Abstract
Background
This pilot study assesses the ability of plasma collected from Canadian blood donors in the first wave of the SARS‐CoV‐2 pandemic to neutralize later SARS‐CoV‐2 variants of concern (VOCs).
Study design and methods
A repeated cross‐sectional design was used, and a random cross‐sectional sample of all available Canadian Blood Services retention samples (n = 1500/month) was drawn monthly for April and May of 2020. Qualitative IgG analysis was performed on aliquots of specimens using anti‐spike, anti‐receptor binding domain, and anti‐nucleocapsid protein enzyme‐linked immunosorbent assays as well as the Abbott Architect SARS CoV‐2 IgG assay (Abbott Laboratories) against the anti‐nucleocapsid protein. Selected plasma specimens were then assessed for neutralization against VOCs using pseudotyped lentivirus inhibition assays as well as plaque reduction neutralization test 50% (PRNT50).
Results
Six specimens with a high neutralizing titer against wild‐type SARS‐CoV‐2 and three specimens with a low neutralizing titer against wild‐type SARS‐CoV‐2 were chosen for further analysis against VOCs. Four of six high neutralizing titer specimens had a reduced neutralizing capacity against beta VOCs by both neutralization methods. Three of six high neutralizing titer specimens had reduced neutralization capacity against gamma VOCs.
Conclusions
This preliminary data can be used as a justification for limiting the use of first wave plasma products in upcoming clinical trials but cannot be used to speculate on general trends in the immunity of Canadian blood donors to SARS‐CoV‐2.
Abbreviations
- anti‐S
anti‐spike
- anti‐N
anti‐nucleocapsid protein
- anti‐RBD
anti‐receptor binding domain
- FSA, first threecharacters of postal code
Forward Sortation Area
- NML
NationalMicrobiology Laboratory
- SARS‐CoV‐2
Severe acute respiratory syndrome coronavirus‐2
- PRNT50
plaque reduction neutralization test 50%
- VOCs
variants of concern
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) virus was first identified in Canada during the first quarter of 2020 and between January 2020 and July of 2021 there were three distinct waves in that country. 1 Nationally, a low amplitude first wave occurred between late February and early July of 2020. 2 , 3 The true amplitude of this wave may have been underestimated as molecular and serological assays were still in development. 4 In Canada, a broad and higher amplitude second wave started sometime in early August, peaked in late December, and reached a trough in the period of mid/late January to early/mid‐March 2021. In late March 2021, a narrower but equal amplitude wave followed. 3 Prior seroprevalence surveys of Canadian Blood donors by Canadian Blood Services suggested that donors had very low levels of seropositivity (e.g., <5%) between April and September of 2020. 5 , 6 In April and early May of 2020. Canadian Blood Services engaged a broad group of laboratories in Canada and the United States to attempt to understand the neutralizing capacity of blood donor antibodies to SARS‐CoV‐2. 7 , 8 The preliminary work was generated to support SARS‐CoV‐2 convalescent plasma studies in Canada to understand anti‐SARS‐CoV‐2 responses as well as anti‐SARS‐CoV‐2 neutralizing titers in Canadian blood donors. 7 , 8 During the study planning process, operational and access issues led us to utilize the Abbott Architect SARSCoV‐2 IgG test (Abbott Laboratories), which detects anti‐nucleocapsid (N) IgG. 5 The identification of waning neutralizing antibody responses in blood donors led to the development of a further “correlates of immunity” project which had the stated goal of understanding changes in anti‐SARS‐CoV‐2 neutralizing capacity as the COVD‐19 pandemic advanced.
National Institutes of Health and Infectious Diseases Society of America practice guidelines indicate that for non‐hospitalized ambulatory patients, COVID‐19 convalescent plasma trials may still be undertaken to fill knowledge gaps from prior clinical trials. 9 , 10 Blood collection for convalescent plasma studies in Canada, as well as the majority of studies in the United States, have ceased. Our hypothesis was whether routinely collected plasma specimens collected in the first wave of the pandemic would be able to continue to neutralize later variants of concern (VOCs). This is an important question, as the epidemiology of the SARS‐CoV‐2 pandemic continues to change in Canada. As of early July 2021, from a cumulative total of approximately 1.4 million reported COVID‐19 cases, the following VOCs have been publicly reported; alpha (B.1.1.7, n = 219,811), beta (B.1.351, n = 1968), gamma (P.1, n = 16,367), Delta (B.1.617.2, n = 3053). 11 The data provided on variants is publicly available information from different provinces and territories and may be biased by variability in provincial surveillance and sequencing strategies. To be conservative, the presented data should be considered a sampling of convenience. This pilot study assesses the ability of routinely collected Canadian blood donor plasma collected in the first wave of the pandemic to neutralize VOCs.
