Abstract
Quercetin, widely distributed in fruits and vegetables, is a flavonoid known for its antioxidant, antiviral, antimicrobial, and antiinflammatory properties. Several studies highlight the potential use of quercetin as an antiviral, due to its ability to inhibit the initial stages of virus infection, to be able to interact with proteases important for viral replication, and to reduce inflammation caused by infection. Quercetin could also be useful in combination with other drugs to potentially enhance the effects or synergistically interact with them, in order to reduce their side effects and related toxicity. Since there is no comprehensive compilation about antiviral activities of quercetin and derivates, the aim of this review is providing a summary of their antiviral activities on a set of human viral infections along with mechanisms of action. Thus, the following family of viruses are examined: Flaviviridae, Herpesviridae, Orthomyxoviridae, Coronaviridae, Hepadnaviridae, Retroviridae, Picornaviridae, Pneumoviridae, and Filoviridae.
Keywords: antiviral, coronavirus, HCV, HIV, influenza virus H1N1, quercetin
1. INTRODUCTION
Quercetin (3,3′,4′,5,7‐pentahydroxy‐2‐phenylchromen‐4‐one) is the major representative of the flavonoid subclass of flavonols. It is found in many fruits and vegetables and, among vegetables, the highest levels of quercetin have been found in onions (Allium cepa L.), asparagus (Asparagus officinalis L.), and red leaf lettuce (Lactuca sativa L.), while lower levels in broccoli, green peppers, peas, and tomatoes. Apples are the fruits with the highest quercetin content, together with cherries and various berries (Nishimuro et al., 2015).
The quercetin in foods is not present as aglycone (i.e., without sugar groups), but as quercetin glycosides (Kawabata et al., 2015). Once ingested, quercetin glycosides are hydrolyzed and the released aglycone is absorbed and metabolized, giving rise to glucuronidated, methylated, and sulfated derivatives.
The dietary intake of all flavonoids has been estimated to over 200 mg/day, while intake of flavonols is about 20 mg/day, of which quercetin is more than 50%, with a daily intake of approximately 10 mg/day (Kawabata et al., 2015). A study carried out in Japan supported these estimates, as daily intake of quercetin was determined to be 16 mg/day (Guo & Bruno, 2015).
Quercetin supplementation may promote antioxidant (Xu et al., 2019), antiinflammatory, immunoprotective effects (Saeedi‐Boroujeni & Mahmoudian‐Sani, 2021), anticarcinogenic, antidiabetic activities (Carrasco‐Pozo et al., 2016; Carullo et al., 2017; Rauf et al., 2018) and can prevent many chronic diseases (Zeng et al., 2020), added to the ability to inhibit lipid peroxidation, platelet aggregation, capillary permeability, and to stimulate mitochondrial biogenesis (Aguirre et al., 2011). Due to its high solubility and bioavailability, quercetin is being used, increasingly, in new preparations for human health care (Aytac et al., 2016). Furthermore, quercetin has been studied in various types and models of viral infection due to its promising antiviral effects in inhibiting polymerases, reverse transcriptase, proteases, suppressing DNA gyrase, and binding viral capsid proteins (Bachmetov et al., 2012; Debiaggi et al., 1990; Shinozuka et al., 1988; Spedding et al., 1989). A large number of small molecules extracted from plants are known for their antiviral effects (di Petrillo et al., 2017; Singh et al., 2020), while no antiviral drugs coming from plant constituents have been approved so far. The reason could be the problematic assessing of the safety and effectiveness of herbal medicines, due to herbal adverse events and herb–drug interactions (Izzo et al., 2016).
A strategy could be to enhance people's antiviral immune response through a nutritious diet including pure quercetin, isolated from natural extracts, in order to minimize the risk of infections.
Recently, quercetin has been used as adjuvant therapy in COVID‐19 symptomatic patients and the result was an improvement in clinical symptoms and a reduction in length of hospitalization (di Pierro et al., 2021).
This review aims to collect and present the current knowledge of the antiviral property of quercetin and its mechanisms of action, focusing the attention on the major human viruses belonging to families of Flaviviridae, Herpesviridae, Orthomyxoviridae, Coronaviridae, Hepadnaviridae, Retroviridae, Picornaviridae, Pneumoviridae, and Filoviridae.
2. ANTIVIRAL ACTIVITY OF QUERCETIN
2.1. Flaviviridae
Flaviviridae is a family of enveloped positive‐strand RNA viruses. The family includes the genera of Flavivirus, Pestivirus, Hepacivirus, and Pegivirus. Hepatitis C virus belongs to the Hepacivirus genus and is the major cause of viral hepatitis, with an estimated 71.1 million individuals chronically infected worldwide (Roudot‐Thoraval, 2021). Treatment with direct‐acting antivirals drugs has dramatically changed outcomes of hepatitis C. Indeed, the sustained viral response rates have reached unprecedented levels (>95%) without relevant adverse events (Kowdley et al., 2014; Webster et al., 2015). However, the price is still one of the major barriers to achieve hepatitis C eradication mainly in low‐ and middle‐income countries.
Several studies, summarized in Table 1, show the role of quercetin as an antiviral against HCV, in which it was reported that quercetin acts at different levels.
TABLE 1.
Anti‐HCV activities of quercetin and derivates
| Compound | Mechanism | References |
|---|---|---|
| Quercetin | Reduces HSP70 and NS5A levels demonstrating its effect on IRES translation | Khachatoorian et al. (2012) |
| Quercetin | Inhibits the cleavage of the engineered NS3 | Bachmetov et al. (2012) |
| Quercetin | Inhibits HCV‐induced ROS and RNS formation in HCV‐replicating cells through its antioxidant activity | Pisonero‐Vaquero et al. (2014) |
|
Quercetin‐7‐O‐arylmethylquercetins Quercetin‐3‐O‐benzoic acid esters |
Compounds are capable of establish key coordination with the two magnesium ions as well as interactions with residues at the active site of HCV NS5B | Zhong et al. (2015) |
| Quercetin | Docking results provide that Que is a potent inhibitor of NS2 protease of HCV | Sajitha Lulu et al. (2016) |
Note: HCV, hepatitis C virus; HSP70, heat shock protein 70; IRES, internal ribosome entry site; Que, Quercetin; RNS, reactive nitrogen species; ROS, reactive oxygen species.
Recently, in vitro studies performed on cells have identified the possible interaction of quercetin with HCV's nonstructural (NS) protein.
Khachatoorian et al. (2012), tested the flavonoid for their antiviral activity using the HCV cell culture system treating it 3 h after infection. The study showed how quercetin markedly blocks viral translation, completely blocks NS5A‐augmented Internal Ribosome Entry Site (IRES)‐mediated translation in an IRES reporter assay and inhibits HSP70 induction, assuming that the antiviral activity of quercetin is mediated through different mechanisms (Khachatoorian et al., 2012). Moreover, the inhibitory effect of quercetin was also obtained using a model system in which NS3 engineered substrates were introduced in NS3‐expressing cells, providing evidence that inhibition could be directed to the NS3. In particular, these cells expressed only NS3 protease and did not carry additional HCV sequences, neither NS5A activities nor IRES translation (Bachmetov et al., 2012). In another study, quercetin showed a marked anti‐HCV activity in replicon containing cells when combined with interferon (IFN)α. Quercetin decreased HCV‐induced reactive oxygen and nitrogen species (ROS/RNS) generation and lipoperoxidation in replicating cells. Quercetin also inhibited liver X receptor (LXR) α‐induced lipid accumulation in LXRα‐overexpressing and replicon‐containing Huh7 cells. This activity might contribute to the inhibitory effect of quercetin on HCV replication (Pisonero‐Vaquero et al., 2014). The effect of quercetin on the expression of key genes involved in lipid metabolism was confirmed by other studies. In particular, quercetin appears to reduce diglyceride acyltransferase 1 (DGAT1) mRNA expression increased by viral infection. Since HCV particle formation requires DGAT1, this could be another quercetin mechanism of action (Rojas et al., 2016).
Molecular docking, increasingly used for the research of new drugs, provides that quercetin and its derivates can establish key coordination with NS5B, an RNA‐dependent RNA polymerase, by two magnesium ions as well as interactions with residues at the active site (Zhong et al., 2015), this gives further evidence to previous studies in which N5 was the protagonist of a possible mechanism of action on HCV inhibition. Meanwhile, another molecular docking study showed that quercetin is a potential inhibitor of NS2 protease (Sajitha Lulu et al., 2016), as they possess minimum binding energy of consecutively −7.97 and −7.95 kcal/mol—which is even lower than the three drugs used as control, including ribavirin (−5.89 kcal/mol) and telbivudine (−6.39 kcal/mol) (Lu et al., 2016).
