shameli) archived swab samples collected in 2010 (Hul et al., 2021). Moreover, R. shameli bats are not distributed in China (Lin et al., 2017) which implies that SARS‐CoV‐2‐related viruses might have a much broader geographic spread than earlier thought and proposes that Southeast Asia signifies a crucial zone to ponder in the continuing examination for the origins of SARS‐CoV‐2, and in monitoring the horseshoe bats for coronavirus diversity. Another virus called Rc‐o319 was identified in Japanese horseshoe bat's (Rhinolophus cornutus) frozen droppings collected in 2013. This one has 81% genome similarity with SARS‐CoV‐2 (Figure 2). The Japanese strain is too distant from SARS‐CoV‐2 and cannot bind to SARS‐CoV‐2′s receptor in human cells (Mallapaty, 2020). So, this strain will not infect humans easily. This discovery provides valuable information about the probable transmission of SARS‐CoV‐2 from bats to people.
Another species Rhinolophus acuminatus harbours SARS‐CoV‐2‐related coronavirus, RacCS203, in Thailand (Wacharapluesadee et al., 2021). Moreover, antibodies against SARS‐CoV‐2 were found in this same bat colony in Southern Thailand (Wacharapluesadee et al., 2021). However, three distinct points indicate that bats are not responsible for transmitting SARS‐CoV‐2 to humans (Jo et al., 2020). First, during the pandemic, local bat species were out and hibernated (Jo et al., 2020). Second, though other animal species were present in the market for sale, bats were not sold at that time. Finally, genomes of SARS‐CoV‐2 and other bat CoV sequences have 96% similarity (Figure 2) (Jo et al., 2020). As the science evolves regularly, the most closely related viruses (>95%) to SARS‐CoV‐2 have been detected in bats from Laos lately. The scientists tested saliva, faeces and urine samples from 645 bats in northern Laos and detected three viruses (BANAL‐52, 103, 236) from Rhinolophus bats with almost identical receptor binding domains like SARS‐CoV‐2 (Mallapaty, 2021).
3.1.2. SARS‐CoV‐2‐related coronaviruses in pangolin
Pangolins (Pholidota, Mammalia) are insects eating nocturnal animals, which have eight species in Africa and Asia (Figure 3) (Heighton & Gaubert, 2021). These critically endangered small animals are trafficked illegally for their meat and scales, which have high demand in China for traditional medicine purposes (Wong et al., 2020). The first SARS‐CoV like CoV was named pangolin‐CoV when it was identified in two Malayan pangolin (Manis javanica) carcasses that were trafficked for illegal wild animal market trade (Liu et al., 2020a). This was supported by the fact that pangolin CoV was closely related to SARS‐CoV‐2 (Figure 1) (Zhang et al., 2020b). The pangolin coronavirus genome shares 89% nucleotide and 98% amino acid resemblances with SARS‐CoV‐2 (Figure 2) (Zhang et al., 2020b).
FIGURE 3.
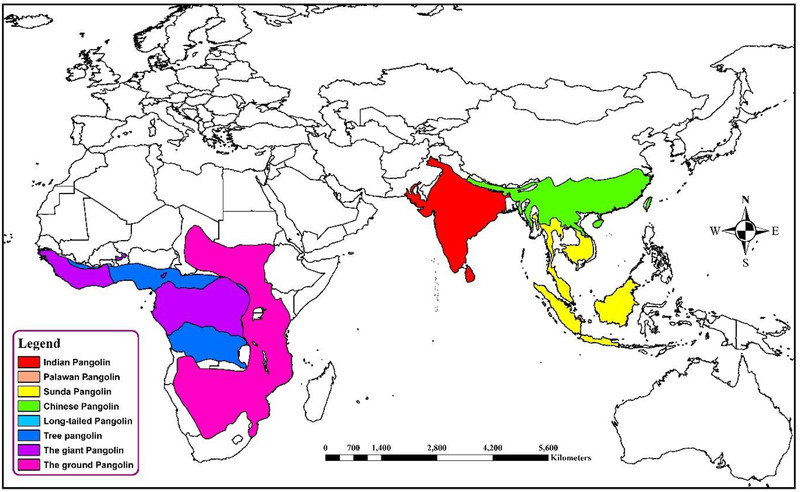
Geospatial distribution of different pangolin species. The common names presented in the map and the scientific names of different colored legends is ginger pink: Smutsia termminckii; amethyst: Smutsia gigantea; cretan blue: Phataginus tricuspis; big sky blue: Phataginus tetradactyla; medium apple: Manis pentadactyla; solar yellow: M. javanica; cantaloupe: M. culionensis; mars red: M. crassicaudata
Along with the bat CoV, pangolin CoV was the second relative of SARS‐CoV‐2 (Zhang et al., 2020c). Besides, the spike protein of SARS‐CoV is more like that of pangolin than that of bat (Goh et al., 2020; Liu et al., 2020a; Zhang et al., 2020c). The genetic analysis of the S glycoprotein of SARS‐CoV‐2 and related coronaviruses presumed that the RBD of SARS‐CoV‐2 S protein is a series of recombination events between the bat‐CoV (RaTG13) and pangolin‐CoV (MP789) and might have ultimately been directed to the advent of this novel coronavirus (Flores‐Alanis et al., 2020). Furthermore, being an insectivorous animal, the pangolin has the possibility of sharing roost with bats especially with the insectivorous ones. This may increase the chance of viral spillover from bat to pangolin. But no literature is available to state as evidence of this fact.
But SARS‐CoV‐2 cannot be the descendant of pangolin CoV as the genetic variation is too high between them (Liu et al., 2020a). Besides, after screening 334 pre‐pandemic samples from Sunda pangolins from Peninsular Malaysia and Sabah between August 2009 and March 2019, no RNA from coronavirus could be detected (Lee et al., 2020). It was concluded that the earlier detection of SARS‐CoV‐2‐related viruses in pangolin reflects their exposure to infected humans, wildlife and other animal species along the trading pathway (Lee et al., 2020). More interestingly, records from Wuhan's market showed that 17 shops of the market sold 36,295 animals between May 2017 to November 2019, but no bat or pangolin was traded during that period (Xiao et al., 2021). So it is of utmost interest to the scientific community to know if pangolin is the natural host (Zhang et al., 2020b) or the dead‐end host (Frutos et al., 2020) of SARS‐CoV‐2. Moreover, antibodies against SARS‐CoV‐2 were found in pangolin in southern Thailand (Wacharapluesadee et al., 2021). Thus, future studies are required to prove the link between pangolin and SARS‐CoV‐2 (Cagliani et al., 2020; Jaimes et al., 2020).
3.2. Transmission dynamics of SARS‐CoV‐2 from humans to domestic and wild animals
After the emergence of SARS‐CoV‐2 in December 2019, several animal species have been infected naturally by humans (Figure 1). Domestic animals like dogs, cats and wild animals like tigers, lions, gorillas, leopards, puma, cougar, ferret, otter and mink have been infected by SARS‐CoV‐2 via reverse zoonoses (Table 2) (Enserink, 2020; Kiros et al., 2020; Oude Munnink et al., 2021). Infected animal species were primarily in contact with humans in settings like a household with pets, zoo, safari park, zoological centres, farms etc. We compiled potential animal species which infected naturally and can act as a reservoir or intermediate or susceptible host.
TABLE 2.
. Natural infection of SARS‐CoV‐2 in animal
| Animal species | Susceptibility | Infection level | Transmission | Reference |
|---|---|---|---|---|
| Companion animals | ||||
| Domestic cat (Felis catus) | High | Sub‐clinical | Between cats | (OIE, 2020d) |
| Dog (Canis lupus familiaris) | Less | Clinical and sub‐clinical | Not between dogs | (OIE, 2020c) |
| Zoo animals | ||||
| Tiger (Panthera tigris) | High | Clinical mostly | To another tiger | (OIE, 2020c) |
| Lion (Panthera leo) | High | Clinical mostly | To another lion | (OIE, 2020c) |
| Gorilla (Gorilla gorilla) | High | Clinical | – | (OIE, 2020c) |
| Puma (Puma concolor) | – | Clinical | From human | (OIE, 2020c) |
| Cougar (Puma concolor) | – | – | – | (USDA, 2021b) |
| Snow leopard (Prionailurus bengalensis euptilurus) | Less | Mild clinical | From human | (OIE, 2020c) |
| Asian small‐clawed otter (Aonyx cinereus) | Unknown | Clinical | From human | (USDA, 2021a) |
| Farmed wild animals | ||||
| American mink (Neovison vison) | High | Clinical | To and from human; to and from other mink; to feral cats | (OIE, 2020c) |
| Ferret (Mustela furo) | From human | (OIE, 2020c) |
In most cases of natural transmission of SARS‐CoV‐2 from human to animal, transmission occurred via direct contact with infected human patients or asymptomatic human carriers (Hamer et al., 2020) but the exact route is still unclear (McAloose et al., 2020). Experimental data indicated that the virus could transmit via droplets or airborne routes from humans to animals (Hossain et al., 2021). Infection through fomites can also be possible. But there is no proof of faecal–oral, bloodborne, vertical or other modes of transmission yet.
3.2.1. SARS‐CoV‐2 spillover events in companion animals
Domestic cat: SARS‐CoV‐2 can produce subclinical infections in cats. To date, 55 cats have been tested positive for SARS‐CoV‐2 by RT‐PCR (Maurin et al., 2021). Natural infection in the cat has been reported in different countries including Spain, China, Hong Kong, Belgium, the United States, France, Germany, Russia and the United Kingdom (Garigliany et al., 2020; Musso et al., 2020; Newman et al., 2020; Sailleau et al., 2020; Segalés et al., 2020). At the beginning of the SARS‐CoV‐2 outbreak in China, antibodies against SARS‐CoV‐2 were detected in three different cats owned by three different patients, suggestive of human to cat transmission (Figure 2). Another study detected a considerable amount of antibodies against SARS‐CoV‐2 in serum samples from cats in Wuhan during and prior to the COVID‐19 outbreak (Zhang et al., 2020b). In addition, serum samples collected from stray cats and hospital cats showed lower antibody titres. In contrast, SARS‐CoV‐2 was detected from symptomatic cat faeces and vomitus in Belgium. Pet cats were also tested positive in New York, USA, which suggests human‐to‐cat transmission (OIE, 2020a). In Spain, eight cats were infected by humans (Ruiz‐Arrondo & Portillo, 2021). At least one infected human has one pet dog or cat in almost 25% of households in Texas, USA (Hamer et al., 2020). Cats from the abandoned household of SARS‐CoV‐2 patients and veterinary clinics showed seropositivity to SARS‐CoV‐2 in Wuhan, China (Zhang et al., 2020b). Furthermore, the alpha (α) variant of concern (VOC) (lineage B.1.1.7) has been identified in a pet cat after being contacted by an infected COVID‐19 owner in the United States (Hamer et al., 2021). The alpha VOC was also detected in cats from Thailand and Italy (Shu & McCauley, 2017). Interestingly, cats can be protected from SARS‐CoV‐2 if they become infected with other feline CoVs previously (Stout et al., 2020). In conclusion, SARS‐CoV‐2 can affect cats more than dogs. To date, no spillback events have been reported from cats to humans. But the shedding of the virus in cats increases the risk of cat‐to‐cat transmission. Besides, there is always the possibility of emerging new variants in animal species and subsequent infection to humans.
Domestic dog: A natural infection of SARS‐CoV‐2 was found in domestic dogs in Hong Kong, the United States, Germany, Japan, Canada, Brazil, Argentina, Mexico, Bosnia & Herzegovina (OIE, 2021). The first report of asymptomatic SARS‐CoV‐2 infection was found on 26 February 2020 in a Pomeranian dog in Hong Kong (Sit et al., 2020). The owner of this dog was also tested positive for COVID‐19 a few days ago (Sit et al., 2020). After that, several dogs were found to be infected without showing any signs in Hong Kong. All of these dogs had a history of mutual living places with infected humans (Figure 2) (Goumenou et al., 2020; Patterson et al., 2020). Another dog was infected by its owner in the Netherlands (Delong, 2020). Fritz et al. (2021) and Patterson (2020) detected high seroprevalence of SARS‐CoV‐2 in dogs from laboratory‐confirmed COVID patients’ households in France and Italy, respectively. SARS‐CoV‐2 can reduce the smelling power of dogs (hyposmia, anosmia) (McNamara et al., 2020). Most recently a pet dog was found to be infected with α VOC, B.1.1.7 after being exposed to a COVID‐19 patient (Hamer et al., 2021). Dogs from Thailand were also infected by the α VOC (Shu & McCauley, 2017).
Even scientists found limited productive replication of the virus in the canine nasal cavity with high ACE2 levels. The tropism and presence of the suitable receptor in dogs made are suitable for virus adaptation and reassortment (Bui et al., 2021). Due to the species’ suitability, continuous surveillance should be implemented in the dog population.
3.2.2. SARS‐CoV‐2 spillover and spillback events in wild animals
SARS‐CoV‐2 spillover events from human to captive wild animals
Several wild animal species are naturally infected by SARS‐CoV‐2 from humans which have been shown in Figure 1. To date, 15 tigers, 22 lions and 3 gorillas have been infected by SARS‐CoV‐2 (OIE, 2021). Two Malayan tigers (Panthera tigris), three Siberian tigers and three African lions (P. leo krugeri) from Wildlife Conservation Society's Bronx Zoo in New York acquired SARS‐CoV‐2 infection from the COVID‐19‐positive caretakers in the zoo (McAloose et al., 2020) and all these animals developed mild respiratory signs and the infection might have occurred at asymptomatic or mild symptomatic animal caretakers in the zoo animals (OIE, 2020a). Nine genomes were identified from these tigers, lions and their keepers (McAloose et al., 2020) of which, two distinct genotypes were detected (Lam et al., 2018). It has been confirmed that transmission occurred from human to tiger (Figure 2) but the exact route is still unclear (McAloose et al., 2020). Two gorillas at the San Diego Zoo safari park in the United States have been infected by COVID‐19. Gorillas had mild symptoms like coughing and congestion. The zoo authority suspects that an asymptomatic worker who tested positive for SARS‐CoV‐2 might infect gorillas. And most recently in a zoo in Hyderabad, India, eight Asiatic lions were found to be infected by COVID‐19 (Steve, 2021). Natural infection was recorded in one cougar (Puma concolor) and two snow leopards (Prionailurus bengalensis euptilurus) from the United States, one leopard from the Czech Republic, two pumas (P. concolor) from Argentina and South Africa, one ferret (Mustela furo) from Slovenia (OIE, 2021). Moreover, the emerging α‐variant (B.1.1.7) of SARS‐CoV‐2 was detected in dogs and cats in the United States; gorillas, lions, leopards and tigers in the Czech Republic and lions from Sri Lanka (Shu & McCauley, 2017). Asiatic lions in India were infected with emerging delta variants (B.1.617.2) (Mishra et al., 2021) .