2. MATERIALS AND METHODS
2.1. Ethical considerations
The project had ethics board clearance from Canadian Blood Services, the University of Alberta and the Sinai Health, Toronto (Lunenfeld‐Tanenbaum Research Institute).
2.2. CIHR correlates of immunity study participants and samples
Canadian Blood Services is responsible for collecting blood donations from in all provinces except Quebec and collection sites are concentrated in large and small cities. Blood donors (≥17 years age) must meet numerous health selection criteria. Blood donations are used to manufacture products for transfusion; an additional EDTA plasma retention sample is also collected alongside each donation. 12
A repeated cross‐sectional design was employed, and a random cross‐sectional sample of all available Canadian Blood Services retention samples (n = 1500/month) was drawn monthly for April and May of 2020. Sample selection was a two‐stage process: first blood donor clinics were randomly selected by region proportional to regional collections and then samples from each donor clinic were randomly selected proportional to collections such that larger clinics had more donations. There are generally about 1000 clinics per month from which about 60,000 donations are collected. About 80% of these had a sample, available for the study; the other 20% were required for operational purposes (e.g., further routine donor testing). All samples were from unique donors and all donation types were included. Samples were anonymized. Donation date, birth year, sex, collection site, and residential Forward Sortation Area (FSA, first three characters of postal code) were extracted from the donor database.
Plasma specimens were stored at 4°C until aliquoted within 7 days at Canadian Blood Services frozen at −80°C and transported to test sites. One freeze to two thaw cycles occurred to aliquot the sample at the test site. One aliquot (250 ul) was stored at −80°C for the remainder of the study.
2.3. Enzyme‐linked immunosorbent assays for detecting IgG
Laboratory‐developed qualitative IgG analysis was performed at the Lunenfeld‐Tanenbaum Research Institute, Sinai Health, on specimens using the anti‐spike (anti‐S), anti‐receptor binding domain (anti‐RBD), and anti‐nucleocapsid protein (anti‐N). Although formal binary assay cutoffs were still being developed in this population, ratio‐converted ELISA reads were generated as previously described. 8 , 13
2.4. Abbott architect SARSCoV‐2 IgG test
Plasma samples were tested with the Abbott Architect SARS‐CoV‐2 IgG test (Abbott Laboratories), which detects anti‐N IgG, as directed by the manufacturer, using an antibody index (AI) cut‐off of 1.4. 5 , 6
2.5. Selection of specimens for neutralizing analysis
Since work was still being undertaken to establish assay cutoffs in this population, a tiered testing approach of specimens with any potential evidence of a signal for anti‐S or anti‐RBD (with or without anti‐N) was used to select specimens for further analysis by neutralization methods. 8 , 14
2.6. Wild‐type PRNT50 testing
Selected EDTA plasma specimens were tested at the National Microbiology Laboratory (NML) (Winnipeg, MB, Canada). Wild‐type D614G SARS‐CoV‐2 stocks at NML were titrated and used in a plaque reduction neutralization test 50% (PRNT50) modified from a previously published method and described in detail elsewhere. 15 The biological variability of the NML PRNT result falls within 2 doubling dilutions, or a four‐fold dilution. 7 Samples were not titrated past a certain dilution (e.g., 1:640). Criteria originally established for convalescent plasma were used to identify high titer plasma specimens with a PRNT50 ≥ 1:160 defined as high titer plasma and specimens with a PRNT50 < 1:160 defined as low titer plasma. 7
2.7. Lentivirus neutralization of wild‐type (D614G) and VOC
Selected EDTA specimens were also assessed for neutralizing capacity against pseudotyped lentivirus particles generated from wild‐type SARS‐CoV‐2 (Wuhan Hu‐1 sequence harboring the D614G mutation) and the VOCs alpha (B.1.1.7), beta (B.1.351), and gamma (P.1) spike protein constructs (a kind gift from Yuko Arita and Eric Marcusson at Providence Therapeutics) as previously described. The 50% inhibitory dose (ID50) values were determined as previously described. Briefly, neutralization curves were used to calculate the ID50 values of each sample with the immune escape of the virus‐like particles corresponding to lower ID50 values. 16
2.8. Plaque‐reduction neutralization‐50 (PRNT50 ) assays: VOC
Selected EDTA plasma specimens were also analyzed with PRNT50 utilizing the Wuhan wild‐type (B1, GISAID#EPI_ISL_425177) as well as variant of concern strains (alpha [B.1.1.7], beta [B.1.351], gamma [P.1], Delta [B.1.617.2]) were undertaken as described above but at the University of Alberta (Edmonton, Canada). Dilutions were established from <1:20 to 1:5120. As with the NML assays, the biological variability of these PRNT result falls within 2 doubling dilutions, or a four‐fold dilution. The identity of the viruses used in the study was confirmed by whole genome sequencing.