The high inhibition efficiency of viral production could be due to its ability to block virus replication by interacting on different proteases and its ability to reduce HCV‐induced ROS/RNS. Quercetin could be used as a support in conventional therapies, considering it has excellent tolerability.
2.2. Herpesviridae
Herpesviridae comprises a large, enveloped family of double‐stranded DNA viruses. Herpesviruses are divided into three subfamilies, alpha (α), beta (β), and gamma (γ) herpesviridae based on biological properties. The α herpesviruses, herpes simplex virus types 1 and 2 (HSV‐1, HSV‐2), and varicella‐zoster virus (VZV), have a short replicative cycle, induce cytopathology in monolayer cell cultures, and have a broad host range; β herpesviruses, cytomegalovirus (CMV), and human herpesviruses 6 and 7, with a long replicative cycle and restricted host range; and γ herpesviruses, Epstein–Barr virus (EBV) and human herpesvirus 8, with a very restricted host range.
The herpesviruses that infect humans characteristically establish a latent infection that may be reactivated later. The consequences of reactivation range from asymptomatic shedding to severe disseminated infection (Whitley, 1996). Therapies that can target the latent phase of these viral infections could potentially result in eradication.
It has long been known that quercetin and derivates show antiviral effect on Herpesviridae, particularly HSV‐1 and HSV‐2 (Table 2).
TABLE 2.
Anti Herpesviridae activities of quercetin and derivates
| Compound | Mechanism | References |
|---|---|---|
| Quercetin | Inhibits production of IEG and IEP with IC50 145 μM for HSV and 2.89 μM for HCMV | Cotin et al. (2012) |
| Quercetin Isoquercetin | Inhibits HSV entry and inhibit NF‐κB activation | Hung et al. (2015) |
| Quercetin | Suppresses the activations of IRF3 and NF‐jB induced by HSV‐1 infection in a TLR3‐dependent manner, and this resulted in reduced TNF‐α production in raw 264.7 cells | Lee et al. (2017) |
| Quercetin | Counteracts EBV‐driven immortalization of B cells and LCL outgrowth. This effect seems to occur interrupting the crosstalk between IL‐6 and STAT3, promoted autophagy, and reduced ROS levels and p62 accumulation | Granato et al. (2019) |
| Quercetin | Inhibit viral lytic gene expression and replication through the downregulation of IEG of VZV and HCMV (IE2) | Kim et al. (2020) |
| Isoquercetin |
Note: EBV, Epstein–Barr virus; IC50, half‐maximal inhibitory concentration; IEG, immediate early genes; IEP, immediate early protein; HCMV, human cytomegalovirus; HSV‐1, Herpes Simplex Virus type‐1; IL‐6, interleukin‐6 virus; LCL, lymphoblastoid cell line; TLR, toll‐like receptor, TNF, tumor necrosis factor; VZV, Varicella Zoster virus.
In vitro studies showed the reduction of intracellular replication of HSV1‐2 and human cytomegalovirus (HCMV) when cell monolayers were infected and subsequently cultured in medium containing quercetin, instead preincubation of tissue culture cell monolayers with quercetin did not affect the ability of the viruses to infect or replicate in the tissue culture monolayers.
Quercetin showed antiviral activity toward HCMV infected cells in a concentration of 4.8 μM. It was found that quercetin partially inhibited the production of Immediate Early Protein and strongly inhibited Early Protein production, suggesting that the flavonol operates at a time point between immediate early and early protein expression (Cotin et al., 2012).
To evaluate the anti‐HSV‐1 effect of quercetin, Raw 264.7 cells were infected with HSV‐1 at 0.1 multiplicity of infection (MOI) in presence or absence of quercetin. In another set of experiment cells were first infected with HSV‐1 at 0.1 MOI and, 2 h later, quercetin was added. In both cases a similar decrease in plaque formation was found (50% decrease for 10 μg/ml). In order to find the molecular mechanism responsible for the anti‐HSV‐1 effect, western blotting and real time PCR were performed on several proteins and HSV genes. Interestingly, quercetin specifically inhibited the expressions of HSV proteins: glycoprotein D (gD) and Infected Cell Protein 0 (ICP0) gD is essential for successful viral entry and ICP0 is encoded after infection. These results suggest that quercetin affects both viral entry and viral replication. Moreover, quercetin suppressed the expression of TLR‐3, member of the toll‐like receptor (TLR) family which plays a fundamental role in pathogen recognition, and this led to the inhibition of inflammatory transcriptional factors (NF‐κB and IRF3). These findings suggest that the anti‐HSV‐1 effects of quercetin are related to the suppression of TLR‐3 dependent inflammatory responses in infected cells (Lee et al., 2017).
Quercetin and isoquercitrin displayed potent antiviral activities against both VZV and HCMV. Both compounds strongly suppressed the expression of lytic immediate–early genes (IEG) (Kim et al., 2020).
Nevertheless, Hung et al., (2015), have shown how Houttuynia cordata water extract, which has quercetin and isoquercetin among the major components, has an antiviral activity against HSV‐1 and HSV‐2 and is HSV aciclovir resistant (HSV‐AR). To elucidate the mechanism of action, cells infected with 100 pfu of the viruses were co‐treated or pretreated with the extract. Pretreatment showed that plaque formation was largely inhibited, assuming that the anti‐HSV effects of the extract might target virus particles directly and inhibit further stages of HSV infection. In the co‐treatment, on the other hand, the extract inhibited HSV‐1, HSV‐2, and HSV‐AR binding ability in a dose‐dependent manner. By analyzing the major components, it was found, that quercetin and isoquercetin targeted virus particles directly and inhibited viral entry. Furthermore, it was identified that both isoquercitin and quercetin inhibited NF‐κB activation in HSV infection (Hung et al., 2015).
Regarding EBV, latent EBV infection can lead to serious malignancies, such as, Burkitt's lymphoma, Hodgkin's disease, and gastric carcinoma, and EBV associated gastric carcinoma is one of the most common EBV‐associated cancers.
In vitro study performed on EBV‐driven B cell immortalization showed that quercetin inhibits the activation of signal transducer and activator of transcription 3 (STAT3) induced by EBV infection and reduce molecules such as interleukin‐6 (IL‐6) and ROS known to be essential for the immortalization process. Moreover, it was found that quercetin promoted autophagy and counteracted the accumulation of sequestosome1/p62, ultimately leading to the prevention of B cell immortalization. These findings suggest that quercetin may have the potential to be used to counteract EBV‐driven lymphomagenesis, especially if its stability is improved (Granato et al., 2019).
These results indicate that quercetin could be a promising candidate anti‐HSV and HSV‐AR since its mechanism of action seems to be able to bind the virus in the early stages and prevent its entry.
2.3. Orthomyxoviridae
The Orthomyxoviridae is a family of viruses that possesses segmented, single‐stranded, and negative‐sense RNA genome. It comprises the genera Influenzavirus A, Influenzavirus B, Influenzavirus C, Influenzavirus D Thogotovirus, Quaranjavirus, and Isavirus (Noda, 2012). Influenza A virus is one of the most important pathogens to our public health, causing worldwide outbreaks and seasonal pandemics, seriously impacting public health, as well as the economy.
Currently, two classes of anti‐influenza drugs are available, one targeting the matrix 2 (M2) ion channel and the other targeting neuraminidase (NA) expressed on the virus envelope, and important for virus entry. Along with the determination of the NA structure and the discovery of Structure–Activity Relationship (SAR) in recent years, the basis of rational design for novel, potent NA inhibitors (NAIs) is valid. Unfortunately, the resistance against current anti‐influenza drugs and the emerging mutations of influenza virus itself limits their development and effectiveness (McKimm‐Breschkin, 2000; Sheu et al., 2011; Spanakis et al., 2014). Thus, there is a strong need to explore new antiviral drugs against influenza virus. The new approaches consist in finding new active small molecular inhibitors such as metal ion chelator that inhibit the RNA‐dependent RNA polymerase and immunomodulators (Wu et al., 2017).
Natural products appear to be a major source of anti‐influenza drug discovery and offer new prospects for influenza management.