The serological evidence of SARS‐CoV‐2 was also reported in two household pet ferrets in Spain and antibodies endured at detectable levels in a seropositive SARS‐CoV‐2 status beyond 129 days of first detection (Giner et al., 2021). As a concern, serological assays may signify a viable opportunity to illuminate a host range of SARS‐CoV‐2 in susceptible species, including ferrets. Recently, SARS‐CoV‐2 has been detected in Asian small‐clawed otters (Aonyx cinereus) at an aquarium in Georgia, USA (USDA, 2021a). SARS‐CoV‐2 antibodies were detected in 40% of pre‐ and post‐pandemic serum samples from wild deer (Odocoileus virginianus) sampled from four US states in 2021 (Chandler et al., 2021). SARS‐CoV‐2 has infected several wild animal species and gradually increasing its host range. Though the wild animals were found to be infected by humans, the infection then can be transmitted among co‐housed animals. Regular screening of staff and animals for SARS‐CoV‐2 should be done for early detection of the virus and any variants of concern in zoos and animal facilities.
SARS‐CoV‐2 spillover and spillback events between farmed minks and humans
Mink farming became popular due to the quality production of fur (Figure 4). Mink's fur is very valuable around the world. The global fur industry has grown recently, and around 95 million mink and foxes were sacrificed for fur in 2014. The first farm was established in south Scotland in 1938 and industry expanded very rapidly and the number of farms rose to more than 100 in the late 1940s and 1950s (Cuthbert, 1973). Fur production in Europe during 2018 was 34.7 million. Due to concerns about animal welfare, ethics and inhuman killing, farming of mink for fur production was banned in the United Kingdom (2000), Austria (2005), Slovenia (2013), Republic of Macedonia (2014), Croatia (2018) and Serbia (2019). Outside Europe, mink farming is declared illegal in Japan, New Zealand and California, USA. Moreover, trading mink fur is banned in New Zealand, India, Brazil and some states of the United States (California, Los Angeles and San Francisco) (Anon, 2018).
FIGURE 4.
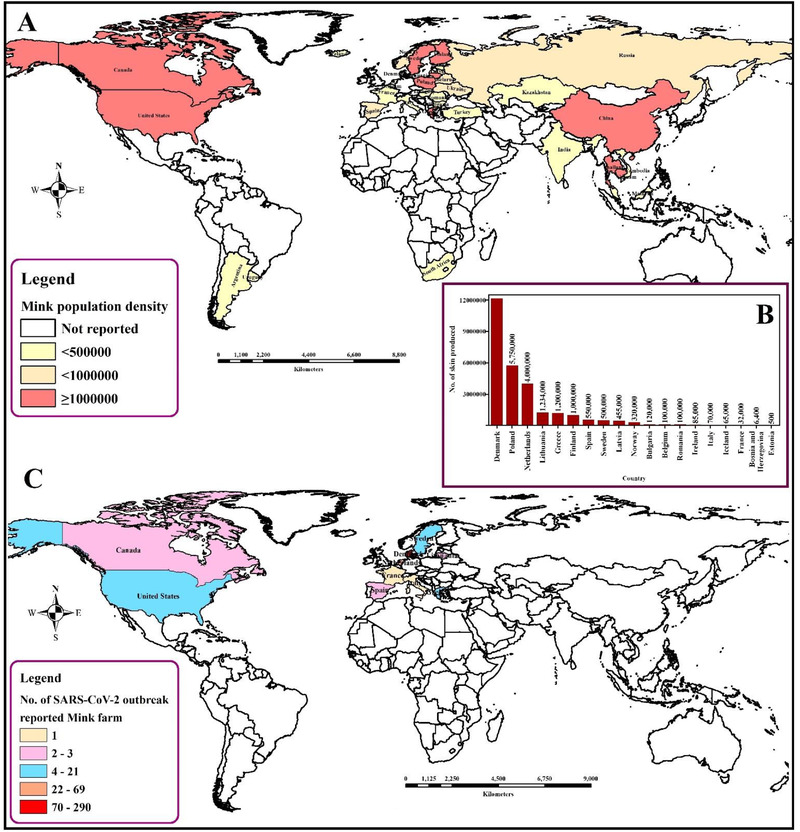
The spatial distribution of mink population along with fur production status and SARS‐CoV‐2 virus infection. (a) Spatial distribution of farm mink population density. (b) Country‐specific fur production status. (c) Country‐specific number of SARS‐CoV‐2‐reported mink farms
The susceptibility of American mink (Neovison vison) to SARS‐CoV‐2 has already been established (Table 2). The virus causes respiratory disease with typical viral pneumonia in histopathology, which can be transmitted from each other. Until January 2021, 400 infected mink farms were identified in 8 countries in Europe – 290 farms in Denmark, 69 in the Netherlands, 21 in Greece, 13 in Sweden, 3 in Spain, 2 in Lithuania, 1 in France and 1 in Italy (Figure 4) (Boklund et al., 2021). Minks from two different farms in the Netherlands showed respiratory and gastrointestinal disorders during April 2020. The mortality rate was estimated as 1.2–2.4% which was higher among pregnant animals. No variation in kid mortality was found. Interstitial pneumonia and other lung lesions were evident in necropsy findings. Viral RNA was detected from lung, throat and rectal swabs and also from the liver and intestines of the dead animals. The record of SARS‐CoV‐2‐infected workers is evidence of possible human to animal transmission. However, sequencing of the initial data is suggestive in favor of mink to human transmission within mink farms (Figure 2) (Munnink et al., 2021).
In December 2020, a free‐ranging wild American mink was infected with SARS‐CoV‐2 in the United States. The animal was asymptomatic, and the infection was transmitted from a nearby affected commercial mink farm (Shriner et al., 2021). Besides, a mink farm in Denmark had a reemergence of SARS‐CoV‐2 after 2 months of testing negative. The mutated virus produces asymptomatic infection and the antibodies persist for a long period (OIE, 2021). The recent SARS‐CoV‐2 detected in two wild American minks, in Spain, highlights the potential significance of indirect transmission pathways of natural infection, seemingly wastewater, as a basis for infection (Islam et al., 2021b) which suggests other aquatic roaming species of carnivores to investigate for their susceptibility to SARS‐CoV‐2 infection. Twelve feral cats and two dogs were infected by SARS‐CoV‐2 in the Netherlands. The study concluded that the feral cats were infected from minks but whether source of infection for the dogs was mink or humans was not clear (Enserink, 2020; van Aart et al., 2021).
A total of 644 human cases have been linked to mink farming in Denmark since June 2020. Moreover, more than half of these cases (N = 338 cases) had a history of working with mink pelting, in six factories and two small facilities. It suggests that people who are involved in farming, culling and pelting of mink have an increased risk of COVID‐19 infection. Besides, till 1 December 2020, approximately 20% (289 farms) of all mink farms in Denmark have been infected with COVID‐19 (WHO, 2020). The family dog of a mink farm owner has also been diagnosed with SARS‐CoV‐2. Immediately after identifying COVID‐19 in mink farms, the authorities took some steps to restrict the transmission. The control measures included entry and egress restriction, boosting of normal hygiene protocols for visitors to mink farms which include washing hands and changing clothes before and after animal handling (Hobbs & Reid, 2021). The Danish government decided (1) depopulating the infected mink farms till March 2021, (2) mandatory reporting of suspected or confirmed SARS‐CoV‐2 infections in Danish fur farms, (3) sampling and testing of fur animals (mink and ferrets), (4) safe handling of feed and manure and (5) the quarantining, depopulation and disinfection of the infected premises (Hobbs & Reid, 2021). Depopulation of mink farms is an inhumane act from an ethical point of view. Moreover, without characterizing the lethality or infectiousness of the isolated viruses from minks, farms were depopulated randomly. If we knew that the mink strain would not be lethal to humans, we could avoid the unnecessary killing of millions of minks. For this reason, we suggest banning mink farming for fur production as well as elaborate molecular characterization of mink variant viruses.
3.3. Experimental infection of SARS‐CoV‐2 in domestic and wild animals
The animal model for SARS‐CoV‐2 infection is important to understand the virus’ pathogenesis and to develop antivirals and vaccines. Non‐human primates are best for this kind of experimental model, but they are expensive and difficult to restrain. Small animals like mice, ferrets and hamsters have receptors for SARS‐CoV and they were used for experimental studies (Glass et al., 2004; Martina et al., 2003; Roberts et al., 2005). Domestic dogs and cats were also used for experimental purposes (Cleary et al., 2020; Sarkar & Guha, 2020). To check the antibody status, ELISA kits were also used (Zhou et al., 2020). ACE‐2 receptors are found only in a few specific animal species (Damas et al., 2020). Animal species which are in contact with human cases of SARS‐CoV‐2 or in zoo or safari parks, zoological centres, rehabilitation centres or farms are mainly used for experimental inoculation. We compiled animal species which can act as reservoirs, intermediate or susceptible hosts (Table 3).
TABLE 3.
. Experimental infection of SARS‐CoV‐2 in different domestic and wild animal species
| Susceptibility to infection (none/extremely low/low/medium/high) | Symptoms | Transmission | References | |
|---|---|---|---|---|
| Domestic animals | ||||
| Dog (Canis lupus familiaris) | Less | No | No | (OIE, 2020c) |
| Black‐tailed prairie dog (Cynomys ludovicianus) | Less | Subclinical | Not reported | (Bosco‐Lauth et al., 2021) |
| Cat (Felis catus) | High | No | Yes | (OIE, 2020c) |
| Pigs (American Yorkshire crossbred pigs, Sus scrofa) | Not susceptible | No | No | (OIE, 2020c) |
| Bird (chicken, duck, turkey, quail, goose) | Not susceptible | No | No | (OIE, 2020c) |
| Cattle (Bos taurus) | Very low | No | No | (OIE, 2020c) |
| Camel (Camelius dromedarius) | Less | – | – | (Wong et al., 2020) |
| Wild animals | ||||
| Mice | Low | No | Yes | (Lei et al., 2020) |
| Deer mouse (Peromyscus maniculatus) | Low | Subclinical | Yes | (Bosco‐Lauth et al., 2021) |
| Bushy tailed woodrat (Neotoma cinerea), wild house mouse (Mus musculus) | Very low | Subclinical | Not reported | (Bosco‐Lauth et al., 2021) |
| Wyoming ground squirrel (Urocitellus elegans), fox squirrel (Sciurus niger) | Very low | Subclinical | Not reported | (Bosco‐Lauth et al., 2021) |
| Golden Syrian hamsters (Mesocricetus auratus) | High | Yes, but it depends on the age | Yes | (OIE, 2020c) |
| Chinese hamster (Cricetulus griseus) | Low to medium | Only weight loss | Not reported | (Bertzbach et al., 2021) |
| Djungarian dwarf hamster (Phodopus sungorus) & Campbell's dwarf hamster (P. campbelli) | Medium | Subclinical | Not reported | (Trimpert et al., 2020) |
| Roborovski dwarf hamster (P. roborovskii) | Medium | Clinical | Not reported | (Trimpert et al., 2020) |
| Striped skunk (Mephitis mephitis) | Medium | Subclinical | Not reported | (Bosco‐Lauth et al., 2021) |
| Mustelids (Ferrets) | High | Only in a small no of cases | Yes | (OIE, 2020c) |
| Mink | High | In some cases | Yes | (OIE, 2020c) |
| Raccoon (Nyctereutes procyonoides) | High | No | Yes | (OIE, 2020c) |
| Raccoon (Procylon lotor) | Very low | Subclinical | Not reported | (Bosco‐Lauth et al., 2021) |
| Reptiles | None | – | – | (Luan et al., 2020) |
| Macaque (Macaca fascicularis, M. mulatta) | High | Yes | Yes | (OIE, 2020c) |
| African green monkey (Chlorocebus aethiops) | High | Clinical | Not reported | (Blair et al., 2021) |
| Egyptian fruit bat (Rousettus aegyptiacus) | High | No | Yes | (OIE, 2020c) |
| Big brown bat (Eptesicus fuscus) | Very low | Subclinical | Not reported | (Hall et al., 2020) |
| Tree Shrews (Tupaia belangeri chinensis) | Medium | Maybe, depending on age and sex | Yes | (Zhao et al., 2020) |
| Baboon (Papio hamadryas) | Very low | No | Not reported | (Singh et al., 2021) |
| New Zealand White rabbit (Oryctolagus cuniculus) | High | No | No | (OIE, 2020c) |
| Cottontail rabbit (Sylvilagus sp.) | Low | No | No | (Bosco‐Lauth et al., 2021) |
| White‐tailed deer (Odocoileus virginianus) | High | No | Yes | (OIE, 2020c) |
| Marmosets (Callithrix jacchus) | High | No | No | (OIE, 2020c) |
3.3.1. Susceptibility of SARS‐CoV‐2 in domestic animals
Domestic dogs: Dogs were found to be less susceptible to SARS‐CoV‐2 during experimental inoculation (Hobbs & Reid, 2020). The virus can accumulate in the kidney and the heart of dogs whereas in humans the predilection site is the lungs (Zhai et al., 2020). Simultaneous activities of ACE‐2 and TMPRSS2 were not found in dogs’ lungs (Chen et al., 2020). Moreover, the required amino acids for the ACE‐2 receptor cannot be found in canids (Mathavarajah & Dellaire, 2020). There is no evidence of SARS‐CoV‐2 detection from an oro‐pharyngeal sample from the dog. However, the rectal swabs tested positive for viral RNA in experimental infection (Hobbs & Reid, 2020). However, the two dogs were reported to be SARS‐CoV‐2 positive at a minute degree, which can be described as a low infection with a minimum likelihood of virus transmission (Leroy et al., 2020). After 4 days of intranasal inoculation of a Wuhan‐like SARS‐CoV‐2 in a dog breed beagle, the virus was found only in rectal swabs but not in other organs. The viable virus could not be found in inoculated dogs. Dogs shared housing with inoculated ones had no antibody or virus suggesting SARS‐CoV‐2's lower affinity for dogs (Shi et al., 2020). Canids may play a role of the intermediate or dead‐end host but not a reservoir of SARS‐CoV‐2 and they have very low ability for virus shedding and transmission.