2.9. Data storage
All donor data were stored on a Microsoft Excel (Redmond, WA) spreadsheet.
3. RESULTS
3.1. Enzyme immunoassay screening of specimens
For this study, 1500 specimens from April 2020 and 1500 specimens from May 2020 (3000 total) were analyzed with previously published assays for anti‐S, anti‐ RBD, and anti‐N as well as the Abbott IgG anti‐N assay. As illustrated in Figure 1, based on previously developed approaches, 113 (8.9%) specimens were sent to the NML for PRNT50 determination.
FIGURE 1.
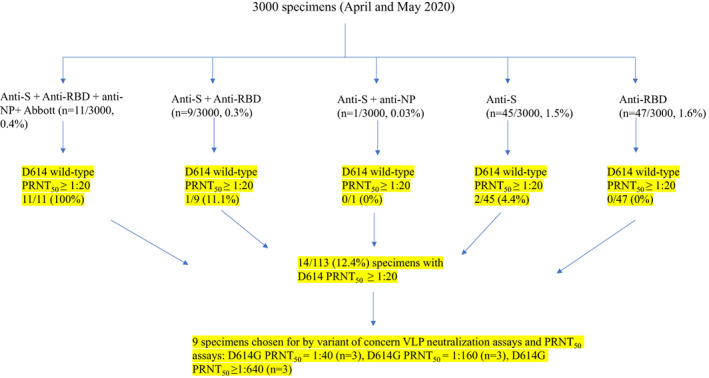
A tiered approach to identify specimens sent for wild ‐type PRNT50 and neutralization testing outcomes. From April and May 2020, 3000 specimens were first tested by SARS‐CoV‐2 enzyme immunoassays. One hundred and thirteen selected specimens were then analyzed by D614 wild‐type PRNT50. Fourteen specimens were available to assess for neutralization capacity against VOCs [Color figure can be viewed at wileyonlinelibrary.com]
3.2. PRNT50 results at NML with wild‐type D614G SARS‐CoV‐2
Fourteen of 113 (12.4%) of selected specimens were identified as having some neutralizing capacity by PRNT50 against wild‐type D614 SARS‐CoV‐2. These PRNT50 values were ≥1:640 (n = 3), 1:320 (n = 1), 1:160 (n = 3), 1:80 (n = 3), 1:40 (n = 3), 1:20 (n = 1) (Figure 1). A total of six specimens representing a high neutralizing titer against wild‐type D614 (≥1:640, [n = 3]; 1:160 [n = 3]), and three specimens representing low neutralizing titer against wild‐type D614 (1:40, [n = 3]) were chosen for analysis of neutralizing capacity against VOCs.
3.3. Neutralizing capacity of plasma collected in April/May 2020 against SARS‐CoV‐2 VOC by lentivirus neutralization
As in Table 1, 2 of 3 high neutralizing titer specimens, had at least a 10‐fold decrease in ID50 beta compared to D614G wild‐type. CIHR000491, CIHR000496, and CIHR000503 were deemed high titer because they had D614G wild‐type PRNT50 results of 1:160 (NML). CIHR002076, CIHR002309, and CIHR0002774 were also deemed high titer because they had wild‐type PRNT50 of 1:640 (NML). For 2 of 3 low neutralizing titer specimens [CIHR001226, CIHR002290, and CIHR002377, all with a D614G wild‐type PRNT50 of 1:40), there was at least a 100‐fold decrease in ID50 beta compared to D614G wild‐type (Table 1). Similarly, 1 of 2 high neutralizing titer specimens had at least a 10‐fold decrease in ID50 gamma compared to D614G wild‐type (Table 1). Also, 1 of 3 low neutralizing titer specimen had at least a 100‐fold decrease in ID50 gamma compared to D614G wild‐type (Table 1). There was no measurable change in ID50 alpha compared to D614G wild‐type (Table 1).