Several in vitro studies performed on cells showed anti‐influenza A activity of quercetin and derivates (Table 3), particularly they seem to have effect in the initial stage of infection. In a study, influenza virus was inoculated to the cells at a MOI of 0.05 or 5, and the virus‐infected cells were incubated in the presence of quercetin and isoquercetin in different concentration and different stage of infection. Isoquercetin showed the highest antiviral activity and the incubation of cells with isoquercetin prior to viral infection followed by extensive washing significantly inhibited viral replication, with an effective dose for 50% reduction on viral replication of 1.2 μM. The addition of isoquercetin to cells up to 4 h following viral infection reduced virus replication in a time‐dependent manner. These results suggest that the antiviral mechanism of isoquercetin may involve early stages of viral replication (Kim et al., 2010). The antiviral activity of quercetin and isoquercetin was also confirmed by a subsequent study by the same authors (Thapa et al., 2012).
TABLE 3.
Anti‐influenza activities of quercetin and derivates
| Compound | Mechanism | References |
|---|---|---|
| Quercetin | Shows strong binding abilities to NA from H1N1 (A/PR/8/34) comparable with zanamivir | Liu et al. (2016) |
| Quercetin‐7‐O‐glucoside | Blocks RNA polymerase of influenza viruses A and B and completely inhibits or reduces AVO formation | Gansukh et al. (2016) |
| Quercetin‐3‐O‐α‐L‐rhamnopyranoside | Decreases the H1N1 viral titer by 6 logs (p < 0.01) in MDCK cells with IC50 25 μg/ml, decreases NP and M2 genes copy numbers and the expression of cytokines | Mehrbod et al. (2018) |
| Quercetin | Molecular docking exhibit relatively high potential for binding quercetin to the active site of neuraminidase N1 | Sadati et al. (2019) |
| Quercetin derivates | Show high binding activity on cap‐binding site of the PB2 of influenza viral RNA polymerase | Gansukh et al. (2021) |
Note: AVO, acidic vesicular organelles; IC50, half‐maximal inhibitory concentration; M2, matrix 2 ion channel; MDCK, Madin–Darby canine kidney; NA, neuroamminidase; PB2, polymerase basic 2.
A quercetin derivative, quercetin‐3‐O‐α‐l‐rhamnopyranoside (Q3R), isolated from Rapanea melanophloeos, was able to significantly decrease copy numbers of M2 and NP genes in co‐penetration treatment, which confirms the blockage of the viral particle receptors from penetration inside the cell. Thus, fewer viral particles propagated inside the cell. No significant effect in pre‐ and post‐penetration treatments verified the inability of the compound to influence the cellular receptors and probably the cellular pathways. Moreover, the study revealed that Q3R, especially in the co‐penetration treatment, alters the status of cytokine production, increasing the IL‐27 production significantly, which has antiinflammatory properties, and decreasing the TNF‐α production, a pro‐inflammatory substance (Mehrbod et al., 2018).
In vivo study on mouse model of influenza virus, showed how quercetin derivatives significant decreased mortality, reduced the levels of IFN‐γ, iNOS, and RANTES in the lungs compared to the untreated group and histological evaluation showed that delayed the development and progression of pulmonary lesions (Choi et al., 2012; Kim et al., 2010; Liu et al., 2016).
Molecular docking studies showed the potential target of quercetin. In a study, quercetin appears to have a high binding potential to NA comparable with oseltamivir and zanamivir. In a first study, (Liu et al., 2015) it was showed how a R294K mutation in NA could remarkably decrease the binding energies for oseltamivir, while other small molecules, like quercetin, showed stable binding abilities with mutated NA. In a second study, it was observed that there were 21 H‐bonds between zanamvir and 11 residues of NA and that there were 17 H‐bonds between quercetin and NA. Abundant H‐bonds indicated that the H‐bonds were important for the binding between small molecules and NA, so quercetin could serve as a leading molecule as NAI (Liu et al., 2015; Liu et al., 2016; Sadati et al., 2019).
Another potential target of quercetin could be M2 protein: docking simulations predicted that the binding affinity of quercetin (−5.35 kcal/mol) for the M2 proton channel protein is stronger than amantadine (−4.52 kcal/mol) (Moorthy et al., 2014).
Quercetin derivatives have shown the ability to block viral RNA polymerase, in particular quercetin‐7‐O‐glucoside (Q7G) showed strong inhibition activity (Gansukh et al., 2016). A recent study was conducted to confirm this inhibitory activity: 410 quercetin derivatives were screened using molecular docking on cap‐binding site of the polymerase basic 2 (PB2) subunit of influenza viral RNA polymerase; all quercetin derivatives showed high binding affinity, with quercetin 3′‐glucuronide (Q3G) that showed strongest binding affinity toward cap‐binding site of the PB2 subunit with −9.6 kcal of binding affinity and 0.00054 mM of Ki value (Gansukh et al., 2021).
As already mentioned, quercetin seems to block virus entry, the initial step of the viral replication cycle, through the interaction with influenza NA protein. Moreover, other mechanisms of action could be the interaction with M2 and NA genes, RNA polymerase, and reduction of cytokines expression.
2.4. Coronaviridae
Coronaviridae is a family of enveloped and positive‐strand RNA viruses, which includes two subfamilies: Coronavirinae and Torovirinae (Payne, 2017). Human coronaviruses, such as HCoV‐229E and HCoV‐OC43, have long been known to circulate in the population and they, together with the more recently identified HCoV‐ NL63 and HCoV‐ HKU1, cause seasonal and usually mild respiratory tract infections associated with symptoms of the “common cold.” In strong contrast, severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East Respiratory Syndrome coronavirus (MERS‐CoV) are highly pathogenic (van den Brand et al., 2015). The global pandemic of the new human coronavirus called SARS‐CoV‐2 causes respiratory tract disease COVID‐19. Currently, there are several safe and effective vaccines against SARS‐CoV‐2 but there is just one treatment authorized in the European Union (EU) to treat COVID‐19, consisting in remdesivir (“COVID‐19 treatments: authorised | European Medicines Agency,” 2020).
Recently several articles have been published on quercetin and its ability to protect against coronaviruses (Table 4). One of the most studied targets are main protease (Mpro), such as 3‐chymotrypsin‐like protease (3CLpro) and papain‐like protease (PLpro). The Mpro are responsible for the processing of the viral polyproteins synthesized from the viral RNA after infection, rendering the individual viral proteins active and functional and genes are highly conserved (Helmy et al., 2020). These characteristics making Mpro a good target for developing effective drugs against SARS‐CoV‐2 and other future coronavirus variants.
TABLE 4.
Anti‐coronavirus activities of quercetin and derivates
| Compound | Mechanism | References |
|---|---|---|
| Quercetin‐3‐β‐galactoside | Binds to the catalytic pocket of SARS‐CoV 3CLpro | Jo et al. (2020) |
| Quercetin | Top scoring ligand for the S protein: ACE2 receptor interface | Smith and Smith (2020) |
| Quercetin | Inhibits 3CLpro and PLpro, with a docking binding energy corresponding to −6.25 and −4.62 kcal/mol | di Pierro et al. (2021) |
| Quercetin | Interacts with furin with a docking score of −7.77 kcal/mol at 2.02 μM | Milanović et al. (2021) |
Note: ACE2, angiotensin‐converting enzyme II; HIV, human immunodeficiency virus; IC50, Half‐maximal inhibitory concentration; SARS‐CoV, severe acute respiratory syndrome coronavirus.
Molecular docking showed that quercetin inhibits 3CLpro and PLpro, with a docking binding energy corresponding to −6.25 and −4.62 kcal/mol, respectively (Derosa et al., 2021). So, quercetin has a theoretical, but significant, capability to interfere with SARS‐CoV‐2 replication (Abian et al., 2020). Previously quercetin inhibitory activity was tested on 3CLpro showing an inhibition rate of 82% at 200 μM (Nguyen et al., 2012).
Quercetin‐3‐β‐galactoside was also found as a potent binder to the catalytic pocket of SARS‐CoV 3CLpro. The predicted binding free energy, inhibitory constant, and the predicted LogP are −9.24 kcal/mol, 1.70 × 10−7, and 0.43, respectively. This compound showed inhibitory activity against SARS‐CoV 3CLpro with IC50 of 42.79 ± 4.97 μM in a competitive mode. Molecular modeling strongly suggested that the residue Q189 plays a key role in the binding between quercetin‐3‐β‐galactoside and SARS‐CoV 3CLpro (Chen et al., 2006; Jo et al., 2020). These studies show that quercetin have a good potential to act as COVID‐19 Mpro inhibitors (Agrawal et al., 2020; Derosa et al., 2021).