Domestic cats: Experimental studies mainly used cats aged between 3 and 18 months (Bosco‐Lauth et al., 2021; Shi et al., 2020). Experimental infections in cats resulted in viral loads in the respiratory tract and small intestine. The airborne transmission was also documented in cats (Halfmann et al., 2020; Shi et al., 2020). Though the virus was isolated and antibody was found in cats after experimental inoculation, it did not show any signs. But recently a study on sub‐adult cats aged between 8 and 18 months reported signs like arched back, weight loss and diarrhoea after experimental inoculation (Bao et al., 2021). So, cats, especially the juvenile ones, are more susceptible to SARS‐CoV‐2 than dogs. Even the cats can transmit the virus to other cats via direct (Bosco‐Lauth et al., 2020) and indirect contact (Shi et al., 2020). Transmission via indirect contact like aerosols was not that effective like direct contact (Shi et al., 2020). Moreover, serial passaging of SARS‐CoV‐2 in cats resulted in viral attenuation (Bao et al., 2021), which mechanism is not clear yet. Interestingly, cats recovered from SARS‐CoV‐2 have protective immunity from being re‐infected via experimental inoculation (Bosco‐Lauth et al., 2020).
Pigs: The intra‐nasal inoculation of SARS‐CoV‐2 in domestic pigs (Sus scrofa domesticus) did not produce any antibody or reveal any virus (Schlottau et al., 2020; Shi et al., 2020). Even the in‐house contact pigs had no virus or antibody (Shi et al., 2020). It suggests pigs are not susceptible to SARS‐CoV‐2. But pigs’ ACE‐2 receptor may favor the binding of SARS‐CoV‐2 (Qiu et al., 2020).
Birds: Avian species are not susceptible to SARS‐CoV‐2. During the experiment, the Wuhan strain and the German strain of SARS‐CoV‐2 showed neither RNA nor antibodies from the sample even they did not show any clinical signs as well (Schlottau et al., 2020; Wu et al., 2019). Further studies showed neither RNA nor antibodies against the virus from the duck sample (Wu et al., 2019). However, to date, chicken, duck, turkey, quail and geese did not show any clinical signs or evidence of viral replication or antibodies during challenges with the SARS‐CoV‐2 viral strain (Suarez et al., 2020). But SARS‐CoV‐2 can use pigeon ACE‐2 receptors (Qiu et al., 2020).
Camel: Camels are less susceptible to SARS‐CoV‐2 (Wong et al., 2020). Experimental studies are still very few regarding camels susceptibility to the virus. (Gai et al., 2021) immunized camel with SARS‐CoV‐2 spike protein RBD and collected the antibodies used in humans to reduce the interaction with ACE‐2 receptors.
3.3.2. Susceptibility of SARS‐CoV‐2 in wild animals
Rodents: Free‐ranging rodents can catch the virus from humans and there is also the chance of reverse zoonosis of SARS‐CoV‐2 (Gryseels et al., 2020). It is also perhaps a concern that SARS‐CoV‐2 can adapt to wild rodents in natural environments and then again can transmit to humans (Konda et al., 2020).
Mice: Wild and transgenic mice were used for experimental purposes. Nevertheless, SARS‐CoV‐2 has a less binding capacity for murine ACE2 receptors (Lei et al., 2020; Letko et al., 2020; Wan et al., 2020). So, the researcher used transgenic mice with human ACE2 receptors rather than wild mice (Bao et al., 2020b). SARS‐CoV‐2 can exert a slight effect on hACE2 mice but not on WT‐HB‐01 mice. SARS‐CoV‐2 has a low affinity to mice without the hACE2 receptor. It was also observed that mice that were treated with human convalescent serum had fewer viral loads in their lungs (Boudewijns et al., 2020). Deer mice are susceptible to SARS‐CoV‐2 after experimental inoculation but do not show any signs of illness. They can also transmit the virus to un‐inoculated susceptible mice (Fagre et al., 2020). Sometimes, a serum sample from other rodent species gives negative ELISA test results for SARS‐CoV‐2 (Zhou et al., 2020). Most recently, two variants of concern (B.1.351 and P.1) of SARS‐CoV‐2 were able to infect laboratory mice with high titres in the lungs (Montagutelli et al., 2021). But there is also the possibility of wild rodents being the reservoirs of the virus (OIE, 2021).
House mouse (Mus musculus) is resistant to SARS‐CoV‐2 experimental infection (Bosco‐Lauth et al., 2021). Deer mice (Peromyscus maniculatus), commonly found in North America, are also susceptible to SARS‐CoV‐2 and showed subclinical infection in the experimental condition. They showed only weight loss, lesions in the brain and olfactory epithelium. Infectious viruses and RNA were isolated from the oral and rectal swab (Griffin et al., 2021), trachea, lungs, nasal turbinate, colon and small intestine. Viral RNA was also detected in the faeces and urine of mice. Infected mice can directly transmit the virus to naïve co‐housed mice. Future research should be directed to assess the spreading capability of SARS‐CoV‐2 to deer mice and deer mice to house mice and humans. Rodents are a model animal for SARS‐CoV‐2 vaccine development (Dagotto et al., 2020; Dinnon et al., 2020; Jiang et al., 2020; Tian et al., 2020).
Rats : Bushy tailed woodrats (packrats/Neotoma cinerea) are commonly found in the western United States and Canada. These rats are susceptible to SARS‐CoV‐2 when inoculated in lab conditions. Though no clinical signs were observed, they shed the virus orally. Some lesions were found in the lungs. The virus was detected in the nose, trachea and lungs.
Hamsters: Golden Syrian hamsters (Mesocricetus auratus) were inoculated nasally with a strain from Hong Kong. Though they developed different signs, no animal died (Chan et al., 2020b). They transmitted the virus to naive co‐housed hamsters (Sia et al., 2020). Passive immunity increased the body's defence and reduces viral loads in hamsters (Chan et al., 2020b). STAT2 protein in hamsters impeded the viral spread throughout the system, though lung pathology increased (Boudewijns et al., 2020). The same susceptibility pattern was observed in the case of Chinese hamsters (Cricetulus griseus), Campbell's dwarf hamster (Phodopus campbelli) and Djungarian hamster (Phodopus sungorus) (Bertzbach et al., 2021; Trimpert et al., 2020). Chinese dwarf hamster is very susceptible to SARS‐CoV‐2 and lose weight after experimental infection (Bertzbach et al., 2020). Viral RNA and infectious viruses were found in the nose oropharynx and trachea of the Chinese hamster (Bertzbach et al., 2020). Campbell's dwarf hamster and Djungarian hamster both are susceptible to SARS‐CoV‐2, but they suffer from mild infection unlike the Chinese hamster and golden Syrian hamster (Trimpert et al., 2020). Infectious viruses and RNA were found in their lungs and oral swabs, respectively. However, Roborovski dwarf hamsters (Phodopus roborovskii) suffered from acute clinical signs like human COVID‐19 (Trimpert et al., 2020). They developed clinical signs like reduced body temperature, weight loss, dyspnea, depression etc. Viral RNA was detected in oral swabs and blood.
Mustelids: Environmental samples from the Wuhan seafood market and samples from a COVID‐19 patient were tested for virulence by Shi et al. (2020). Intranasal inoculation of the virus in ferrets resulted in viral excretion in the upper respiratory tract but no virus was detected in the trachea, lungs, heart, liver, spleen, kidney, pancreas, small intestine and brain. Rectal swabs had no detectable viral RNA. Fever and appetite loss were observed in only two ferrets on the 10th and 12th days of post‐infection. Antibodies against SARS‐CoV‐2 were detected in ELISA and serum neutralization tests (SNT). The same researchers detected viral RNA in the upper respiratory tract and trachea 8 days after intra‐nasal post‐infection in ferrets (Shi et al., 2020). Kim et al. (2020) inoculated a Korean strain of SARS‐CoV‐2 intra‐nasally in ferrets. Signs included high body temperature, decreased physical activity and sometimes coughing. Virus RNA is detected in serums, nostril washes, saliva, urine, faeces, trachea, lungs, intestines and kidneys. Other ferrets, housed together with these experimental animals, had antibodies that suggested transmission of the virus by direct contact and by indirect contact via air (Kim et al., 2020).
Intranasal inoculation of a German strain of the virus was done in ferrets and then the ferrets were placed with naive ferrets in the same house. It was done to determine whether there is airborne transmission or not. After 19 days of inoculation, viral RNA was detected in challenged animals. Whereas naïve ferrets shed the virus after 17 days of exposure. The naïve ferrets then transmitted the virus to other ferrets via indirect contact. Viral RNA was found 13–19 days after airborne transmission. Nasal swabs had more viral concentration than that of the throat and rectal swabs, but viable viruses were found from nasal and throat swabs but not from rectal swabs. Interestingly, whatever the route of transmission was (experimentally infected, direct or indirect contact), all the ferrets had the same antibody level at days of post‐infection (Richard et al., 2020). Moreover, another experiment showed that a German strain of SARS‐CoV‐2 can infect other ferrets housed together with experimental ferrets but showed no signs of disease (Beer, 2020; Schlottau et al., 2020).
Raccoon: Moderate susceptibility of raccoon (Nyctereutes procyonoides) was found in experimental infection by 105 median tissue culture infectious dose (TCID50) intra‐nasally. No clinical signs were observed but a viable virus was found in the raccoon. Antibody was detected also. The virus transmitted to contact animals had a higher viral load in the nose and throat than the rectum (Freuling et al., 2020). The ACE‐2 receptors of raccoon and domestic dogs are similar (Zhai et al., 2020). On the other hand, striped skunks (Mephitis mephitis) are susceptible to SARS‐CoV‐2 (Bosco‐Lauth et al., 2021).
Reptiles: Turtles’ and snakes’ ACE‐2 receptors interact with S protein RBD, thus making them potential intermediate hosts of SARS‐CoV‐2 (Liu et al., 2020b). But later many researchers proved it wrong. Similarly, the many‐branded krait (Bungarus multicinctus) and the Chinese cobra (Naja atra) use the same protein patterns as humans. That is why some hypothesized that these two snake species can act as wildlife reservoirs of SARS‐CoV‐2 (Ji et al., 2020). But this study could not be replicated and proved to be not right due to the small number of protein sequences, vertebrate diversity and outdated codon usage (Gong & Bao, 2018; Luan et al., 2020; Zhang et al., 2020a). Moreover Luan et al. (2020) snakes’ ACE2 cannot interact with the S protein of SARS‐CoV‐2 anymore. So, the snake cannot act as an intermediate host of SARS‐CoV‐2.
Non‐human primates: Experimental studies have been done in rhesus macaques (Macaca mulatta) (Bao et al., 2020a; Hedman et al., 2021; Munster et al., 2020), cynomolgus macaques (Macaca fascicularis) (Hedman et al., 2021), New World monkey (Callithrix jacchus) (Maurin et al., 2021) and green monkeys (Chlorocebus sabaeus) (Woolsey et al., 2021). Rhesus macaques had a recurrent fever and there were no respiratory symptoms after inoculation of SARS‐CoV‐2 experimentally through oral, nasal, ocular and tracheal routes (Maurin et al., 2021; Munster et al., 2020). Some degrees of loss of weight were observed with altered respiratory patterns, piloerection, anorexia, abdominal pain, dehydration and paleness. Recovery after viral inoculation was observed between 9 and 17 days. The virus was identified from several organs of the respiratory and gastrointestinal tract. Viral antibodies persisted until the 10th day of inoculation (Munster et al., 2020). Shan et al. (2020) inoculated SARS‐CoV‐2 in the trachea of rhesus macaques. But only one macaque showed recurrent anorexia while others showed no signs. Moreover, the virus could not be detected in blood though a high level was found in oropharyngeal swabs. Another study also inoculated SARS‐CoV‐2 intratracheally in rhesus macaques. Subsequent signs included loss of body weight, transient anorexia and hunched posture (Bao et al., 2020a). The virus was detected in several organs including the nose, lungs, GI tract, heart, bladder etc. Antibodies against SARS‐CoV‐2 were identified in 14, 21 and 28 days of post‐infection. When the macaques were re‐infected with SARS‐CoV‐2 after 28 days of post‐infection, no viral RNA or antibody was found in them. This indicated that the macaques were protected against re‐infection (Bao et al., 2020a). If macaques are vaccinated with adenovirus serotype 26 (Ad26) vector vaccine, they can be protected against SARS‐CoV‐2 (Mercado et al., 2020).
A comparison conducted by Lu et al. (2020b) found similar findings as others, but there were higher viral titres in Macaca sp. compared to C. jacchus. Moreover, no virus was detected in the pulmonary tissues of C. jacchus, there were no macroscopic lesions observed in the lung tissues and finally animal did not develop a specific antibody response to SARS CoV‐2. In summary, none of the non‐human primates developed critical diseases but Macaca sp. was more susceptible to SARS CoV‐2 infection than C. jacchus.
Bats: Experimental intra‐nasal inoculation of a German strain in Rousettus fruit bats, which are considered a putative reservoir of virus, resulted in oral viral shedding up to 12 days of inoculation but there were no symptoms (Schlottau et al., 2020). The virus is detected in the respiratory, heart, skin and intestinal tissue. Inoculated and contact bats were antibodies against SARS‐CoV‐2. Bat to bat transmission was observed between inoculated bats and contact bats (Schlottau et al., 2020). Experimental infection in Egyptian fruit bats (R. aegyptiacus) results in no clinical signs. However, viral RNA was detected in the oral cavity, trachea, lungs, lymph nodes, heart, skin, duodenum and adrenal gland tissues, whereas infectious viruses were found in the nose and trachea. The virus was transmitted to other contact animals (Schlottau et al., 2020).
Tree shrews : Chinese Tree shrews (Tupaia belangeri chinensis) were experimentally infected via the intranasal route. Most females had elevated body temperature but no other signs. The virus was detected in younger animals especially, until 12 days of inoculation (Zhao et al., 2020). But tree shrews can act as intermediate hosts or asymptomatic carriers of SARS‐CoV‐2 (Zhao et al., 2020).