TABLE 1.
Neutralizing capacity of plasma collected in April/May 2020 against SARS‐CoV‐2 variants of concern by VLP neutralization
| Specimen # | Wild‐type titer category | PRNT50 Wuhan D614 wild‐type (NML) | ID50 Wuhan D614G | ID50 alpha | ID50 beta | ID50 gamma |
|---|---|---|---|---|---|---|
| CIHR001226 | Low titer | 1:40 | 1.0 | 1.0 | 1.0 | 1.0 |
| CIHR002290 | 1:40 | 161.68 | 157.80 | 1.0 a | 1.0 a | |
| CIHR002377 | 1:40 | 150.29 | 85.32 | 1.0 a | 1.0 a | |
| CIHR000491 | High titer | 1:160 | 1050.53 | 638.57 | 98.72 b | 109.33 b |
| CIHR000496 | 1:160 | 2430.13 | 2293.58 | 116.80 b | 129.38 b | |
| CIHR000503 | 1:160 | 975.61 | 410.00 | 234.03 | 260.69 | |
| CIHR002076 | ≥1:640 | 7917.66 | 2539.66 | 396.35 b | 712.25 b | |
| CIHR002309 | ≥1:640 | 1762.11 | 481.23 | 118.67 b | 369.14 | |
| CIHR002774 | ≥1:640 | 1267.43 | 723.59 | 263.02 | 378.93 |
Denotes a ≥ 100‐fold drop in ID50 compared to Wuhan D614G wild‐type.
Denotes a ≥ 10‐fold drop in ID50 compared to Wuhan D614G wild‐type.
3.4. Neutralizing capacity of plasma collected in April/May 2020 against SARS‐CoV‐2 VOC by PRNT50
As in Table 2, 2 of 3 high neutralizing titer specimens, had at least a fourfold decrease in PRNT50 beta compared to Wuhan wild‐type. Also, 1 of 2 high neutralizing titer specimens had at least a fourfold decrease in PRNT50 gamma compared to Wuhan wild‐type (Table 2). There was no measurable change in PRNT50 alpha and PRNT50 Delta compared to Wuhan wild‐type. No measurable change was defined as <fourfold change in PRNT50 compared to Wuhan wild‐type (Table 2).
TABLE 2.
Neutralizing capacity of plasma collected in April/May 2020 against SARS‐CoV‐2 variants of concern by PRNT50
| Specimen # | Wild‐type titer category | PRNT50 Wuhan | PRNT50 alpha | PRNT50 beta | PRNT50 gamma | PRNT50 delta |
|---|---|---|---|---|---|---|
| CIHR001226 | Low titer a | <1:20 | <1:20 | <1:20 | <1:20 | <1:20 |
| CIHR002290 | 1:20 | 1:40 | <1:20 | <1:20 | 1:20 | |
| CIHR002377 | 1:40 | 1:40 | <1:20 | 1:20 | 1:40 | |
| CIHR000491 | High titer b | 1:160 | 1:320 | <1:20 c | <1:20 c | 1:160 |
| CIHR000496 | 1:320 | 1:320 | 1:40 c | <1:20 c | 1:160 | |
| CIHR000503 | 1:160 | 1:160 | 1:160 | 1:80 | 1:160 | |
| CIHR002076 | 1:640 | 1:640 | 1:160 | 1:320 | 1:640 | |
| CIHR002309 | 1:640 | 1:320 | 1:20 c | 1:160 | 1:160 | |
| CIHR002774 | 1:640 | 1:160 | 1:80 c | 1:40 c | 1:160 |
All low value specimens had a D614 wild‐type PRNT50 (NML) of 1:40.
CIHR000491, CIHR000496, and CIHR000503 had D614G wild‐type PRNT50 results of 1:160 (NML). CIHR002076, CIHR002309, and CIHR0002774 had D614 wild‐type PRNT50 results of 1:640 (NML).
Denotes a > fourfold drop in PRNT50 titers compared to Wuhan wild‐type.