Another coronavirus target is viral the spike protein (S protein). In fact, a reasonable target for structure‐based drug discovery was identified to be the disruption of the viral S protein‐ angiotensin‐converting enzyme 2 (ACE2) receptor interface. A computational model of the S‐protein of SARS‐CoV‐2 interacting with the human ACE2 receptor was used to identify small molecules to potentially limit viral recognition of host cells and/or to disrupt host–virus interactions (Smith & Smith, 2020). A recent article assayed molecular docking between quercetin and two proteins: S protein and another protein, furin. Furin is a host‐cell enzyme responsible for the nonclathoin mediated fusion of membranes, which increases the probability of the entanglement of S protein with ACE2. The specific inhibitors of furin could prevent the cleavage of spikes and syncytium, therefore suppressing the virus reproduction. The results suggests that quercetin could interacts with furin since showed a high binding affinity for the neutral form of quercetin (−7.77 kcal/mol, 2.02 μM). In contrast the reactivity of quercetin on S protein is lower than for the investigated drugs, this was proved by the lower number of hydrogen and carbon–hydrogen bonds (Milanović et al., 2021). In Figure 1 we have schematized the interaction between quercetin‐3‐β‐galactoside and the COVID protease most represented in the works, 3CLpro. Quercetin, as well as other flavonoids, interact with target amino acids located around the catalytic site of each 3CLpro protomer. Quercetin‐3‐β‐galactoside forms hydrogen bonds specifically with Gln189 and Glu166 amino acids located inside a specific pocket hollowed in the protein surface (Waterhouse et al., 2018).
FIGURE 1.
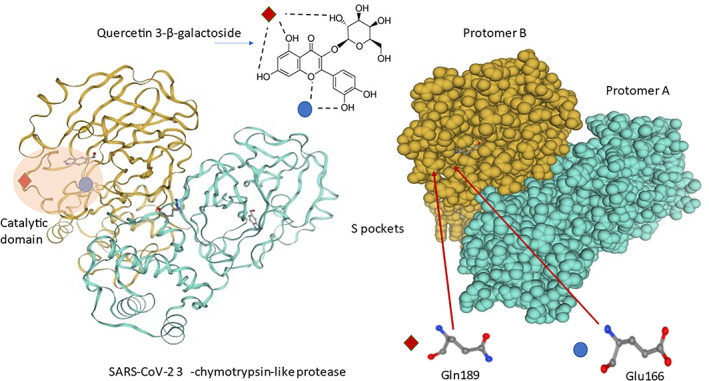
Molecular interactions between Quercetin‐3‐β‐galactoside and SARS‐CoV‐2 3CLpro. Note: Quercetin‐3‐β‐galactoside forms hydrogen bonds specifically with Gln189 and Glu166 amino acids located inside a specific pocket hollowed in 3CLpro surface. The three‐dimensional (3D) protein structures were created by using SWISS MODEL program
A clinical study evaluated the possible role of quercetin on prophylaxis and treatment of COVID‐19 since the immunomodulatory activity of quercetin could benefit patients with SARS‐CoV2.
In this study, quercetin supplement statistically shortens the timing of molecular test conversion from positive to negative and reducing at the same time symptoms severity (di Pierro et al., 2021).
In conclusion, quercetin seems to protect against coronavirus through different mechanisms of action: the most important studies regard the inhibition of proteins S, furin, and Mpro, important for cell recognition and viral replication. In addition, quercetin supplements could help to decrease symptoms and to reduce the time of positive molecular test. On this basis it would be interesting to carry out new clinical studies.
2.5. Hepadnaviridae
Hepadnaviridae is a family of small, enveloped viruses with partially double‐stranded DNA. It contains two genera: hepatitis viruses, specific for man and other mammals, are grouped in the genus Orthohepadnavirus, while those of birds are placed into the genus Avihepadnavirus.
Hepatitis B virus (HBV), the hepadnavirus infecting humans, is classified into eight genotypes today and numerous subgenotypes (Schaefer, 2007).
Nearly 350 million people are chronically infected with HBV in the world, which is one of major global health problems. It is estimated that about 780,000 people die each year due to consequences of hepatitis B (HB), such as liver cirrhosis and liver cancer (“Hepatitis B,” 2021). Current treatments include nucleoside/nucleotide analog therapy and interferon therapy. Long‐term, nucleoside/nucleotide analog therapy often leads to drug resistance, and interferon therapy can be used only for a limited duration due to its many side effects (Zoulim & Locarnini, 2009). B can be prevented by vaccines that are safe, available, and effective (“Hepatitis B,” 2021).
Several in vitro studies shown how quercetin inhibits HBV antigen, Hepatitis B surface antigen (HBsAg) and Hepatitis B e antigen (HBeAg), secretion and genome replication in human hepatoma cell lines (Table 5), which suggests that quercetin may be a potentially effective anti‐HBV agent (Alam et al., 2017; Cheng et al., 2015).
TABLE 5.
Anti‐HBV activities of quercetin
| Compound | Mechanism | References |
|---|---|---|
| Quercetin | Reduces HBsAg, HBeAg and the secretion of HBV genomic DNA levels. | Cheng et al. (2015) |
| Quercetin | Anti‐HBV potential, inhibiting HBsAg and HBeAg synthesis. Molecular docking shows that quercetin forms very stable complexes with HBV polymerase. | Parvez et al. (2019) |
| Quercetin | Has higher binding affinity toward Pol/RT than lamivudine. | Parvez et al. (2020) |
Note: HBeAg, HBV secretory protein “e” antigen; HBsAg, Hepatitis B surface antigen; HBV, Hepatitis B virus; IC50, half‐maximal inhibitory concentration; Pol/RT, polymerase/reverse‐transcriptase.
Furthermore, quercetin extracted from Guiera senegalensis has demonstrated the high anti‐HBV potential inhibiting HBsAg and HBeAg synthesis by 68% and 73%, respectively in cultured HepG2.2.2.15. In order to find the mechanism of action molecular docking was performed against HBV Polymerase (Pol/RT). Data shown that quercetin binds the active site of HBV Pol by forming nine hydrogen bonds that stabilized the quercetin‐pol complex with an estimated free energy of −7.4 kcal/mol (Parvez et al., 2019). The anti‐HBV modes of action and the molecular interaction patterns of quercetin with viral Pol/RT and Core proteins were determined as well as a host‐encoded receptor sodium taurocholate co‐transporting polypeptide. Quercetin shown better affinity toward Pol/RT as compared to lamivudine. The HBV‐Core protein is considered as a promising viral target for drug development because of its multiple roles in the viral life cycle (Parvez et al., 2020).
These in vitro studies show how quercetin can block HBV replication by acting on HBsAg, HBeAg, HBV polymerase, and Core protein.
2.6. Retroviridae
The family Retroviridae is a large family of RNA viruses that replicate through a DNA intermediate. It is divided into two subfamilies (Orthoretrovirinae and Spumaretrovirinae) and seven genera (Ryu, 2017). Since the mid 1980s, retroviruses have been the focus of an intensive research effort, due primarily to the causative association between the human immunodeficiency virus (HIV) and the acquired immunodeficiency syndrome (AIDS).
Highly active antiretroviral therapy (HAART) is a medication regimen used in the management and treatment of human immunodeficiency virus type 1 (HIV‐1). However, the treatment is required to be administered for the remainder of an individual's lifetime due to latent HIV‐1 reservoirs. The “shock and kill” strategy, which involves using agents to reactivate latent HIV‐1 and subsequently killing latently infected cells in the presence of HAART, was recently proposed. Unfortunately, the agents used showed a high toxicity. Therefore, the identification of novel latency activators is required (Yang et al., 2018).
In vitro enzyme inhibitory properties showed a quercetin inhibition power against HIV‐1 integrase (HIV‐1 IN) and topoisomerase II with IC50 11.0 and 19.4 μM respectively (Fesen et al., 1993).