Lagomorphs (rabbit): Damas et al. (2020) reported that the SARS‐CoV‐2 can bind with lagomorphs’ ACE‐2 receptor. New Zealand white rabbits (Oryctolagus cuniculus) shed the virus after experimental infection (Mykytyn et al., 2021). Rabbits are less infected by SARS‐CoV‐2 than hares and ferrets (Mykytyn et al., 2021). Farmed rabbits can infect with viruses, like mink (Enserink, 2020), and can infect humans (Yekta et al., 2020). Another species, the cottontail rabbit (Sylvilagus sp.) is not susceptible to intranasal inoculation of SARS‐CoV‐2. This rabbit did not shed any virus after the experimental infection. However, the underlying reason for the low susceptibility of cottontail rabbits to the virus may be due to the use of a low dose of the virus particle (Bosco‐Lauth et al., 2021). For this reason, advanced studies should be done to explore the adaptability of SARS‐CoV‐2 in the rabbit population and its infectiousness in humans.
Cervids (deer): White‐tailed deer (WTD) (Odocoileus virginianus) has been identified as highly susceptible to infection with SARS‐CoV‐2 due to the high degree of resemblance of ACE2 receptors of white‐tailed deer with human ACE2 (Palmer et al., 2021). Furthermore, infected animals were able to shed the SARS‐CoV‐2 virus through nasal secretions and faeces. Notably, indirect contact WTD were infected and shed from the virus, signifying effective SARS‐CoV‐2 transmission from experimental animals. As a result, the spillover of SARS‐CoV‐2 from humans to cervids might happen at captive deer farms and zoos. For example, farmed deer can mix with wild deer and other species where biosecurity measures are insufficient; this might stipulate possibilities to transmit the virus in feral wild animals.
3.4. Sustainable One Health surveillance for prevention and control of future epidemics and pandemics like Disease X
The word ‘One Health’ was flourished based on the popular One Medicine concept by Calvin Schwabe after 1970. The theme of the concept was that human, animal and environmental health all are interconnected (Saylors et al., 2021). The three organizations – Food and Agricultural Organization (FAO), OIE and the World Health Organization (WHO) – emphasize preventing diseases directly or indirectly arising from animals at the human–animal interface. One ninety‐three countries endorsed the 2015–2030 Sustainable Development Goals which admitted the importance of a united approach to society and the environment. From a health care standpoint, they highlighted the importance of a One Health approach that embraces human, animal and environmental health and realizes their serious interdependence. The World Bank referred to an integrated surveillance‐response for zoonoses control back in 2012 (Anon, 2012). The World Bank also suggested including the environment, wildlife and water reservoirs as they can be the source of zoonotic pathogens (Zinsstag et al., 2020). The US Agency for International Development (USAID) funded several projects to uplift the One Health capacities to prevent and control zoonotic diseases in human–animal interfaces. They also identified that hunting wildlife and sharing food and resources predisposes the risk of viral spillover (Saylors et al., 2021).
The contemporary pandemics (like SARS, MERS, 2009 H1N1 influenza and COVID‐19) remind the inter‐relatedness of our molecular universe. This recaps the necessity for us all to make and respond more efficiently to infection spillover from animals to humans as well as the challenges posed by the degradation of the natural environment (Figure 5). Ecosystem factors should be considered which promote infectious disease transmission. The economic benefits of this approach sufficiently warrant the costs involved (Dobso et al., 2020). The earlier the pathogens can be detected in the environment, wildlife or domestic animals, the more efficiently the One Health surveillance can be integrated. However, Figure 5 represents the overview of a planetary One Health perspective of the emerging infectious disease transmission dynamics using SARS‐CoV‐2 as a case study to visualize the importance of One Health surveillance to mitigate the risk of outbreak prevention (Zinsstag et al., 2020). The advent of the COVID‐19 pandemic is a One Health challenge that pleads for collective attempts from various fields and experts. But a roadmap must be executed first, by placing a One Health approach in place. Though it is unknown when the next threat will arrive, or what it will be, health systems and decision‐makers can grab this COVID‐19 pandemic as a chance to focus the multifaceted contacts among humans, animals, plants and the environment and to reassemble our health systems to be better prepared for the next complex One Health confront that we will unavoidably tackle.
FIGURE 5.
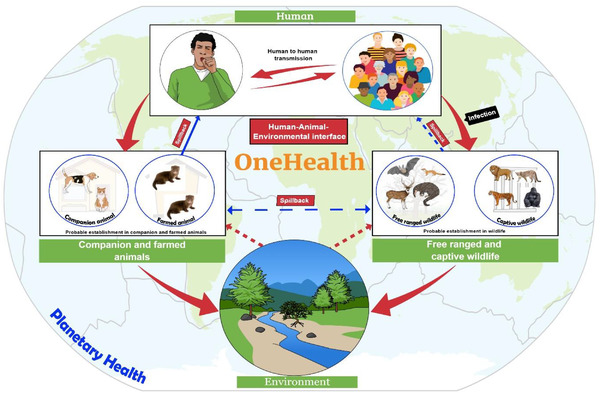
One health perspective of infectious disease transmission and susceptibility dynamics: SARS‐CoV‐2 as a case study
SARS‐CoV‐2 is a hazard for free‐ranging wild and aquatic animals. Climate change along with pollution of water by sewage and other contaminants helps the virus to spread (Islam et al., 2021c). The sharp decline in the health of our planet also increases the chance of generating more mutant viruses and adapting to a new host. Thus, the food production system has also been affected. As every component relates to each other, planetary health is at risk due to the global burden of the disease. Therefore, a risk assessment should be conducted in a qualitative way (very low to high) to identify the risk of release, exposure and infection by SARS‐CoV‐2. The risk will be determined by combining the ‘likelihood assessment scale’ and ‘consequence assessment scale’ (Logeot et al., 2021). Moreover, biosecurity measures should be taken to reduce the risk of human–animal contact and to prevent exposure of free‐ranging wild animals to the virus. A guideline has been published in this regard (OIE, 2020b). The emergence of some deadly viruses including Ebola, Zika, Dengue, Nipah encephalitis, Bird flu, Swine flu, MERS‐CoV and current pandemic SARS‐CoV‐2 (Dhama et al., 2018; Dhama et al., 2012; Munjal et al., 2017; Singh et al., 2019) has taught us the lesson for practicing strict biosecurity measures to prevent introduction or reemergence of the virus into the population which has already proved effective for preventing transboundary epizootics.
Functional wildlife health surveillance programs are absent in most of the countries of the world. Wildlife and the environment are not getting priority in health security plans despite their importance for pandemic prevention. To reduce known and novel disease risks, wildlife health capacity and operations should be strengthened in the One Health approach (Machalaba et al., 2021). OIE expert groups have given a statement on wildlife trade and emerging zoonotic diseases also (OIE, 2020a). The virus can transmit from humans to domestic animals and subsequently to wildlife. Some wild animal species like bats, primates and rodents live in groups. It facilitates the spreading of viruses from a single animal to a large group of animals. This spread increases the risk of the establishment of the virus in wildlife and the emergence of viral strains capable of transmitting back to humans or domestic animals through reverse zoonoses. Though several species of animals have been infected to date, we still do not know exactly which animal species will be adapted to the virus. Knowledge of animal reservoirs and the transmission cycle will mitigate the gaps between animal and human spread in the future.
Moreover, veterinary laboratories all over the world were well equipped, which helped to support the public health response for COVID‐19 during the pandemic (OIE, 2021). This resource should be used in the future also for surveillance of both humans and animals worldwide. We should also eliminate the possibility of SARS‐CoV‐2 establishment in novel animal hosts to reduce the animal to human transmission (Olival et al., 2020; Sun et al., 2020). Continuous sero‐ and genomic surveillance will guarantee effective vaccine production. We need to think about vaccines also for pet animals as they can be an asymptomatic carrier of the virus. A candidate vaccine (LinearDNATM) against COVID‐19 is under trial in domestic felines after receiving approval from the United States Department of Agriculture (Brook, 2020; Sharun et al., 2021). In addition, another candidate vaccine for dogs and cats has been found to be effective in a preliminary trial, which is also used experimentally in captive bonobos and orangutans at a Zoo in the United States (Daly, 2021). However, vaccination of pets and farmed animals is complex. Vaccination in companion animals will be feasible only when they are in contact with the immunocompromised human. But it is very difficult for farmed animals. We saw that minks were infected by humans and it can be prevented by strict biosecurity and immunization of workers in those farms. Vaccination of mink can only be then complementing the biosecurity measures.
In addition, rapid diagnosis, observance, isolation and quarantine measures are needed to formulate for the future prevention and control of pandemic potential disease spread. Moreover, intensive medical care facilities, public health awareness buildup programs, networking, rapid communication and international collaboration are needed to develop against any pandemic potential emerging virus to prevent haunting the lives of billions of human populations (Bonilla‐Aldana et al., 2020; Malik et al., 2020; Rodriguez‐Morales et al., 2020). Identifying behavioural risk factors in human–animal interfaces is another crucial portion of One Health research. Proper intervention should be implemented in these interfaces to reduce the risk factors and preventing spillover (Saylors et al., 2021) from animals to humans.
The WHO Research and Development Blueprint publishes an annual list of priority diseases to guide governmental concentration in research and development on conditions that pose significant public health threats (Mehand et al., 2018). ‘Disease X’, a surrogate name serving as a reminder that the most critical disease risk is likely one as yet unknown, capped the 2018 list (Mehand et al., 2018). The current Disease X is COVID‐19, but there will certainly be more. The pandemic is not under full control yet, but we need to think about future mitigation strategies from now on. We will get over the COVID‐19 pandemic eventually. Nevertheless, we should not wait until the next pandemic before applying the One Health approach to secure a healthier future for our nation and the world.
4. CONCLUSION
SARS‐CoV‐2 is assumed to emerge from an animal source and later spill over to humans. Even though SARS‐CoV‐2‐related viruses have been detected in Rhinolophus bats and pangolin, the exact source and route of introduction into the human population have not been established yet. Multiple domestic and wild animal species have been infected naturally by humans and some species demonstrated susceptibility in experimental settings. Moreover, alpha variant, B.1.1.7, has been detected in pet dog and cat after contacting with COVID‐19‐positive owner whereas beta variant, B.1.351, has been detected in rodents in the experimental condition. A wider host range of SARS‐CoV‐2 makes the path easier to select a species for experimental purposes. Several animal species can be used as pre‐clinical models in the laboratory, especially the non‐human primates and mice. But the resistance of most of the livestock species like cattle and poultry is a relaxing matter in terms of food safety issues. As a consequence of a natural infection in animals, humans are at risk of contracting mutated strains from animals as they can be an asymptomatic carriers of the virus. Reverse zoonoses have already been established from humans to mink to humans. The continuing spillover and spillback of SARS‐CoV‐2 in a wide range of animals in farming, captive and free‐ranging interfaces make inferences for human and animal health, welfare and conservation. In addition, authorities should take steps to make traditional animal markets safer to reduce public health risks. But capturing wild mammals for food or breeding should be banned also. Moreover, we recommend a combined One Health surveillance in both humans and animals to prevent future epidemics and pandemics. Vaccination of pets, farmed and captive wild animals in accordance with vaccination in humans should be done.
ETHICAL APPROVAL
No ethical approval was needed.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Supporting Information
ACKNOWLEDGEMENTS
We acknowledge all researchers for submitting articles regarding SARS‐CoV‐2‐related viruses in the animal that was used for writing this manuscript. The authors are thankful to the Institute of Epidemiology, Disease Control and Research (IEDCR), EcoHealth Alliance, NY, USA, and Chattogram Veterinary and Animal Sciences University (CVASU) for their continued support to our research team. The authors did not receive any external funds to conduct this research. However, the research team was partially supported by NIH, National Institute of Allergy and Infectious Diseases (NIAID) Award U01AI153420 (PI Jonathan H. Epstein) through EcoHealth Alliance.
Islam, A. , Ferdous, J. , Islam, S. , Sayeed, M. A. , Rahman, M. K. , Saha, O. , Hassan, M. M. , & Shirin, T. (2021). Transmission dynamics and susceptibility patterns of SARS‐CoV‐2 in domestic, farmed and wild animals: Sustainable One Health surveillance for conservation and public health to prevent future epidemics and pandemics. Transboundary and Emerging Diseases, 1–21. 10.1111/tbed.14356
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/.
REFERENCES
- Anon . (2012). People, pathogens and our planet: The economics of one health Retrieved from https://openknowledge.worldbank.org/handle/10986/11892 [Accessed June 3, 2021]. World Bank.
- Anon . (2018). Fur farming bans. Retrieved from https://www.furfreealliance.com/fur‐bans/ [Accessed June 3, 2021].