4. DISCUSSION
The purpose of this analysis was to evaluate the neutralizing capacity of stored plasma from wave 1 but not to estimate seroprevalence in Canadian blood donors. Two separate analyses have identified that the seroprevalence within Canadian Blood donors in the period of April/May 2020 was <1%. 5 , 6 Other work by members of this group identified that direct binding assays for RBD could potentially be used to pre‐screen specimens for further neutralization studies. 8 For this study, a liberalization of this approach selected specimens that were also positive for anti‐S positive regardless of the a‐N. This data‐driven approach utilized cutoffs that were more liberal than previously described by our group and undertook a tiered sampling of 113 (3.8%) of 3000 specimens. 6 With this approach, only 14 (12.4%) of the 113 specimens had a measurable level of neutralizing activity against wild type SARS‐CoV‐2 when measured by PRNT50. This low level of neutralizing capacity within the first wave of the pandemic has previously been shown in convalescent plasma donors. 7 , 8
There may be a variability in plasma specimens collected from Canadian blood donors in the first wave to neutralize beta and gamma variants. A Strasbourg cohort of convalescent plasma collected prior to the emergence of the VOC neutralized alpha but did show a reduction of neutralizing capacity against beta and Delta variants. 17 , 18 This difference in activity against Delta may be a product of the relatively small specimen numbers or neutralization methodology. Furthermore, we did not have a Delta construct available for neutralization in a pseudovirus assay system. Convalescent plasma collected from patients in the United States during the Spring of 2020 was able to effectively neutralize alpha but showed a reduced neutralizing capacity against both beta and gamma in separate studies. 19
There are several caveats to this study including the small number of specimens (1:40 [n = 3], 1:160 [N = 3], ≥1:640 [n = 3]) analyzed with nine of 14 available specimens tested. Specimens with D614 wild‐type PRNT50 of 1:20 and 1:320 were not tested because there were no other specimens with equal PRNT50 values to allow for duplicate or triplicate testing. Three specimens with D614 wild‐type PRNT50 values of 1:80 were held in reserve and not tested. There was also no Delta construct for the pseudovirus assay system. This study was limited to Canadian blood donors. 20 Specimens were also collected from routine blood donors and not qualified convalescent plasma donors in Canada. 7 Data on donor infection and symptom history were not available. This preliminary data can be used as a justification for limiting the use of first wave plasma products in upcoming clinical trials but cannot be used to estimate general trends in the immunity of Canadian blood donors to SARS‐CoV‐2.
CONFLICT OF INTEREST
The authors Sheila F. O'Brien, Kento T. Abe, Queenie Hu, Reuben Samson, Karen Colwill, Bhavisha Rathod, Jenny Wang, Mahaya Fazel‐Zarandi, Heidi Wood, Alyssia Robinson, Heidi Wood, Qi‐Long Yi, Ashleigh Tuite have no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Providence Therapeutics for the kind gift of lentivirus constructs for the VOCs.
Drews SJ, Abe KT, Hu Q, Samson R, Gingras A‐C, Colwill K, et al. Resistance of SARS‐CoV‐2 beta and gamma variants to plasma collected from Canadian blood donors during the spring of 2020. Transfusion. 2022;62:37–43. 10.1111/trf.16713
Funding informationSteven J. Drews received funding through the Canadian Institutes of Health Research (VR2‐172723) and Alberta Innovates (G2020000360 Drews), Anne‐Claude Gingras received funding through the Krembil Foundation to the Sinai Health System Foundation. The robotics equipment used for the ELISA assays is housed in the Network Biology Collaborative Centre at the Lunenfeld‐Tanenbaum Research Institute (ACG), a facility supported by Canada Foundation for Innovation funding, by the Ontarian Government and by Genome Canada and Ontario Genomics (OGI‐139). Commercial Abbott Architect SARS‐Cov‐2 IgG assay kit costs were partially supported by Abbott Laboratories, Abbott Park, Illinois. Abbott analyzers used at Canadian Blood Services were provided by the COVID‐19 Immunity task Force (CITF). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Steven J Drews has acted as a content expert for respiratory viruses for Johnson & Johnson (Janssen). Anne‐Claude Gingras receives funds from a research contract with Providence Therapeutics Holdings, Inc. for other projects. David H. Evans consults for, and fold research contracts from Tonix Pharma, New York relating to the construction of COVID‐19 vaccines. Support for the University of Alberta BSL3 facility was also received from the World Health Organization, Li Ka Shing Institute of Virology, and Alberta Innovates. David Fisman has served on advisory boards for Pfizer, Sanofi‐Pasteur, and Seqirus vaccines.