Compounds isolated from Dioscorea bulbifera, such as, quercetin‐3‐O‐β‐d‐glucopyranoside and quercetin‐3‐O‐β‐d‐galactopyranoside, showed inhibitory activity against HIV‐1 IN inhibitors with IC50 value19.39 and 21.80 μM respectively. The docking results showed that all compounds bound to the IN with similar patterns (Chaniad et al., 2016). This result confirms the inhibitory activity against integrase, established in the previous study.
Quercetin reactivated latent HIV‐1 gene expression. In addition, there is evidence that quercetin may reactivate HIV‐1 expression by inducing nuclear factor κB nuclear translocation and that the toxicity of quercetin is lower when compared with various additional activators of HIV‐1 (Yang et al., 2018).
In conclusion, as shown in Table 6, quercetin seems to inhibit in vitro HIV‐1 replication acting on integrase and topoisomerase II; however, these results need to be confirmed in other studies to fully demonstrate its inhibitory potential.
TABLE 6.
Anti‐HIV activities of quercetin and derivates
| Compound | Mechanism | References |
|---|---|---|
| Quercetin | Strong inhibitory activity on HIV‐1 IN with IC50 11.0 μM and TOP2, IC50 19.4 μM | Fesen et al. (1993) |
|
Quercetin‐3‐O‐β‐d‐glucopyranoside; Quercetin‐3‐O‐β‐d‐galactopyranoside |
Compounds inhibit HIV‐1 IN with IC50 19.4 and 21.8 μM, respectively | Chaniad et al.( 2016) |
| Quercetin | Reactivates latent HIV‐1 gene expression and induces nuclear factor κB nuclear translocation | Yang et al. (2018) |
Note: HIV‐1, human immunodeficiency virus type 1; IC50, half‐maximal inhibitory concentration; IN, integrase; TOP2, topoisomerase 2.
2.7. Picornaviridae
Picornaviruses are small, nonenveloped, icosahedral RNA viruses with positive‐strand polarity. Although many of picornavirus infections remain asymptomatic, some are important human and animal pathogens and cause diseases that affect different tissues. Genera associated with Picornaviridae include erbovirus, teschovirus, kobuvirus, aphthovirus, cardiovirus, enterovirus, coxsackievirus, hepatovirus, parechovirus, and rhinovirus (Zell, 2018).
Enteroviruses are implicated in many diseases, including undifferentiated febrile illnesses, upper and lower respiratory tract infections, gastrointestinal disturbances, conjunctivitis, skin and mucous membrane lesions, and diseases of the central nervous system, muscles, heart, and liver. There is no established specific therapy. Treatment is symptomatic and supportive. Clinical studies show that ribavirin shortens respiratory illnesses and interferon nasal sprays have prophylactic value for common colds.
In in vitro studies, quercetin was demonstrated to possess concentration‐dependent anti‐enteroviral activities with the IC50 values of 39.63 μg/ml for Enterovirus 71 (EV71) and 59.53 μg/ml for Coxsackievirus A16 (CVA16). Cells were infected with EV71 or CVA16 at a titer of 100 a plaque‐forming unit (pfu) and simultaneously treated with quercetin (Wang et al., 2012). Further investigation determined that quercetin acts on EV71 with different mechanism of action: inducing apoptosis and cell damage, inhibiting viral RNA and protein synthesis, and inhibiting the protease 3Cpro (Yao et al., 2018).
Dihydroquercetin (DHQ) showed antiviral properties against Coxsackievirus B4 (CVB4) reducing viral titers at 100 μg/ml. The highest efficacy of the antiviral therapy was reached when DHQ is added 1–3 h postinfection demonstrating, even in this case, that it acts in the early stages of virus reproduction. Moreover, the effect of DHQ on the course of pancreatitis of white mice caused by CVB4 was analyzed. Mice were infected with CVB4 and infectious titer reached 6.0 ± 0.7 TCID50 (Median Tissue Culture Infectious Dose) per 20 mg tissue by day 5 and then gradually decreased. Based on these results, the effect of DHQ on the peak of virus titer was measured, applying it intraperitoneally at doses of 75 or 150 mg/kg/day. The compounds decreased the virus titer in dose‐dependent manner and the maximal effect was achieved at the highest dose of DHQ with a result comparable with the reference compound ribavirin (Galochkina et al., 2016).
Rhinovirus (RV), an Enterovirus, is responsible for majority of common colds. There are no approved drugs are available to treat rhinovirus infection. Quercetin shown, both in vitro and in vivo an anti‐rhinovirus activity. Quercetin pretreatment significantly decreased endocytosis of both RV1B and RV39 by BEAS‐2B cells with a MOI of 1.0. The cells treated with quercetin 8 h prior to infection there were no antiviral effects instead addition of quercetin during or immediately after viral infection may be necessary to effectively limit viral infection. In vivo, quercetin treatment decreased viral replication, expression of pro‐inflammatory cytokines and chemokines, and airways hyperresponsiveness to methacholine challenge. (Ganesan et al., 2012).
Quercetin and derivatives seem to inhibit Picornaviridae family by acting on early stage of virus replication, even in this case the maximum antiviral activity was found by treating the cells with quercetin after they were infected or during the infection. Furthermore, the ability to inhibit pro‐inflammatory cytokines, hence the ability of quercetin to act on the immune system, was observed also in this case.
In Table 7 the summary of the antiviral activity against picornavirus is reported.
TABLE 7.
Anti picornavirus activities of quercetin and derivates
| Compound | Mechanism | References |
|---|---|---|
| Quercetin | Inhibits viral infection at multiple stages, including endocytosis, transcription of the viral genome and viral protein synthesis. | Ganesan et al.(2012) |
| Dihydroquercetin | Shows antiviral properties against CVB4 | Galochkina et al. (2016) |
| Quercetin | Inhibits EV71 replication in RD cells with IC50 12.1 μM and Vero cells with IC50 8.8 μM. Moreover, Que acts inhibiting 3Cpro with IC50 30.3 μM. | Yao et al. (2018) |
Note: 3Cpro, 3C protease; CVA16, coxsackievirus A16; EV71, Enterovirus 71; CVB4, coxsackievirus B4; IC50, half‐maximal inhibitory concentration; RD cells, rhabdomyosarcoma cells; Que, Quercetin.
2.8. Pneumoviridae
The family Pneumoviridae comprises large enveloped negative‐sense RNA viruses. This family has two genera, Orthopneumovirus and Metapneumovirus. Some viruses are specific and pathogenic for humans, such as human respiratory syncytial virus (RSV) and human metapneumovirus (Rima et al., 2017).
HumanRSV causes a globally prevalent respiratory infection, which can cause life‐threatening illness, particularly in the young, elderly, and immunocompromised. The mainstay of treatment for patients with RSV is supportive care.
Several studies investigated the quercetin mechanism of action by molecular docking (Table 8). A research analyzed Non‐Structural Protein 1 (NS1)‐quercetin interaction. NS1 is an RSV non‐structural protein plays an important role in the modulation of the host response to infection, antagonizing the interferon‐mediated antiviral state (Atreya et al., 1998). Experimental and in silico approaches showed that the interaction between RSV‐NS1 and quercetin is stable, with a dissociation constant of the order of 10−6 M. Thus, NS1 could be a quercetin potential target (Gomes et al., 2016).
TABLE 8.
Anti Pneumoviridae activities of quercetin and derivates
| Compound | Mechanisms | References |
|---|---|---|
| Quercetin | Protects cells form MPV infection and reduces cytokine and chemokine secretion. | Komaravelli et al. (2015) |
| Quercetin | Interacts with NS1 protein, the enthalpy and entropy balanced forces indicated that the NS1‐quercetin interaction presented both hydrophobic and electrostatic contributions. | Gomes et al. (2016) |
| Quercetin | Interacts with M2‐1 protein and that hydrogen bonds and stacking interactions are important contributions for stabilization of the complexes. | Guimarães et al. (2018); Teixeira et al. (2017) |
| Quercetin and acetylated derivatives | Acetylated derivatives protect HEp‐2 cells infected with RSV and interact with F‐protein showing ΔG = −14.22 kcal/mol | Lopes et al. (2020) |
Note: HEp‐2 cells, human epithelial type 2 cells; MPV, metapneumovirus; NS1, nonstructural protein 1; RSV, respiratory syncytial virus.
Other research suggest that quercetin and its derivatives exert their action by interacting with the M2‐1 protein, involved in genome replication and transcription by form the complex RNA‐dependent RNA polymerase. Molecular docking showed that these compounds could interact with M2‐1 in important domains for its activity (Guimarães et al., 2018; Teixeira et al., 2017).