- Anthony, S. J. , Epstein, J. H. , Murray, K. A. , Navarrete‐Macias, I. , Zambrana‐Torrelio, C. M. , Solovyov, A. , Ojeda‐Flores, R. , Arrigo, N. C. , Islam, A. , & Ali Khan, S. (2013). A strategy to estimate unknown viral diversity in mammals. MBio, 4(5), e00598‐00513. 10.1128/mBio.00598-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour, H. M. , Elkhatib, W. F. , Rahman, M. , & Elshabrawy, H. A. (2020). Insights into the recent 2019 novel coronavirus (SARS‐CoV‐2) in light of past human coronavirus outbreaks. Pathogens, 9(3), 186. 10.3390/pathogens9030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, L. , Deng, W. , Gao, H. , Xiao, C. , Liu, J. , Xue, J. , Lv, Q. , Liu, J. , Yu, P. , & Xu, Y. (2020a). Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. bioRxiv, 1–21. 10.1101/2020.03.13.990226 [DOI] [Google Scholar]
- Bao, L. , Deng, W. , Huang, B. , Gao, H. , Liu, J. , Ren, L. , Wei, Q. , Yu, P. , Xu, Y. , Qi, F. , Qu, Y. , Li, F. , Lv, Q. , Wang, W. , Xue, J. , Gong, S. , Liu, M. , Wang, G. , Wang, S. , … Qin, C. (2020b). The pathogenicity of SARS‐CoV‐2 in hACE2 transgenic mice. Nature, 583(7818), 830–833. 10.1038/s41586-020-2312-y [DOI] [PubMed] [Google Scholar]
- Bao, L. , Song, Z. , Xue, J. , Gao, H. , Liu, J. , Wang, J. , Guo, Q. , Zhao, B. , Qu, Y. , & Qi, F. (2021). Susceptibility and Attenuated Transmissibility of SARS‐CoV‐2 in Domestic Cats. Journal of Infectious Diseases, 223(8), 1313–1321. 10.1093/infdis/jiab104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs, V. R. , Peiris, M. , Tam, K. W. , Law, P. Y. , Brackman, C. J. , To, E. M. , Yu, V. Y. , Chu, D. K. , Perera, R. A. , & Sit, T. H. (2020). SARS‐CoV‐2 in Quarantined domestic cats from COVID‐19 households or close contacts, Hong Kong, China. Emerging Infectious Diseases, 26(12), 3071–3074. 10.3201/eid2612.202786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer, M. (2020). COVID‐19: Experimental infection of fruit bats, ferrets, pigs and chicken with SARS‐CoV‐2 at Friedrich‐Loeffler‐Institut. ProMed‐Mail. Archive, (20200407.7196506). [Google Scholar]
- Bertzbach, L. D. , Vladimirova, D. , Dietert, K. , Abdelgawad, A. , Gruber, A. D. , Osterrieder, N. , & Trimpert, J. (2020). SARS‐CoV‐2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID‐19 pneumonia in a well‐established small animal model. Transboundary and Emerging Diseases, 68(3), 1075–1079. 10.1111/tbed.13837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertzbach, L. D. , Vladimirova, D. , Dietert, K. , Abdelgawad, A. , Gruber, A. D. , Osterrieder, N. , & Trimpert, J. (2021). SARS‐CoV‐2 infection of Chinese hamsters (Cricetulus griseus) reproduces COVID‐19 pneumonia in a well‐established small animal model. Transboundary Emerging Diseases, 68(3), 1075–1079. 10.1111/tbed.13837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair, R. V. , Vaccari, M. , Doyle‐Meyers, L. A. , Roy, C. J. , Russell‐Lodrigue, K. , Fahlberg, M. , Monjure, C. J. , Beddingfield, B. , Plante, K. S. , & Plante, J. A. (2021). Acute respiratory distress in aged, SARS‐CoV‐2–infected African green monkeys but not rhesus macaques. The American Journal of Pathology, 191(2), 274–282. 10.1016/j.ajpath.2020.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklund, A. , Gortázar, C. , Pasquali, P. , Roberts, H. , Nielsen, S. S. , Stahl, K. , Stegeman, A. , Baldinelli, F. , & Broglia, A. (2021). Monitoring of SARS‐CoV‐2 infection in mustelids. EFSA Journal, 19(3), e06459. 10.2903/j.efsa.2021.6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla‐Aldana, D. K. , Dhama, K. , & Rodriguez‐Morales, A. J. (2020). Revisiting the one health approach in the context of COVID‐19: A look into the ecology of this emerging disease. Advances in Animal and Veterinary Sciences, 8(3), 234–237. [Google Scholar]
- Bosco‐Lauth, A. M. , Hartwig, A. E. , Porter, S. M. , Gordy, P. W. , Nehring, M. , Byas, A. D. , VandeWoude, S. , Ragan, I. K. , Maison, R. M. , & Bowen, R. A. (2020). Experimental infection of domestic dogs and cats with SARS‐CoV‐2: Pathogenesis, transmission, and response to reexposure in cats. Proceedings of the National Academy of Sciences, 117(42), 26382–26388. 10.1073/pnas.2013102117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco‐Lauth, A. M. , Root, J. J. , Porter, S. M. , Walker, A. E. , Guilbert, L. , Hawvermale, D. , Pepper, A. , Maison, R. M. , Hartwig, A. E. , & Gordy, P. (2021). Peridomestic mammal susceptibility to severe acute respiratory syndrome coronavirus 2 infection. Emerging Infectious Diseases, 27(8), 2073. 10.3201/eid2708.210180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudewijns, R. , Thibaut, H. , Kaptein, S. , Li, R. , Vergote, V. , Seldeslachts, J. , De Keyzer, C. , Sharma, S. , Jansen, S. , & Weyenbergh, J. V. (2020). STAT2 signaling as double‐edged sword restricting viral dissemination but driving severe pneumonia in SARS‐CoV‐2 infected hamsters. bioRxiv , 2020.2004.2023.056838., 23, 1–23. 10.1101/2020.04.23.056838 [DOI] [Google Scholar]
- Brook, C. E. , & Dobson, A. P. (2015). Bats as ‘special’ reservoirs for emerging zoonotic pathogens. Trends in Microbiology, 23(3), 172–180. 10.1016/j.tim.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook, S. (2020). Applied DNA, EvviVax, and GVS receive regulatory approval to conduct veterinary clinical trial for linear COVID‐19 vaccine candidate. Retrived from https://www.businesswire.com/news/home/20201130005340/en/Applied‐DNA‐EvviVax‐andGVS‐Receive‐Regulatory‐Approval‐to‐Conduct‐Veterinary‐Clinical‐Trial‐for‐Linear‐COVID‐19‐Vaccine‐Candidate [Accessed March 28, 2021].
- Bui, C. H. T. , Yeung, H. W. , Ho, J. C. W. , Leung, C. Y. H. , Hui, K. P. Y. , Perera, R. , Webby, R. J. , Schultz‐Cherry, S. L. , Nicholls, J. M. , Peiris, J. S. M. , & Chan, M. C. W. (2021). Tropism of SARS‐CoV‐2, SARS‐CoV and influenza virus in canine tissue explants. Journal of Infectious Diseases, 224(5), 821–830. 10.1093/infdis/jiab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliani, R. , Forni, D. , Clerici, M. , & Sironi, M. (2020). Computational inference of selection underlying the evolution of the novel coronavirus, severe acute respiratory syndrome coronavirus 2. Journal of Virology, 94(12), e00411–00420. 10.1128/JVI.00411-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. F.‐W. , Yuan, S. , Kok, K.‐H. , To, K. K.‐W. , Chu, H. , Yang, J. , Xing, F. , Liu, J. , Yip, C. C.‐Y. , & Poon, R. W.‐S. (2020a). A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: A study of a family cluster. The Lancet, 395(10223), 514–523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. F.‐W. , Zhang, A. J. , Yuan, S. , Poon, V. K.‐M. , Chan, C. C.‐S. , Lee, A. C.‐Y. , Chan, W.‐M. , Fan, Z. , Tsoi, H.‐W. , Wen, L. , Liang, R. , Cao, J. , Chen, Y. , Tang, K. , Luo, C. , Cai, J.‐P. , Kok, K.‐H. , Chu, H. , Chan, K.‐H. , … Yuen, K.‐Y. (2020b). Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID‐19) in a golden Syrian hamster model: Implications for disease pathogenesis and transmissibility. Clinical Infectious Diseases, 71(9), 2428–2446. 10.1093/cid/ciaa325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler, J. C. , Bevins, S. N. , Ellis, J. W. , Linder, T. J. , Tell, R. M. , Jenkins‐Moore, M. , Root, J. J. , Lenoch, J. B. , Robbe‐Austerman, S. , & DeLiberto, T. J. (2021). SARS‐CoV‐2 exposure in wild white‐tailed deer (Odocoileus virginianus). bioRxiv, 2021.07.29.454326; 10.1101/2021.07.29.454326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Liu, B. , Yang, J. , & Jin, Q. (2014). DBatVir: The database of bat‐associated viruses. Database, 2014, bau21. 10.1093/database/bau021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D. , Sun, J. , Zhu, J. , Ding, X. , Lan, T. , Zhu, L. , Xiang, R. , Ding, P. , Wang, H. , & Wang, X. (2020). Single‐cell screening of SARS‐CoV‐2 target cells in pets, livestock, poultry and wildlife. bioRxiv, 1–15. 10.1101/2020.06.13.149690 [DOI] [Google Scholar]
- Cleary, S. J. , Pitchford, S. C. , Amison, R. T. , Carrington, R. , Robaina Cabrera, C. L. , Magnen, M. , Looney, M. R. , Gray, E. , & Page, C. P. (2020). Animal models of mechanisms of SARS‐CoV‐2 infection and COVID‐19 pathology. British Journal of Pharmacology, 177(21), 4851–4865. 10.1111/bph.15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert, J. (1973). The origin and distribution of feral mink in Scotland. Mammal Review, 3(3), 97–103. 10.1111/j.1365-2907.1973.tb00176.x [DOI] [Google Scholar]
- Dagotto, G. , Yu, J. , & Barouch, D. H. (2020). Approaches and challenges in SARS‐CoV‐2 vaccine development. Cell Host & Microbe, 28(3), 364–370. 10.1016/j.chom.2020.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, N. (2021). First great apes at U.S. zoo receive COVID‐19 vaccine made for animals. Retrived from https://www.nationalgeographic.com/animals/article/first‐great‐apes‐at‐uszoo‐receive‐coronavirus‐vaccine‐made‐for‐animals [Accessed March 27, 2021].
- Damas, J. , Hughes, G. M. , Keough, K. C. , Painter, C. A. , Persky, N. S. , Corbo, M. , Hiller, M. , Koepfli, K.‐P. , Pfenning, A. R. , & Zhao, H. (2020). Broad host range of SARS‐CoV‐2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proceedings of the National Academy of Sciences, 117(36), 22311–22322. 10.1073/pnas.2010146117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delong, J. (2020). Dutch Minister confirms dog, three cats have caught novel coronavirus. Retrived from https://www.reporter.am/dutch‐minister‐confirms‐dog‐three‐cats‐have‐caught‐novel‐coronavirus/ [Accessed on June 21, 2021] American Reporter.
- Dhama, K. , Karthik, K. , Khandia, R. , Chakraborty, S. , Munjal, A. , Latheef, S. K. , Kumar, D. , Ramakrishnan, M. A. , Malik, Y. S. , & Singh, R. (2018). Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus. Frontiers in Immunology, 9, 1803. 10.3389/fimmu.2018.01803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama, K. , Verma, A. K. , Rajagunalan, S. , Deb, R. , Karthik, K. , Kapoor, S. , Tiwari, R. , Panwar, P. K. , & Chakraborty, S. (2012). Swine flu is back again: A review. Pakistan Journal of Biological Sciences, 15(21), 1001–1009. 10.3923/pjbs.2012.1001.1009 [DOI] [PubMed] [Google Scholar]
- Dinnon, K. H. , Leist, S. R. , Schäfer, A. , Edwards, C. E. , Martinez, D. R. , Montgomery, S. A. , West, A. , Yount, B. L. , Hou, Y. J. , & Adams, L. E. (2020). A mouse‐adapted model of SARS‐CoV‐2 to test COVID‐19 countermeasures. Nature, 586(7830), 560–566. 10.1038/s41586-020-2708-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobso, A. P. , Pim, S. L. , Hannah, L. , Kaufman, L. , Ahumad, J. A. , Bernstein, A. , Busch, J. , Daszak, P. , Engelmann, J. , & Kinnair, M. F. (2020). Ecology and economics for pandemic prevention: Investments to prevent tropical deforestation and to limit wildlife trade will protect against future zoonosis outbreaks. Science, 369(6502), 379–381. 10.1126/science.abc3189 [DOI] [PubMed] [Google Scholar]
- El‐Sayed, A. , & Kamel, M. (2021). Coronaviruses in humans and animals: The role of bats in viral evolution. Environmental Science and Pollution Research, 28, 1–12. 10.1007/s11356-021-12553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink, M. (2020). Coronavirus rips through Dutch mink farms, triggering culls. (Vol. 368, pp. 1169). American Association for the Advancement of Science. 10.1126/science.368.6496.1169 [DOI] [PubMed] [Google Scholar]
- Fagre, A. , Lewis, J. , Eckley, M. , Zhan, S. , Rocha, S. M. , Sexton, N. R. , Burke, B. , Geiss, B. , Peersen, O. , & Kading, R. (2020). SARS‐CoV‐2 infection, neuropathogenesis and transmission among deer mice: Implications for reverse zoonosis to New World rodents. bioRxiv, 1–15. 10.1101/2020.08.07.241810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Alanis, A. , Sandner‐Miranda, L. , Delgado, G. , Cravioto, A. , & Morales‐Espinosa, R. (2020). The receptor binding domain of SARS‐CoV‐2 spike protein is the result of an ancestral recombination between the bat‐CoV RaTG13 and the pangolin‐CoV MP789. BMC Research Notes, 13(1), 1–6. 10.1186/s13104-020-05242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freuling, C. M. , Breithaupt, A. , Müller, T. , Sehl, J. , Balkema‐Buschmann, A. , Rissmann, M. , Klein, A. , Wylezich, C. , Höper, D. , Wernike, K. , Aebischer, A. , Hoffmann, D. , Friedrichs, V. , Dorhoi, A. , Groschup, M. H. , Beer, M. , & Mettenleiter, T. C. (2020). Susceptibility of raccoon dogs for experimental SARS‐CoV‐2 infection. Emerging Infectious Diseases, 26(12), 2982–2985 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz, M. , Rosolen, B. , Krafft, E. , Becquart, P. , Elguero, E. , Vratskikh, O. , Denolly, S. , Boson, B. , Vanhomwegen, J. , Gouilh, M. A. , Kodjo, A. , Chirouze, C. , Rosolen, S. G. , Legros, V. , & Leroy, E. M. (2021). High prevalence of SARS‐CoV‐2 antibodies in pets from COVID‐19+ households. One Health, 11, 100192. 10.1016/j.onehlt.2020.100192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos, R. , Serra‐Cobo, J. , Chen, T. , & Devaux, C. A. (2020). COVID‐19: Time to exonerate the pangolin from the transmission of SARS‐CoV‐2 to humans. Infection, Genetics and Evolution, 84, 104493. 10.1016/j.meegid.2020.104493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai, J. , Ma, L. , Li, G. , Zhu, M. , Qiao, P. , Li, X. , Zhang, H. , Zhang, Y. , Chen, Y. , & Ji, W. (2021). A potent neutralizing nanobody against SARS‐CoV‐2 with inhaled delivery potential. MedComm, 2, 101–113. 10.1002/mco2.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigliany, M. , Van Laere, A.‐S. , Clercx, C. , Giet, D. , Escriou, N. , Huon, C. , Van Der Werf, S. , Eloit, M. , & Desmecht, D. (2020). SARS‐CoV‐2 natural transmission from human to cat, Belgium, March 2020. Emerging Infectious Diseases, 26(12), 3069. 10.3201/eid2612.202223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner, J. , Villanueva‐Saz, S. , Tobajas, A. P. , Pérez, M. D. , González, A. , Verde, M. , Yzuel, A. , García‐García, A. , Taleb, V. , & Lira‐Navarrete, E. (2021). SARS‐CoV‐2 seroprevalence in household domestic ferrets (Mustela putorius furo). Animals, 11(3), 667. 10.3390/ani11030667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, W. G. , Subbarao, K. , Murphy, B. , & Murphy, P. M. (2004). Mechanisms of host defense following severe acute respiratory syndrome‐coronavirus (SARS‐CoV) pulmonary infection of mice. The Journal of Immunology, 173(6), 4030–4039. 10.4049/jimmunol.173.6.4030 [DOI] [PubMed] [Google Scholar]