REFERENCES
- 1. Silverstein W, Stroud L, Cleghorn G, Leis J. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet. 2020;395:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Public Health Agency of Canada COVID‐19 Surveillance and Epidemiology Team . A retrospective analysis of the start of the COVID‐19 epidemic in Canada, January 15–March 12, 2020. Can Commun Dis Rep. 2020;46(236):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Government of Canada . COVID‐19 daily epidemiology update. 2021. https://health‐infobase.canada.ca/covid‐19/epidemiological‐summary‐covid‐19‐cases.html. Last accessed 2021‐09‐28.
- 4. Rogers R, O'Brien T, Aridi J, Beckwith C. The COVID‐19 diagnostic dilemma: a Clinician's perspective. J Clin Microbiol. 2020;58:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saeed S, Drews SJ, Pambrun C, Yi QL, Osmond L, O'Brien SF. SARS‐CoV‐2 seroprevalence among blood donors after the first COVID‐19 wave in Canada. Transfusion. 2021;61:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saeed S, O'Brien SF, Abe K, Yi QL, Rathod B, Wang J, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) seroprevalence: navigating the absence of a gold standard. PLoS ONE. 2021;16:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drews S, Devine D, McManus J, Mendoza E, Manguiat K, Wood H, et al. A trend of dropping anti‐SARS‐CoV‐2 plaque reduction neutralization test titers over time in Canadian convalescent plasma donors. Transfusion. 2021;61:1440–6. [DOI] [PubMed] [Google Scholar]
- 8. Abe K, Li Z, Samson R, Samavarchi‐Tehrani P, Valcourt E, Wood H, et al. A simple protein‐based surrogate neutralization assay for SARS‐CoV‐2. JCI Insight. 2020;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Health. NIo. COVID‐19 Treatment Guidelines Panel . Coronavirus Disease 2019 (COVID‐19) Treatment Guidelines, 2021, https://www.covid19treatmentguidelines.nih.gov/. [PubMed]
- 10. Bhimraj A, Morgan R, Shumaker A, Lavergne V, Baden L, Cheng V, et al. Infectious Diseases Society of America guidelines on the treatment and Management of Patients with COVID‐19. Clin Infect Dis. 2020:1–37. 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Collaborating Centre for Infectious Diseases Updates on COVID‐19 Variants of Concern, 2021. https://nccid.ca/covid-19-variants/. Last accessed 2021‐09‐28.
- 12. Canadian Blood Services . Surveillance report 2019. https://professionaleducation.blood.ca/en/transfusion/publications/surveillance-report. Last accessed 2021‐09‐28.
- 13. Abe K, Hu Q, Mozafarihashjin M, Samson R, Manguiat K, Robinson A, et al. Neutralizing antibody responses to SARS‐CoV‐2 variants in vaccinated Ontario long‐term care home residents and workers. medRxiv. 2021;21261721. 10.1101/2021.08.06.21261721. [DOI] [Google Scholar]
- 14. Isho B, Abe K, Zuo M, Jamal A, Rathod B, Wang J, et al. Persistence of serum and saliva antibody responses to SARS‐CoV‐2 spike antigens in COVID‐19 patients. Sci Immunol. 2020;5:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mendoza E, Manguiat K, Wood H, Drebot M. Two detailed plaque assay protocols for the quantification of infectious SARS‐CoV‐2. Curr Protoc Microbiol. 2020;57:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu J, Budylowski P, Samson R, Griffin B, Babuadze G, Rathod B, et al. Preclinical evaluation of a SARS‐CoV‐2 mRNA vaccine PTX‐COVID19‐B. bioRxiv. 2021;443286. 10.1101/2021.05.11.443286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C, Ginn H, Dejnirattisai W, Supasa P, Wang B, Tuekprakhon A, et al. Reduced neutralization of SARS‐CoV‐2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel‐Benhassine F, Rajah M, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature. 2021;596:276–80. [DOI] [PubMed] [Google Scholar]
- 19. Wang P, Casner R, Nair M, Wang M, Yu J, Cerutti G, et al. Increased resistance of SARS‐CoV‐2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kunze K, Johnson P, van Helmond N, Senefeld J, Petersen M, Klassen S, et al. Mortality in individuals treated with COVID‐19 convalescent plasma varies with the geographic provenance of donors. Nat Commun. 2021;12:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