Lopes et al., showed alternative anti‐RSV strategy, quercetin, and acetylated derivatives may interact with F‐protein on RSV surface in an important region to adhesion and viral infection. Through molecular docking quercetin acetylated interacted with F‐protein showing ΔG = −14.22 kcal/mol and it was more stable than quercetin (Lopes et al., 2020).
Regarding human metapneumovirus (MPV), a major cause of respiratory tract infections in children, elderly, and immunocompromised hosts, no vaccine or treatment are currently available. There was explored the potential protective role of dietary antioxidants in MPV infection using in vitro model. MPV infection was associated with a significant increase in pro‐inflammatory cytokines, such as IL‐1, IL‐6, and TNF‐α, and the chemokines CXCL10 (IP‐10) and CCL4 (MIP‐1). Quercetin treatment significantly reduced these cytokine and chemokine secretion from infected cells. Moreover, quercetin treatment was associated with a much lower viral titer, compared to untreated infected cells. This study suggest that antioxidant diet interferes with viral assembly and formation of mature virus and can reduce oxidative damage and inflammatory responses (Komaravelli et al., 2015).
In conclusion quercetin could experts its antiviral action interacting with structural and non‐structural protein and reducing pro‐inflammatory cytokines.
2.9. Filoviridae
Filoviridae family comprise large enveloped negative‐sense RNA viruses. Among genus, Ebolavirus (EBOV) and Marburgvirus represent major threats to human health worldwide because they have extremely high death rates and antiviral therapies or vaccines against them are not available (He et al., 2015).
Like showed in Table 9, in vitro and in vivo study demonstrated that a flavonoid derivative called quercetin 3‐β‐O‐d‐glucoside (Q3G) had the ability to protect from EBOV. In vitro Q3G reduced viral titer, in vivo the mice were protected from EBOV even when Q3G was given as little as 30 min prior to infection showing a survival of 10/10. In contrast, only 3/10 mice that received Q3G at 24 h post challenge survived. To test the mechanism of the effect of Q3G on EBOV replication, they utilized wild‐type vesicular stomatitis virus (VSV) and VSV‐Ebola virus constructs in which the outer glycoprotein of VSV was replaced with the glycoprotein of EBOV. While the entry of wild‐type VSV was not inhibited by Q3G, entry of the VSV‐Ebola virus construct was strongly inhibited, suggesting that Q3G affects a glycoprotein‐mediated step in the viral life cycle, that is, viral entry (Qiu et al., 2016).
TABLE 9.
Anti Ebolavirus activities of quercetin and derivates
| Compound | Mechanisms | References |
|---|---|---|
| Quercetin 3‐β‐O‐d‐glucoside | Could inhibit the first stage of infection by a mechanism glycoprotein mediate | Qiu et al. (2016) |
| Quercetin | Interacts with VP24 and significantly suppresses anti‐IFN function | Fanunza et al. (2020) |
Note: IFN, interferon; VP24, viral protein 24.
Recently, a study hypothesized the use of flavonoids against EBOV proteins that cause a protective immune response, such as VP40, VP35, VP24, and VP30 proteins. The docking studies indicated that flavonoid compounds have strong hydrogen binding interactions and high docking score with Ebola proteins (Raj & Varadwaj, 2016). Based on this study, a recent in vitro study tested the effect of quercetin on VP24, one of the main determinants of virulence by virtue of its inhibition of the IFN signaling cascade. Results showed how quercetin significantly suppressed the anti‐IFN function of VP24, restoring IFN stimulation. In order to test if quercetin was also able to inhibit EBOV replication, wild‐type EBOV Makona‐infected cells were treated with quercetin and Q3G. Quercetin inhibited viral replication in HEK293T cells, while it was not able to inhibit EBOV in Vero E6 cells (Fanunza et al., 2020). This confirms the effect of quercetin on evasion of the IFN pathway, since inhibited EBOV only in IFN‐competent HEK293T cells and not in IFN‐incompetent Vero cells. In contrast, Q3G was able to block EBOV replication in both cell lines. It has been previously demonstrated that Q3G targets the viral entry process by Qiu et al. The fact that Q3G is more active in HEK293T cells than in Vero cells might suggest a dual mechanism of action: impairing the IFN antagonism of VP24 and blocking virus entry (Fanunza et al., 2020).
Quercetin could inhibit the first stage of infection interacting with structural protein and reducing anti‐IFN function of VP24. However, furthers and in vivo studies are needed.
3. CONCLUSIONS
Quercetin and its derivatives are naturally occurring phytochemicals with promising bioactive effects such as immunoprotective, antiinflammatory, and antiviral effects. In this review the antiviral activity of quercetin and its derivates against potential human viruses was collected. Quercetin showed a potent antiviral activity in vitro and the different mechanisms of action are reported in Figure 2.
FIGURE 2.
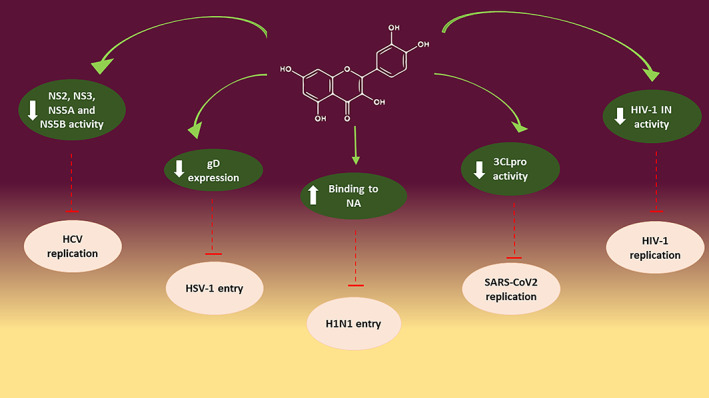
Highlight on different antiviral mechanisms of action of quercetin and derivates. Note: Quercetin blocks virus entry or virus replication through interaction with viral proteins
Particularly, quercetin seems to block virus entry by interacting with membrane glycoproteins such as gD of HSV and NA of H1N1. Moreover, molecular docking studies have shown that quercetin and its derivatives could interact with specific proteases essential for viral replication, such as NS2, NS3, and NS5A of HCV, integrase and TOP2 of HIV, Mpro of Coronaviridae, and 3Cpro of Enterovirus.
All these studies shown how the quercetin and its derivates have a wide spectrum of antiviral activities and a better understanding of quercetin's mechanistic properties could help in the rational design of more potent or bioavailable flavonol‐type compounds.
Despite many in vitro studies, there are very low studies with human subjects. It would be of extreme importance to pay attention to the use of quercetin for preventive purposes, as well as in combination with drugs, in order to enhance or synergistically interact with these chemical agents and consequently reduce their side effects, related toxicity, and increase their overall efficacy and safety.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
Di Petrillo, A. , Orrù, G. , Fais, A. , & Fantini, M. C. (2022). Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytotherapy Research, 36(1), 266–278. 10.1002/ptr.7309
Contributor Information
Amalia Di Petrillo, Email: amaliadipetrillo@gmail.com.
Germano Orrù, Email: orru@unica.it.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [repository name] at http://doi.org/[doi], reference number [reference number].