- Goh, G. K.‐M. , Dunker, A. K. , Foster, J. A. , & Uversky, V. N. (2020). Shell disorder analysis suggests that pangolins offered a window for a silent spread of an attenuated SARS‐CoV‐2 precursor among humans. Journal of Proteome Research, 19(11), 4543–4552. 10.1021/acs.jproteome.0c00460 [DOI] [PubMed] [Google Scholar]
- Gong, S. R. , & Bao, L. L. (2018). The battle against SARS and MERS coronaviruses: Reservoirs and animal models. Animal Models and Experimental Medicine, 1(2), 125–133. 10.1002/ame2.12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumenou, M. , Spandidos, D. A. , & Tsatsakis, A. (2020). Possibility of transmission through dogs being a contributing factor to the extreme Covid‑19 outbreak in North Italy. Molecular Medicine Reports, 21(6), 2293–2295. 10.3892/mmr.2020.11037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, B. D. , Chan, M. , Tailor, N. , Mendoza, E. J. , Leung, A. , Warner, B. M. , Duggan, A. T. , Moffat, E. , He, S. , & Garnett, L. (2021). SARS‐CoV‐2 infection and transmission in the North American deer mouse. Nature Communications, 12(1), 1–10. 10.1038/s41467-021-23848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryseels, S. , De Bruyn, L. , Gyselings, R. , Calvignac‐Spencer, S. , Leendertz, F. H. , & Leirs, H. (2020). Risk of human‐to‐wildlife transmission of SARS‐CoV‐2. Mammal Review, 51(2), 272–292. 10.1111/mam.12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, P. J. , Hatta, M. , Chiba, S. , Maemura, T. , Fan, S. , Takeda, M. , Kinoshita, N. , Hattori, S.‐i. , Sakai‐Tagawa, Y. , & Iwatsuki‐Horimoto, K. (2020). Transmission of SARS‐CoV‐2 in domestic cats. New England Journal of Medicine, 383(6), 592–594. 10.1056/NEJMc2013400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. S. , Knowles, S. , Nashold, S. W. , Ip, H. S. , Leon, A. E. , Rocke, T. , Keller, S. , Carossino, M. , Balasuriya, U. , & Hofmeister, E. (2020). Experimental challenge of a North American bat species, big brown bat (Eptesicus fuscus), with SARS‐CoV‐2. Transboundary Emerging Diseases, 1–10. Online ahead of print. 10.1111/tbed.13949 [DOI] [PubMed] [Google Scholar]
- Hamer, S. A. , Ghai, R. R. , Zecca, I. B. , Auckland, L. D. , Roundy, C. M. , Davila, E. , Busselman, R. E. , Tang, W. , Pauvolid‐Corrêa, A. , Killian, M. L. , Jenkins‐Moore, M. , Torchetti, M. K. , Robbe Austerman, S. , Lim, A. , Akpalu, Y. , Fischer, R. S. B. , Barton Behravesh, C. , & Hamer, G. L. (2021). SARS‐CoV‐2 B.1.1.7 variant of concern detected in a pet dog and cat after exposure to a person with COVID‐19, USA. Transboundary and Emerging Diseases. Online ahead of print. 10.1111/tbed.14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer, S. A. , Pauvolid‐Corrêa, A. , Zecca, I. B. , Davila, E. , Auckland, L. D. , Roundy, C. M. , Tang, W. , Torchetti, M. , Killian, M. L. , & Jenkins‐Moore, M. (2020). Natural SARS‐CoV‐2 infections, including virus isolation, among serially tested cats and dogs in households with confirmed human COVID‐19 cases in Texas, USA. bioRxiv, 1–15. 10.1101/2020.12.08.416339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman, H. D. , Krawczyk, E. , Helmy, Y. A. , Zhang, L. , & Varga, C. (2021). Host diversity and potential transmission pathways of SARS‐CoV‐2 at the human‐animal interface. Pathogens, 10(2), 180. 10.3390/pathogens10020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heighton, S. P. , & Gaubert, P. (2021). A timely systematic review on pangolin research, commercialization, and popularization to identify knowledge gaps and produce conservation guidelines. Biological Conservation, 256, 109042. 10.1016/j.biocon.2021.109042 [DOI] [Google Scholar]
- Hobbs, E. C. , & Reid, T. J. (2020). Animals and SARS‐CoV‐2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transboundary and Emerging Diseases, 68(4), 1850–1867. 10.1111/tbed.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, E. C. , & Reid, T. J. (2021). Animals and SARS‐CoV‐2: Species susceptibility and viral transmission in experimental and natural conditions, and the potential implications for community transmission. Transboundary and Emerging Diseases, 68, 1850–1867. 10.1111/tbed.13885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, M. , Kleine‐Weber, H. , Schroeder, S. , Krüger, N. , Herrler, T. , Erichsen, S. , Schiergens, T. S. , Herrler, G. , Wu, N.‐H. , & Nitsche, A. (2020). SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell, 181(2), 271–280. e278. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain, M. G. , Javed, A. , Akter, S. , & Saha, S. (2021). SARS‐CoV‐2 host diversity: An update of natural infections and experimental evidence. Journal of Microbiology, Immunology and Infection, 54(2), 175–181. 10.1016/j.jmii.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hul, V. , Delaune, D. , Karlsson, E. A. , Hassanin, A. , Tey, P. O. , Baidaliuk, A. , Gámbaro, F. , Tu, V. T. , Keatts, L. , & Mazet, J. (2021). A novel SARS‐CoV‐2 related coronavirus in bats from Cambodia. bioRxiv, 1–15. 10.1101/2021.01.26.428212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. , Ferdous, J. , Islam, S. , Sayeed, M. , Dutta Choudhury, S. , Saha, O. , Hassan, M. M. , & Shirin, T. (2021a). Evolutionary dynamics and epidemiology of endemic and emerging coronaviruses in humans, domestic animals, and wildlife. Viruses, 13(10), 1908. 10.3390/v13101908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. , Hossain, M. E. , Rostal, M. K. , Ferdous, J. , Islam, A. , Hasan, R. , Miah, M. , Rahman, M. , Rahman, M. Z. , & Daszak, P. (2020). Epidemiology and molecular characterization of rotavirus A in fruit bats in Bangladesh. EcoHealth, 17(3), 398–405. 10.1007/s10393-020-01488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. , Kalam, M. , Sayeed, M. , Shano, S. , Rahman, M. , Islam, S. , Ferdous, J. , Choudhury, S. D. , & Hassan, M. M. (2021b). Escalating SARS‐CoV‐2 circulation in environment and tracking waste management in South Asia. Environmental Science Pollution Research, 1–18. Online ahead of print. 10.1007/s11356-021-16396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. , Sayeed, M. , Kalam, M. , Ferdous, J. , Rahman, M. , Abedin, J. , Islam, S. , Shano, S. , Saha, O. , & Shirin, T. (2021c). Molecular epidemiology of SARS‐CoV‐2 in diverse environmental samples globally. Microorganisms, 9(8), 1696. 10.3390/microorganisms9081696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, A. , Sayeed, M. A. , Rahman, M. K. , Ferdous, J. , Islam, S. , & Hassan, M. M. (2021d). Geospatial dynamics of COVID‐19 clusters and hotspots in Bangladesh. Transboundary and Emerging Diseases, 1–15. Online ahead of print. 10.1111/tbed.13973 [DOI] [PubMed] [Google Scholar]
- Islam, A. , Sayeed, M. A. , Rahman, M. K. , Zamil, S. , Abedin, J. , Saha, O. , & Hassan, M. M. (2021e). Assessment of basic reproduction number (R0), spatial and temporal epidemiological determinants, and genetic characterization of SARS‐CoV‐2 in Bangladesh. Infection, Genetics Evolution, 92, 104884. 10.1016/j.meegid.2021.104884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN . (2021). Spatial Daa download: The IUCN Red List of Threatened Species. Retrieved from https://www.iucnredlist.org/resources/spatial‐data‐download [Accessed September 9, 2021].
- Jaimes, J. A. , André, N. M. , Chappie, J. S. , Millet, J. K. , & Whittaker, G. R. (2020). Phylogenetic analysis and structural modeling of SARS‐CoV‐2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. Journal of Molecular Biology, 432(10), 3309–3325. 10.1016/j.jmb.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, W. , Wang, W. , Zhao, X. , Zai, J. , & Li, X. (2020). Cross‐species transmission of the newly identified coronavirus 2019‐nCoV. Journal of Medical Virology, 92(4), 433–440. 10.1002/jmv.25682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, S. , Hillyer, C. , & Du, L. (2020). Neutralizing antibodies against SARS‐CoV‐2 and other human coronaviruses. Trends in Immunology, 41(5), 355–359. 10.1016/j.it.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, W. K. , de Oliveira‐Filho, E. F. , Rasche, A. , Greenwood, A. D. , Osterrieder, K. , & Drexler, J. F. (2020). Potential zoonotic sources of SARS‐CoV‐2 infections. Transboundary and Emerging Diseases, 68(4), 1824–1834. 10.1111/tbed.13872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , & Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30(4), 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.‐I. , Kim, S.‐G. , Kim, S.‐M. , Kim, E.‐H. , Park, S.‐J. , Yu, K.‐M. , Chang, J.‐H. , Kim, E. J. , Lee, S. , & Casel, M. A. B. (2020). Infection and rapid transmission of SARS‐CoV‐2 in ferrets. Cell Host & Microbe, 27(5), 704–709. e702. 10.1016/j.chom.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiros, M. , Andualem, H. , Kiros, T. , Hailemichael, W. , Getu, S. , Geteneh, A. , Alemu, D. , & Abegaz, W. E. (2020). COVID‐19 pandemic: Current knowledge about the role of pets and other animals in disease transmission. Virology Journal, 17(1), 1–8. 10.1186/s12985-020-01416-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konda, M. , Dodda, B. , Konala, V. M. , Naramala, S. , & Adapa, S. (2020). Potential zoonotic origins of SARS‐CoV‐2 and insights for preventing future pandemics through One Health approach. Cureus, 12(6). 10.7759/cureus.8932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, S. , Stecher, G. , & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. J Molecular Biology Evolution, 33(7), 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, H. M. , Ratmann, O. , & Boni, M. F. (2018). Improved algorithmic complexity for the 3SEQ recombination detection algorithm. Molecular Biology and Evolution, 35(1), 247–251. 10.1093/molbev/msx263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, T. T.‐Y. , Jia, N. , Zhang, Y.‐W. , Shum, M. H.‐H. , Jiang, J.‐F. , Zhu, H.‐C. , Tong, Y.‐G. , Shi, Y.‐X. , Ni, X.‐B. , & Liao, Y.‐S. (2020). Identifying SARS‐CoV‐2‐related coronaviruses in Malayan pangolins. Nature, 583(7815), 282–285. 10.1038/s41586-020-2169-0 [DOI] [PubMed] [Google Scholar]
- Lan, J. , Ge, J. , Yu, J. , Shan, S. , Zhou, H. , Fan, S. , Zhang, Q. , Shi, X. , Wang, Q. , & Zhang, L. (2020). Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature, 581(7807), 215–220. 10.1038/s41586-020-2180-5 [DOI] [PubMed] [Google Scholar]
- Lee, J. , Hughes, T. , Lee, M.‐H. , Field, H. , Rovie‐Ryan, J. J. , Sitam, F. T. , Sipangkui, S. , Nathan, S. K. S. S. , Ramirez, D. , Kumar, S. V. , Lasimbang, H. , Epstein, J. H. , & Daszak, P. (2020). No evidence of coronaviruses or other potentially zoonotic viruses in Sunda pangolins (Manis javanica) entering the wildlife trade via Malaysia. EcoHealth, 17(3), 406–418. 10.1007/s10393-020-01503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, C. , Qian, K. , Li, T. , Zhang, S. , Fu, W. , Ding, M. , & Hu, S. (2020). Neutralization of SARS‐CoV‐2 spike pseudotyped virus by recombinant ACE2‐Ig. Nature Communications, 11(1), 1–5. 10.1038/s41467-020-16048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy, E. M. , Ar Gouilh, M. , & Brugere‐Picoux, J. (2020). The risk of SARS‐CoV‐2 transmission to pets and other wild and domestic animals strongly mandates a one‐health strategy to control the COVID‐19 pandemic. One Health, 10, 100133. 10.1016/j.onehlt.2020.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko, M. , Marzi, A. , & Munster, V. (2020). Functional assessment of cell entry and receptor usage for SARS‐CoV‐2 and other lineage B betacoronaviruses. Nature Microbiology, 5(4), 562–569. 10.1038/s41564-020-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X.‐D. , Wang, W. , Hao, Z.‐Y. , Wang, Z.‐X. , Guo, W.‐P. , Guan, X.‐Q. , Wang, M.‐R. , Wang, H.‐W. , Zhou, R.‐H. , & Li, M.‐H. (2017). Extensive diversity of coronaviruses in bats from China. Virology, 507, 1–10. 10.1016/j.virol.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P. , Jiang, J.‐Z. , Wan, X.‐F. , Hua, Y. , Li, L. , Zhou, J. , Wang, X. , Hou, F. , Chen, J. , & Zou, J. (2020a). Are pangolins the intermediate host of the 2019 novel coronavirus (SARS‐CoV‐2)? PLoS Pathogens, 16(5), e1008421. 10.1371/journal.ppat.1008421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Xiao, X. , Wei, X. , Li, J. , Yang, J. , Tan, H. , Zhu, J. , Zhang, Q. , Wu, J. , & Liu, L. (2020b). Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. Journal of Medical Virology, 92(6), 595–601. 10.1002/jmv.25726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeot, M. , Mauroy, A. , Thiry, E. , De Regge, N. , Vervaeke, M. , Beck, O. , De Waele, V. , & Van den Berg, T. (2021). Risk assessment of SARS‐CoV‐2 infection in free‐ranging wild animals in Belgium. Transboundary and Emerging Diseases, 00, 1–11. 10.1111/tbed.14131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, G. , Wang, Q. , & Gao, G. F. (2015). Bat‐to‐human: Spike features determining ‘host jump'of coronaviruses SARS‐CoV, MERS‐CoV, and beyond. Trends in Microbiology, 23(8), 468–478. 10.1016/j.tim.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , Wang, W. , Song, H. , Huang, B. , & Zhu, N. (2020a). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. The Lancet, 395(10224), 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , Zhao, Y. , Yu, W. , Yang, Y. , Gao, J. , Wang, J. , Kuang, D. , Yang, M. , Yang, J. , & Ma, C. (2020b). Comparison of nonhuman primates identified the suitable model for COVID‐19. Signal Transduction and Targeted Therapy, 5(1), 1–9. 10.1038/s41392-020-00269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan, J. , Lu, Y. , Jin, X. , & Zhang, L. (2020). Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS‐CoV‐2 infection. Biochemical and Biophysical Research Communications, 526(1), 165–169. 10.1016/j.bbrc.2020.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machalaba, C. , Uhart, M. , Ryser‐Degiorgis, M.‐P. , & Karesh, W. B. (2021). Gaps in health security related to wildlife and environment affecting pandemic prevention and preparedness, 2007–2020. Bulletin of the World Health Organization, 99(5), 342. 10.2471/BLT.20.272690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, Y. S. , Sircar, S. , Bhat, S. , Sharun, K. , Dhama, K. , Dadar, M. , Tiwari, R. , & Chaicumpa, W. (2020). Emerging novel coronavirus (2019‐nCoV)—Current scenario, evolutionary perspective based on genome analysis and recent developments. Veterinary Quarterly, 40(1), 68–76. 10.1080/01652176.2020.1727993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallapaty, S. (2020). Coronaviruses closely related to the pandemic virus discovered in Japan and Cambodia. Nature, 588(7836), 15–16. [DOI] [PubMed] [Google Scholar]
- Mallapaty, S. (2021). Closest known relatives of virus behind COVID‐19 found in Laos. Nature, 597(7878), 603‐603. [DOI] [PubMed] [Google Scholar]
- Martina, B. E. , Haagmans, B. L. , Kuiken, T. , Fouchier, R. A. , Rimmelzwaan, G. F. , Van Amerongen, G. , Peiris, J. M. , Lim, W. , & Osterhaus, A. D. (2003). SARS virus infection of cats and ferrets. Nature, 425(6961), 915–915. 10.1038/425915a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathavarajah, S. , & Dellaire, G. (2020). Lions, Tigers and Kittens too: ACE2 and susceptibility to CoVID‐19. Evolution, Medicine, and Public Health, 2020(1), 109–113. 10.1093/emph/eoaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin, M. , Fenollar, F. , Mediannikov, O. , Davoust, B. , Devaux, C. , & Raoult, D. (2021). Current status of putative animal sources of SARS‐CoV‐2 infection in humans: Wildlife, domestic animals and pets. Microorganisms, 9(4), 868. 10.3390/microorganisms9040868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose, D. , Laverack, M. , Wang, L. , Killian, M. L. , Caserta, L. C. , Yuan, F. , Mitchell, P. K. , Queen, K. , Mauldin, M. R. , & Cronk, B. D. (2020). From people to panthera: Natural SARS‐CoV‐2 infection in tigers and lions at the Bronx Zoo. MBio, 11(5). 10.1128/mBio.02220-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, T. , Richt, J. A. , & Glickman, L. (2020). A critical needs assessment for research in companion animals and livestock following the pandemic of COVID‐19 in humans. Vector‐Borne and Zoonotic Diseases, 20(6), 393–405. 10.1089/vbz.2020.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehand, M. S. , Al‐Shorbaji, F. , Millett, P. , & Murgue, B. (2018). The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antiviral Research, 159, 63–67. 10.1016/j.antiviral.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado, N. B. , Zahn, R. , Wegmann, F. , Loos, C. , Chandrashekar, A. , Yu, J. , Liu, J. , Peter, L. , McMahan, K. , Tostanoski, L. H. , He, X. , Martinez, D. R. , Rutten, L. , Bos, R. , van Manen, D. , Vellinga, J. , Custers, J. , Langedijk, J. P. , Kwaks, T. , … Barouch, D. H. (2020). Single‐shot Ad26 vaccine protects against SARS‐CoV‐2 in rhesus macaques. Nature, 586(7830), 583–588. 10.1038/s41586-020-2607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, A. , Kumar, N. , Bhatia, S. , Aasdev, A. , Kanniappan, S. , Thayasekhar, A. , Gopinadhan, A. , Silambarasan, R. , Sreekumar, C. , & Dubey, C. K. (2021). Natural infection of SARS‐CoV‐2 delta variant in Asiatic lions (Panthera leo persica) in India. bioRxiv, 1–15. 10.1101/2021.07.02.450663 [DOI] [Google Scholar]
- Montagutelli, X. , Prot, M. , Levillayer, L. , Salazar, E. B. , Jouvion, G. , Conquet, L. , Donati, F. , Albert, M. , Gambaro, F. , & Behillil, S. (2021). The B1. 351 and P. 1 variants extend SARS‐CoV‐2 host range to mice. bioRxiv, 1–15. 10.1101/2021.03.18.436013 [DOI] [Google Scholar]
- Munjal, A. , Khandia, R. , Dhama, K. , Sachan, S. , Karthik, K. , Tiwari, R. , Malik, Y. S. , Kumar, D. , Singh, R. K. , & Iqbal, H. (2017). Advances in developing therapies to combat Zika virus: Current knowledge and future perspectives. Frontiers in Microbiology, 8, 1469. 10.3389/fmicb.2017.01469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnink, B. B. O. , Sikkema, R. S. , Nieuwenhuijse, D. F. , Molenaar, R. J. , Munger, E. , Molenkamp, R. , Van Der Spek, A. , Tolsma, P. , Rietveld, A. , & Brouwer, M. (2021). Transmission of SARS‐CoV‐2 on mink farms between humans and mink and back to humans. Science, 371(6525), 172–177. 10.1126/science.abe5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster, V. J. , Feldmann, F. , Williamson, B. N. , Van Doremalen, N. , Pérez‐Pérez, L. , Schulz, J. , Meade‐White, K. , Okumura, A. , Callison, J. , & Brumbaugh, B. (2020). Respiratory disease in rhesus macaques inoculated with SARS‐CoV‐2. Nature, 585(7824), 268–272. 10.1038/s41586-020-2324-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, S. , Kitamura, T. , Suzuki, J. , Sato, R. , Aoi, T. , Fujii, M. , Matsugo, H. , Kamiki, H. , Ishida, H. , Takenaka‐Uema, A. , Shimojima, M. , & Horimoto, T. (2020). Detection and characterization of bat sarbecovirus phylogenetically related to SARS‐CoV‐2, Japan. Emerging Infectious Diseases, 26(12), 3025–3029. 10.3201/eid2612.203386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso, N. , Costantino, A. , La Spina, S. , Finocchiaro, A. , Andronico, F. , Stracquadanio, S. , Liotta, L. , Visalli, R. , & Emmanuele, G. (2020). New SARS‐CoV‐2 infection detected in an Italian pet cat by RT‐qPCR from deep pharyngeal swab. Pathogens, 9(9), 746. 10.3390/pathogens9090746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn, A. Z. , Lamers, M. M. , Okba, N. M. , Breugem, T. I. , Schipper, D. , van den Doel, P. B. , van Run, P. , van Amerongen, G. , de Waal, L. , & Koopmans, M. P. (2021). Susceptibility of rabbits to SARS‐CoV‐2. Emerging Microbes & Infections, 10(1), 1–7. 10.1080/22221751.2020.1868951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, A. , Smith, D. , Ghai, R. R. , Wallace, R. M. , Torchetti, M. K. , Loiacono, C. , Murrell, L. S. , Carpenter, A. , Moroff, S. , & Rooney, J. A. (2020). First reported cases of SARS‐CoV‐2 infection in companion animals—New York, March–April 2020. Morbidity and Mortality Weekly Report, 69(23), 710. 10.15585/mmwr.mm6923e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . (2020a). COVID‐19 (SARS‐COV‐2), Hong Kong (SAR ‐ PRC). Retrieved from https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=33832 [Accessed November 9, 2020].
- OIE . (2020b). Guideline for working with free‐ranging wild mammals in the era of the COVID‐19 pandemic. Retrieved from https://www.oie.int/app/uploads/2021/03/a‐whsg‐and‐oie‐covid‐19‐guidelines.pdf [Accessed June 10, 2021].
- OIE . (2020c). OIE technical factsheet: Infection with SARS‐COV‐2 in animals. Retrieved from https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/COV‐19/A_Factsheet_SARS‐CoV‐2.pdf [Accessed on August 10, 2021].
- OIE . (2020d). OIE technical factsheet: Infection with SARS‐COV‐2 in animals. Retrieved from https://www.oie.int/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/COV‐19/A_Factsheet_SARS‐CoV‐2.pdf [Accessed August 10, 2021].
- OIE . (2021). World organization for animal health. Retrieved from https://www.oie.int/en/what‐we‐do/animal‐health‐and‐welfare/animal‐diseases [Accessed November 9, 2020].
- Olival, K. J. , Cryan, P. M. , Amman, B. R. , Baric, R. S. , Blehert, D. S. , Brook, C. E. , Calisher, C. H. , Castle, K. T. , Coleman, J. T. , & Daszak, P. (2020). Possibility for reverse zoonotic transmission of SARS‐CoV‐2 to free‐ranging wildlife: A case study of bats. PLoS Pathogens, 16(9), e1008758. 10.1371/journal.ppat.1008758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink, B. B. , Sikkema, R. S. , Nieuwenhuijse, D. F. , Molenaar, R. J. , Munger, E. , Molenkamp, R. , van der Spek, A. , Tolsma, P. , Rietveld, A. , Brouwer, M. , Bouwmeester‐Vincken, N. , Harders, F. , Hakze‐van der Honing, R. , Wegdam‐Blans, M. C. A. , Bouwstra, R. J. , GeurtsvanKessel, C. , van der Eijk, A. A. , Velkers, F. C. , Smit, L. A. M. , … Koopmans, M. P. G. (2021). Transmission of SARS‐CoV‐2 on mink farms between humans and mink and back to humans. Science, 371(6525), 172–177. 10.1126/science.abe5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani, G. , Lai, A. , Bergna, A. , Rizzo, A. , Stranieri, A. , Giordano, A. , Paltrinieri, S. , Lelli, D. , Decaro, N. , Rusconi, S. , Gismondo, M. R. , Antinori, S. , Lauzi, S. , Galli, M. , & Zehender, G. (2021). Human‐to‐cat SARS‐CoV‐2 transmission: Case report and full‐genome sequencing from an infected pet and its owner in Northern Italy. Pathogens, 10(2), 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, M. V. , Martins, M. , Falkenberg, S. , Buckley, A. , Caserta, L. C. , Mitchell, P. K. , Cassmann, E. D. , Rollins, A. , Zylich, N. C. , Renshaw, R. W. , Guarino, C. , Wagner, B. , Lager, K. , & Diel, D. G. (2021). Susceptibility of white‐tailed deer (Odocoileus virginianus) to SARS‐CoV‐2. Journal of Virology, 95(11), e00083‐21. 10.1128/jvi.00083-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin, C. , Virtuoso, S. , Giovanetti, M. , Angeletti, S. , Ciccozzi, M. , & Borsetti, A. (2021). Animal hosts and experimental models of SARS‐CoV‐2 infection. Chemotherapy, 10.1159/000515341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, E. I. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. 10.1101/2020.07.21.214346 [DOI] [PMC free article] [PubMed]
- Patterson, E. I. , Elia, G. , Grassi, A. , Giordano, A. , Desario, C. , Medardo, M. , Smith, S. L. , Anderson, E. R. , Prince, T. , & Patterson, G. T. (2020). Evidence of exposure to SARS‐CoV‐2 in cats and dogs from households in Italy. Nature Communications, 11(1), 1–5. 10.1038/s41467-020-20097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y. , Zhao, Y.‐B. , Wang, Q. , Li, J.‐Y. , Zhou, Z.‐J. , Liao, C.‐H. , & Ge, X.‐Y. (2020). Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS‐CoV‐2. Microbes and Infection, 22(4‐5), 221–225. 10.1016/j.micinf.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, M. , Kok, A. , de Meulder, D. , Bestebroer, T. M. , Lamers, M. M. , Okba, N. M. , van Vlissingen, M. F. , Rockx, B. , Haagmans, B. L. , & Koopmans, M. P. (2020). SARS‐CoV‐2 is transmitted via contact and via the air between ferrets. Nature Communications, 11(1), 1–6. 10.1038/s41467-020-17367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, A. , Vogel, L. , Guarner, J. , Hayes, N. , Murphy, B. , Zaki, S. , & Subbarao, K. (2005). Severe acute respiratory syndrome coronavirus infection of golden Syrian hamsters. Journal of Virology, 79(1), 503–511. 10.1128/JVI.79.1.503-511.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Morales, A. J. , Bonilla‐Aldana, D. K. , Tiwari, R. , Sah, R. , Rabaan, A. A. , & Dhama, K. J. J. P. A. M. (2020). COVID‐19, an emerging coronavirus infection: Current scenario and recent developments‐an overview. Journal of Pure and Applied Microbiology, 14(1), 5–12. 10.22207/JPAM.14.1.02 [DOI] [Google Scholar]
- Ruiz‐Arrondo, I. , & Portillo, A. (2021). Detection of SARS‐CoV‐2 in pets living with COVID‐19 owners diagnosed during the COVID‐19 lockdown in Spain: A case of an asymptomatic cat with SARS‐CoV‐2 in Europe. Transboundary & Emerging Disease, 68(2), 973–976. 10.1111/tbed.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulli, M. C. , D'Odorico, P. , Galli, N. , & Hayman, D. (2020). Land use change and livestock revolution as contributors to Coronavirus emergence risk. Research Square, 1, 1–18. 10.21203/rs.3.rs-69560/v1 [DOI] [Google Scholar]
- Sailleau, C. , Dumarest, M. , Vanhomwegen, J. , Delaplace, M. , Caro, V. , Kwasiborski, A. , Hourdel, V. , Chevaillier, P. , Barbarino, A. , & Comtet, L. (2020). First detection and genome sequencing of SARS‐CoV‐2 in an infected cat in France. Transboundary and Emerging Diseases, 67(6), 2324–2328. 10.1111/tbed.13659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou, N. , & Nei, M. (1987). The neighbor‐joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4(4), 406–425. 10.1093/oxfordjournals.molbev.a040454 [DOI] [PubMed] [Google Scholar]