REFERENCES
- Abian, O. , Ortega‐Alarcon, D. , Jimenez‐Alesanco, A. , Ceballos‐Laita, L. , Vega, S. , Reyburn, H. T. , … Velazquez‐Campoy, A. (2020). Structural stability of SARS‐CoV‐2 3CLpro and identification of quercetin as an inhibitor by experimental screening. International Journal of Biological Macromolecules, 164, 1693–1703. 10.1016/j.ijbiomac.2020.07.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal, P. K. , Agrawal, C. , & Blunden, G. (2020). Quercetin: Antiviral significance and possible COVID‐19 integrative considerations. Natural Product Communications, 15(12). 10.1177/1934578X20976293 [DOI] [Google Scholar]
- Aguirre, L. , Arias, N. , Macarulla, M. T. , Gracia, A. , & Portillo, M. P. (2011). Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals Journal, 4, 189–198. 10.2174/1876396001104010189 [DOI] [Google Scholar]
- Alam, P. , Parvez, M. K. , Arbab, A. H. , & Al‐Dosari, M. S. (2017). Quantitative analysis of rutin, quercetin, naringenin, and gallic acid by validated RP‐ and NP‐HPTLC methods for quality control of anti‐HBV active extract of Guiera senegalensis. Pharmaceutical Biology, 55, 1317–1323. 10.1080/13880209.2017.1300175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya, P. L. , Peeples, M. E. , & Collins, P. L. (1998). The NS1 protein of human respiratory syncytial virus is a potent inhibitor of minigenome transcription and RNA replication. Journal of Virology, 72, 1452–1461. 10.1128/jvi.72.2.1452-1461.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytac, Z. , Kusku, S. I. , Durgun, E. , & Uyar, T. (2016). Quercetin/β‐cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chemistry, 197, 864–871. 10.1016/j.foodchem.2015.11.051 [DOI] [PubMed] [Google Scholar]
- Bachmetov, L. , Gal‐Tanamy, M. , Shapira, A. , Vorobeychik, M. , Giterman‐Galam, T. , Sathiyamoorthy, P. , … Zemel, R. (2012). Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. Journal of Viral Hepatitis, 19, e81–e88. 10.1111/j.1365-2893.2011.01507.x [DOI] [PubMed] [Google Scholar]
- Carrasco‐Pozo, C. , Tan, K. N. , Reyes‐Farias, M. , de La Jara, N. , Ngo, S. T. , Garcia‐Diaz, D. F. , … Borges, K. (2016). The deleterious effect of cholesterol and protection by quercetin on mitochondrial bioenergetics of pancreatic β‐cells, glycemic control and inflammation: In vitro and in vivo studies. Redox Biology, 9, 229–243. 10.1016/j.redox.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carullo, G. , Cappello, A. R. , Frattaruolo, L. , Badolato, M. , Armentano, B. , & Aiello, F. (2017). Quercetin and derivatives: Useful tools in inflammation and pain management. Future Medicinal Chemistry, 9, 79–93. 10.4155/fmc-2016-0186 [DOI] [PubMed] [Google Scholar]
- Chaniad, P. , Wattanapiromsakul, C. , Pianwanit, S. , & Tewtrakul, S. (2016). Anti‐HIV‐1 integrase compounds from Dioscorea bulbifera and molecular docking study. Pharmaceutical Biology, 54, 1077–1085. 10.3109/13880209.2015.1103272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Li, J. , Luo, C. , Liu, H. , Xu, W. , Chen, G. , … Jiang, H. (2006). Binding interaction of quercetin‐3‐β‐galactoside and its synthetic derivatives with SARS‐CoV 3CLpro: Structure‐activity relationship studies reveal salient pharmacophore features. Bioorganic and Medicinal Chemistry, 14, 8295–8306. 10.1016/j.bmc.2006.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Sun, G. , Guo, W. , Huang, Y. , Sun, W. , Zhao, F. , & Hu, K. (2015). Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virologica Sinica, 30, 261–268. 10.1007/s12250-015-3584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, H. J. , Song, J. H. , & Kwon, D. H. (2012). Quercetin 3‐rhamnoside exerts antiinfluenza A virus activity in mice. Phytotherapy Research, 26, 462–464. 10.1002/ptr.3529 [DOI] [PubMed] [Google Scholar]
- Cotin, S. , Calliste, C. A. , Mazeron, M. C. , Hantz, S. , Duroux, J. L. , Rawlinson, W. D. , … Alain, S. (2012). Eight flavonoids and their potential as inhibitors of human cytomegalovirus replication. Antiviral Research, 96, 181–186. 10.1016/j.antiviral.2012.09.010 [DOI] [PubMed] [Google Scholar]
- COVID‐19 treatments: authorised|European Medicines Agency [WWW Document] , Retrieved from https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/treatments-covid-19/covid-19-treatments-authorised. 2020.
- Debiaggi, M. , Tateo, F. , Pagani, L. , Luini, M. , & Romero, E. (1990). Effects of propolis flavonoids on virus infectivity and replication. Microbiologica, 13, 207–213. [PubMed] [Google Scholar]
- Derosa, G. , Maffioli, P. , D'Angelo, A. , & di Pierro, F. (2021). A role for quercetin in coronavirus disease 2019 (COVID‐19). Phytotherapy Research, 35, 1230–1236. 10.1002/ptr.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Petrillo, A. , Fais, A. , Pintus, F. , Santos‐Buelga, C. , González‐Paramás, A. M. , Piras, V. , … Frau, A. (2017). Broad‐range potential of Asphodelus microcarpus leaves extract for drug development. BMC Microbiology, 17, 159. 10.1186/s12866-017-1068-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Pierro, F. , Derosa, G. , Maffioli, P. , Bertuccioli, A. , Togni, S. , Riva, A. , … Ahmed, S. (2021). Possible therapeutic effects of adjuvant quercetin supplementation against early‐stage COVID‐19 infection: A prospective, randomized, controlled, and open‐label study. International Journal of General Medicine, 14, 2359–2366. 10.2147/IJGM.S318720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanunza, E. , Iampietro, M. , Distinto, S. , Corona, A. , Quartu, M. , MacCioni, E. , … Tramontano, E. (2020). Quercetin blocks ebola virus infection by counteracting the VP24 interferon‐inhibitory function. Antimicrobial Agents and Chemotherapy, 64(7), e00530–20. 10.1128/AAC.00530-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesen, M. R. , Kohn, K. W. , Leteurtre, F. , & Pommier, Y. (1993). Inhibitors of human immunodeficiency virus integrase. Proceedings of the National Academy of Sciences of the United States of America, 90, 2399–2403. 10.1073/pnas.90.6.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galochkina, A. v. , Anikin, V. B. , Babkin, V. A. , Ostrouhova, L. A. , & Zarubaev, V. v. (2016). Virus‐inhibiting activity of dihydroquercetin, a flavonoid from Larix sibirica, against coxsackievirus B4 in a model of viral pancreatitis. Archives of Virology, 161, 929–938. 10.1007/s00705-016-2749-3 [DOI] [PubMed] [Google Scholar]
- Ganesan, S. , Faris, A. N. , Comstock, A. T. , Wang, Q. , Nanua, S. , Hershenson, M. B. , & Sajjan, U. S. (2012). Quercetin inhibits rhinovirus replication in vitro and in vivo . Antiviral Research, 94, 258–271. 10.1016/j.antiviral.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansukh, E. , Kazibwe, Z. , Pandurangan, M. , Judy, G. , & Kim, D. H. (2016). Probing the impact of quercetin‐7‐O‐glucoside on influenza virus replication influence. Phytomedicine, 23, 958–967. 10.1016/j.phymed.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Gansukh, E. , Nile, A. , Kim, D. H. , Oh, J. W. , & Nile, S. H. (2021). New insights into antiviral and cytotoxic potential of quercetin and its derivatives—A biochemical perspective. Food Chemistry, 334, 127508. 10.1016/j.foodchem.2020.127508 [DOI] [PubMed] [Google Scholar]
- Gomes, D. E. , Caruso, Í. P. , de Araujo, G. C. , de Lourenço, I. O. , de Melo, F. A. , Cornélio, M. L. , … de Souza, F. P. (2016). Experimental evidence and molecular modeling of the interaction between hRSV‐NS1 and quercetin. International Journal of Biological Macromolecules, 85, 40–47. 10.1016/j.ijbiomac.2015.12.051 [DOI] [PubMed] [Google Scholar]
- Granato, M. , Gilardini Montani, M. S. , Zompetta, C. , Santarelli, R. , Gonnella, R. , Romeo, M. A. , … Cirone, M. (2019). Quercetin interrupts the positive feedback loop between STAT3 and IL‐6, promotes autophagy, and reduces ROS, preventing EBV‐driven B cell immortalization. Biomolecules, 9(9), 482. 10.3390/biom9090482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães, G. C. , Piva, H. R. M. , Araújo, G. C. , Lima, C. S. , Regasini, L. O. , de Melo, F. A. , … Souza, F. P. (2018). Binding investigation between M2‐1protein from hRSV and acetylated quercetin derivatives: 1H NMR, fluorescence spectroscopy, and molecular docking. International Journal of Biological Macromolecules, 111, 33–38. 10.1016/j.ijbiomac.2017.12.141 [DOI] [PubMed] [Google Scholar]