- Sarkar, J. , & Guha, R. (2020). Infectivity, virulence, pathogenicity, host‐pathogen interactions of SARS and SARS‐CoV‐2 in experimental animals: A systematic review. Veterinary Research Communications, 44, 101–110. 10.1007/s11259-020-09778-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed, M. , Islam, B. , Nahar, N. , Bari, M. , Sultana, S. , Arfin, S. , Haldar, P. , & Islam, A. (2020). Epidemiology of livestock and poultry diseases in Jhenaidah district of Bangladesh. Advances in Animal and Veterinary Sciences, 8(8), 804–812. 10.17582/journal.aavs/2020/8.8.804.812 [DOI] [Google Scholar]
- Saylors, K. , Wolking, D. J. , Hagan, E. , Martinez, S. , Francisco, L. , Euren, J. , Olson, S. H. , Miller, M. , Fine, A. E. , & Thanh, N. N. T. (2021). Socializing One Health: An innovative strategy to investigate social and behavioral risks of emerging viral threats. One Health Outlook, 3(1), 1–16. 10.1186/s42522-021-00036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau, K. , Rissmann, M. , Graaf, A. , Schön, J. , Sehl, J. , Wylezich, C. , Höper, D. , Mettenleiter, T. C. , Balkema‐Buschmann, A. , & Harder, T. (2020). Experimental transmission studies of SARS‐CoV‐2 in fruit bats, ferrets, pigs and chickens. The Lancet Microbe, 1(5), e218‐e225. 10.1016/S2666-5247(20)30089-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalés, J. , Puig, M. , Rodon, J. , Avila‐Nieto, C. , Carrillo, J. , Cantero, G. , Terrón, M. T. , Cruz, S. , Parera, M. , & Noguera‐Julián, M. (2020). Detection of SARS‐CoV‐2 in a cat owned by a COVID‐19− affected patient in Spain. Proceedings of the National Academy of Sciences, 117(40), 24790–24793. 10.1073/pnas.2010817117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, C. , Yao, Y.‐F. , Yang, X.‐L. , Zhou, Y.‐W. , Gao, G. , Peng, Y. , Yang, L. , Hu, X. , Xiong, J. , & Jiang, R.‐D. (2020). Infection with novel coronavirus (SARS‐CoV‐2) causes pneumonia in Rhesus macaques. Cell Research, 30(8), 670–677. 10.1038/s41422-020-0364-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharun, K. , Dhama, K. , Pawde, A. M. , Gortázar, C. , Tiwari, R. , Bonilla‐Aldana, D. K. , Rodriguez‐Morales, A. J. , de la Fuente, J. , Michalak, I. , & Attia, Y. A. (2021). SARS‐CoV‐2 in animals: Potential for unknown reservoir hosts and public health implications. Veterinary Quarterly, 41(1), 181–201. 10.1080/01652176.2021.1921311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , Liu, R. , He, X. , Shuai, L. , & Sun, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368(6494), 1016–1020. 10.1126/science.abb7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriner, S. A. , Ellis, J. W. , Root, J. J. , Roug, A. , Stopak, S. R. , Wiscomb, G. W. , Zierenberg, J. R. , Ip, H. S. , Torchetti, M. K. , & DeLiberto, T. J. (2021). SARS‐CoV‐2 exposure in escaped mink, Utah, USA. Emerging Infectious Diseases, 27(3), 988. 10.3201/eid2703.204444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, Y. , & McCauley, J. (2017). GISAID: Global initiative on sharing all influenza data—From vision to reality. Euro Surveillance, 22(13). 10.2807/1560-7917.es.2017.22.13.30494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia, S. F. , Yan, L.‐M. , Chin, A. W. , Fung, K. , Choy, K.‐T. , Wong, A. Y. , Kaewpreedee, P. , Perera, R. A. , Poon, L. L. , & Nicholls, J. M. (2020). Pathogenesis and transmission of SARS‐CoV‐2 in golden hamsters. Nature, 583(7818), 834–838. 10.1038/s41586-020-2342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. K. , Dhama, K. , Chakraborty, S. , Tiwari, R. , Natesan, S. , Khandia, R. , Munjal, A. , Vora, K. S. , Latheef, S. K. , & Karthik, K. J. V. Q. (2019). Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies–a comprehensive review. Veterinary Quarterly, 39(1), 26–55. 10.1080/01652176.2019.1580827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, D. K. , Singh, B. , Ganatra, S. R. , Gazi, M. , Cole, J. , Thippeshappa, R. , Alfson, K. J. , Clemmons, E. , Gonzalez, O. , & Escobedo, R. (2021). Responses to acute infection with SARS‐CoV‐2 in the lungs of rhesus macaques, baboons and marmosets. Nature Microbiology, 6(1), 73–86. 10.1038/s41564-020-00841-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T. H. , Brackman, C. J. , Ip, S. M. , Tam, K. W. , Law, P. Y. , To, E. M. , Veronica, Y. , Sims, L. D. , Tsang, D. N. , & Chu, D. K. (2020). Infection of dogs with SARS‐CoV‐2. Nature, 586(7831), 776–778. 10.1038/s41586-020-2334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skirmuntt, E. C. , Escalera‐Zamudio, M. , Teeling, E. C. , Smith, A. , & Katzourakis, A. (2020). The potential role of endogenous viral elements in the evolution of bats as reservoirs for zoonotic viruses. Annual Review of Virology, 7, 103–119. 10.1146/annurev-virology-092818-015613 [DOI] [PubMed] [Google Scholar]
- Steve, G. (2021). Gorillas at San Diego Zoo Safari Park diagnosed with COVID‐19. Retrieved from https://www.reuters.com/article/us‐health‐coronavirus‐usa‐gorillas‐idUSKBN29G2L9 [Accessed June 17, 2021].
- Stopsack, K. H. , Mucci, L. A. , Antonarakis, E. S. , Nelson, P. S. , & Kantoff, P. W. (2020). TMPRSS2 and COVID‐19: Serendipity or opportunity for intervention? Cancer Discovery, 10(6), 779–782. 10.1158/2159-8290.CD-20-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout, A. E. , André, N. M. , Jaimes, J. A. , Millet, J. K. , & Whittaker, G. R. (2020). Coronaviruses in cats and other companion animals: Where does SARS‐CoV‐2/COVID‐19 fit? Veterinary Microbiology, 247, 108777. 10.1016/j.vetmic.2020.108777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez, D. L. , Pantin‐Jackwood, M. J. , Swayne, D. E. , Lee, S. A. , Deblois, S. M. , & Spackman, E. (2020). Lack of susceptibility of poultry to SARS‐CoV‐2 and MERS‐CoV. bioRxiv, 1–15. 10.1101/2020.06.16.154658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. , He, W.‐T. , Wang, L. , Lai, A. , Ji, X. , Zhai, X. , Li, G. , Suchard, M. A. , Tian, J. , & Zhou, J. (2020). COVID‐19: Epidemiology, evolution, and cross‐disciplinary perspectives. Trends in Molecular Medicine, 26(5), 483–495. 10.1016/j.molmed.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, J.‐H. , Patel, N. , Haupt, R. , Zhou, H. , Weston, S. , Hammond, H. , Logue, J. , Portnoff, A. D. , Norton, J. , & Guebre‐Xabier, M. (2020). SARS‐CoV‐2 spike glycoprotein vaccine candidate NVX‐CoV2373 elicits immunogenicity in baboons and protection in mice. Nature Communications, 12(1), 1–14. 10.1038/s41467-020-20653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touati, R. , Haddad‐Boubaker, S. , Ferchichi, I. , Messaoudi, I. , Ouesleti, A. E. , Triki, H. , Lachiri, Z. , & Kharrat, M. (2020). Comparative genomic signature representations of the emerging COVID‐19 coronavirus and other coronaviruses: High identity and possible recombination between Bat and Pangolin coronaviruses. Genomics, 112(6), 4189–4202. 10.1016/j.ygeno.2020.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimpert, J. , Vladimirova, D. , Dietert, K. , Abdelgawad, A. , Kunec, D. , Dökel, S. , Voss, A. , Gruber, A. D. , Bertzbach, L. D. , & Osterrieder, N. (2020). The Roborovski dwarf hamster is a highly susceptible model for a rapid and fatal course of SARS‐CoV‐2 infection. Cell Reports, 33(10), 108488. 10.1016/j.celrep.2020.108488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA . (2021a). United States Department of Agriculture (USDA). Confirmation of COVID‐19 in otters at an aquarium in Georgia. https://www.aphis.usda.gov/aphis/newsroom/stakeholder‐info/sa_by_date/sa‐2021/sa‐04/covid‐georgia‐otters [Accessed on 3 June, 2021].
- USDA . (2021b). United States Department of Agriculture (USDA). Confirmation of COVID‐19 in otters at an aquarium in Georgia. Retrived from https://www.aphis.usda.gov/aphis/newsroom/stakeholder‐info/sa_by_date/sa‐2021/sa‐04/covid‐georgia‐otters [Accessed June 3, 2021].
- van Aart, A. E. , Velkers, F. C. , Fischer, E. A. , Broens, E. M. , Egberink, H. , Zhao, S. , Engelsma, M. , Hakze‐van der Honing, R. W. , Harders, F. , de Rooij, M. , Radstake, C. , Meijer, P. A. , Oude Munnink, B. B. , de Rond, J. , Sikkema, R. S. , van der Spek, A. N. , Spierenburg, M. , Wolters, W. J. , Molenaar, R. J. , Koopmans, M. , … Smit, L. (2021). SARS‐CoV‐2 infection in cats and dogs in infected mink farms. Transboundary and Emerging Diseases, 1–7. Advance online publication. 10.1111/tbed.14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacharapluesadee, S. , Tan, C. W. , Maneeorn, P. , Duengkae, P. , Zhu, F. , Joyjinda, Y. , Kaewpom, T. , Chia, W. N. , Ampoot, W. , & Lim, B. L. (2021). Evidence for SARS‐CoV‐2 related coronaviruses circulating in bats and pangolins in Southeast Asia. Nature Communications, 12(1), 1–9. 10.1038/s41467-021-21240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Graham, R. , Baric, R. S. , & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade‐long structural studies of SARS coronavirus. Journal of Virology, 94(7),. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2021). SARS‐CoV‐2 in animals used for fur farming: GLEWS+ risk assessment, 20 January 2021. Retrieved from https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐fur‐farming‐risk‐assessment‐2021.1 [Accessed September 10, 2021].
- Wong, G. , Bi, Y.‐H. , Wang, Q.‐H. , Chen, X.‐W. , Zhang, Z.‐G. , & Yao, Y.‐G. (2020). Zoonotic origins of human coronavirus 2019 (HCoV‐19/SARS‐CoV‐2): Why is this work important? Zoological Research, 41(3), 213. 10.24272/j.issn.2095-8137.2020.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey, C. , Borisevich, V. , Prasad, A. N. , Agans, K. N. , Deer, D. J. , Dobias, N. S. , Heymann, J. C. , Foster, S. L. , Levine, C. B. , Medina, L. J. N. , Melody, K. , Geisbert, J. B. , Fenton, K. A. , Geisbert, T. W. , & Cross, R. W. (2021). Establishment of an African green monkey model for COVID‐19 and protection against re‐infection. Nature Immunology, 22, 86–98. 10.1038/s41590-020-00835-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp, D. , Wang, N. , Corbett, K. S. , Goldsmith, J. A. , Hsieh, C.‐L. , Abiona, O. , Graham, B. S. , & McLellan, J. S. (2020). Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science, 367(6483), 1260–1263. 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y. , Guo, C. , Tang, L. , Hong, Z. , Zhou, J. , Dong, X. , Yin, H. , Xiao, Q. , Tang, Y. , & Qu, X. (2020). Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. The Lancet Gastroenterology & hepatology, 5(5), 434–435. 10.1016/S2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Wang, D. , & Evans, J. A. (2019). Large teams develop and small teams disrupt science and technology. Nature, 566(7744), 378–382. 10.1038/s41586-019-0941-9 [DOI] [PubMed] [Google Scholar]
- Xiao, X. , Newman, C. , Buesching, C. D. , Macdonald, D. W. , & Zhou, Z.‐M. (2021). Animal sales from Wuhan wet markets immediately prior to the COVID‐19 pandemic. Scientific Reports, 11(1), 11898. 10.1038/s41598-021-91470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekta, R. , Vahid‐Dastjerdi, L. , Norouzbeigi, S. , & Mortazavian, A. M. (2020). Food products as potential carriers of SARS‐CoV‐2. Food Control, 123, 107754. 10.1016/j.foodcont.2020.107754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, X. , Sun, J. , Yan, Z. , Zhang, J. , Zhao, J. , Zhao, Z. , Gao, Q. , He, W.‐T. , Veit, M. , & Su, S. (2020). Comparison of severe acute respiratory syndrome coronavirus 2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. Journal of Virology, 9 4(15). 10.1128/JVI.00831-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Xiao, K. , Zhang, X. , Roy, A. , & Shen, Y. (2020c). Emergence of SARS‐like coronavirus in China: An update. Journal of Infection, 80(5), e28–e29. 10.1016/j.jinf.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Gao, J. , Huang, K. , Yang, Y. , Hui, X. , He, X. , Li, C. , Gong, W. , & Zhang, Y. (2020b). A serological survey of SARS‐CoV‐2 in cat in Wuhan. Emerging Microbes & Infections, 9(1), 2013–2019. 10.1080/22221751.2020.1817796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Zheng, W. , Huang, X. , Bell, E. W. , Zhou, X. , & Zhang, Y. (2020a). Protein structure and sequence reanalysis of 2019‐nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV‐1. Journal of Proteome Research, 19(4), 1351–1360. 10.1021/acs.jproteome.0c00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Wang, J. , Kuang, D. , Xu, J. , Yang, M. , Ma, C. , Zhao, S. , Li, J. , Long, H. , & Ding, K. (2020). Susceptibility of tree shrew to SARS‐CoV‐2 infection. Scientific Reports, 10(1), 1–9. 10.1038/s41598-020-72563-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , Si, H. R. , Zhu, Y. , Li, B. , Huang, C. L. , Chen, H. D. , Chen, J. , Luo, Y. , Guo, H. , Jiang, R. D. , Liu, M. Q. , Chen, Y. , Shen, X. R. , Wang, X. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , Zhao, X. , Huang, B. , Shi, W. , Lu, R. , Niu, P. , Zhan, F. , Ma, X. , Wang, D. , Xu, W. , Wu, G. , Gao, G. F. , & Tan, W. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsstag, J. , Utzinger, J. , Probst‐Hensch, N. , Shan, L. , & Zhou, X.‐N. (2020). Towards integrated surveillance‐response systems for the prevention of future pandemics. Infectious Diseases of Poverty, 9(1), 1–6. 10.1186/s40249-020-00757-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
The data that support the findings of this study are openly available in GenBank at https://www.ncbi.nlm.nih.gov/genbank/.