- Guo, Y. , & Bruno, R. S. (2015). Endogenous and exogenous mediators of quercetin bioavailability. Journal of Nutritional Biochemistry, 26, 201–210. 10.1016/j.jnutbio.2014.10.008 [DOI] [PubMed] [Google Scholar]
- He, B. , Feng, Y. , Zhang, H. , Xu, L. , Yang, W. , Zhang, Y. , … Tu, C. (2015). Filovirus RNA in fruit bats, China. Emerging Infectious Diseases, 21, 1675–1677. 10.3201/eid2109.150260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmy, Y. A. , Fawzy, M. , Elaswad, A. , Sobieh, A. , Kenney, S. P. , & Shehata, A. A. (2020). The COVID‐19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. Journal of Clinical Medicine, 9, 1225. 10.3390/jcm9041225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepatitis B [WWW Document] , 2021. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
- Hung, P.‐Y. , Ho, B.‐C. , Lee, S.‐Y. , Chang, S.‐Y. , Kao, C.‐L. , Lee, S.‐S. , & Lee, C.‐N. (2015). Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS One, 10, e0115475. 10.1371/JOURNAL.PONE.0115475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo, A. A. , Hoon‐Kim, S. , Radhakrishnan, R. , & Williamson, E. M. (2016). A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytotherapy Research, 30, 691–700. 10.1002/ptr.5591 [DOI] [PubMed] [Google Scholar]
- Jo, S. , Kim, S. , Shin, D. H. , & Kim, M. S. (2020). Inhibition of SARS‐CoV 3CL protease by flavonoids. Journal of Enzyme Inhibition and Medicinal Chemistry, 35, 145–151. 10.1080/14756366.2019.1690480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata, K. , Mukai, R. , & Ishisaka, A. (2015). Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food and Function, 6, 1399–1417. 10.1039/c4fo01178c [DOI] [PubMed] [Google Scholar]
- Khachatoorian, R. , Arumugaswami, V. , Raychaudhuri, S. , Yeh, G. K. , Maloney, E. M. , Wang, J. , … French, S. W. (2012). Divergent antiviral effects of bioflavonoids on the hepatitis C virus life cycle. Virology, 433, 346–355. 10.1016/J.VIROL.2012.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. H. , Kim, J. E. , & Song, Y. J. (2020). Antiviral activities of quercetin and isoquercitrin against human herpesviruses. Molecules, 25(10), 2379. 10.3390/molecules25102379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y. , Narayanan, S. , & Chang, K. O. (2010). Inhibition of influenza virus replication by plant‐derived isoquercetin. Antiviral Research, 88, 227–235. 10.1016/j.antiviral.2010.08.016 [DOI] [PubMed] [Google Scholar]
- Komaravelli, N. , Kelley, J. P. , Garofalo, M. P. , Wu, H. , Casola, A. , & Kolli, D. (2015). Role of dietary antioxidants in human metapneumovirus infection. Virus Research, 200, 19–23. 10.1016/j.virusres.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley, K. v. , Gordon, S. C. , Reddy, K. R. , Rossaro, L. , Bernstein, D. E. , Lawitz, E. , … Fried, M. W. (2014). Ledipasvir and Sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. New England Journal of Medicine, 370, 1879–1888. 10.1056/nejmoa1402355 [DOI] [PubMed] [Google Scholar]
- Lee, S. , Lee, H. H. , Shin, Y. S. , Kang, H. , & Cho, H. (2017). The anti‐HSV‐1 effect of quercetin is dependent on the suppression of TLR‐3 in raw 264.7 cells. Archives of Pharmacal Research, 40, 623–630. 10.1007/s12272-017-0898-x [DOI] [PubMed] [Google Scholar]
- Liu, Z. , Zhao, J. , Li, W. , Shen, L. , Huang, S. , Tang, J. , … Zhang, R. (2016). Computational screen and experimental validation of anti‐influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Scientific Reports, 6, 1–9. 10.1038/srep19095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Zhao, J. , Li, W. , Wang, X. , Xu, J. , Xie, J. , … Zhang, R. (2015). Molecular docking of potential inhibitors for influenza H7N9. Computational and Mathematical Methods in Medicine, 2015, 1–8. 10.1155/2015/480764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, B. R. P. , da Costa, M. F. , Genova Ribeiro, A. , da Silva, T. F. , Lima, C. S. , Caruso, I. P. , … Toledo, K. A. (2020). Quercetin pentaacetate inhibits in vitro human respiratory syncytial virus adhesion. Virus Research, 276, 197805. 10.1016/j.virusres.2019.197805 [DOI] [PubMed] [Google Scholar]
- Lu, N. T. , Crespi, C. M. , Liu, N. M. , Vu, J. Q. , Ahmadieh, Y. , Wu, S. , … French, S. W. (2016). A phase i dose escalation study demonstrates quercetin safety and explores potential for bioflavonoid antivirals in patients with chronic hepatitis C. Phytotherapy Research, 30, 160–168. 10.1002/ptr.5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm‐Breschkin, J. L. (2000). Resistance of influenza viruses to neuraminidase inhibitors—A review. Antiviral Research, 47, 1–17. 10.1016/S0166-3542(00)00103-0 [DOI] [PubMed] [Google Scholar]
- Mehrbod, P. , Abdalla, M. A. , Fotouhi, F. , Heidarzadeh, M. , Aro, A. O. , Eloff, J. N. , … Fasina, F. O. (2018). Immunomodulatory properties of quercetin‐3‐O‐α‐L‐rhamnopyranoside from Rapanea melanophloeos against influenza a virus. BMC Complementary and Alternative Medicine, 18, 184. 10.1186/s12906-018-2246-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanović, Ž. B. , Antonijević, M. R. , Amić, A. D. , Avdović, E. H. , Dimić, D. S. , Milenković, D. A. , & Marković, Z. S. (2021). Inhibitory activity of quercetin, its metabolite, and standard antiviral drugs towards enzymes essential for SARS‐CoV‐2: The role of acid‐base equilibria. RSC Advances, 11, 2838–2847. 10.1039/d0ra09632f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy, N. S. H. N. , Poongavanam, V. , & Pratheepa, V. (2014). Viral M2 ion channel protein: A promising target for anti‐influenza drug discovery. Mini Reviews in Medicinal Chemistry, 14, 819–830. 10.2174/138955751410141020150822 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. T. H. , Woo, H. J. , Kang, H. K. , Nguyen, V. D. , Kim, Y. M. , Kim, D. W. , … Kim, D. (2012). Flavonoid‐mediated inhibition of SARS coronavirus 3C‐like protease expressed in Pichia pastoris. Biotechnology Letters, 34, 831–838. 10.1007/s10529-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimuro, H. , Ohnishi, H. , Sato, M. , Ohnishi‐Kameyama, M. , Matsunaga, I. , Naito, S. , … Shimamoto, K. (2015). Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients, 7, 2345–2358. 10.3390/nu7042345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda, T. (2012). Orthomyxoviruses. Uirusu, 62, 219–228. 10.2222/jsv.62.219 [DOI] [PubMed] [Google Scholar]
- Parvez, M. K. , Al‐Dosari, M. S. , Arbab, A. H. , Al‐Rehaily, A. J. , & Abdelwahid, M. A. S. (2020). Bioassay‐guided isolation of anti‐hepatitis B virus flavonoid myricetin‐3‐O‐rhamnoside along with quercetin from Guiera senegalensis leaves. Saudi Pharmaceutical Journal, 28, 550–559. 10.1016/j.jsps.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez, M. K. , Tabish Rehman, M. , Alam, P. , Al‐Dosari, M. S. , Alqasoumi, S. I. , & Alajmi, M. F. (2019). Plant‐derived antiviral drugs as novel hepatitis B virus inhibitors: Cell culture and molecular docking study. Saudi Pharmaceutical Journal, 27, 389–400. 10.1016/j.jsps.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne, S. (2017). Family coronaviridae. In Viruses (pp. 149–158). United Kingdom: Elsevier. 10.1016/b978-0-12-803109-4.00017-9 [DOI] [Google Scholar]
- Pisonero‐Vaquero, S. , García‐Mediavilla, M. V. , Jorquera, F. , Majano, P. L. , Benet, M. , Jover, R. , … Sánchez‐Campos, S. (2014). Modulation of PI3K‐LXRα‐dependent lipogenesis mediated by oxidative/nitrosative stress contributes to inhibition of HCV replication by quercetin. Laboratory Investigation, 94, 262–274. 10.1038/labinvest.2013.156 [DOI] [PubMed] [Google Scholar]
- Qiu, X. , Kroeker, A. , He, S. , Kozak, R. , Audet, J. , Mbikay, M. , & Chrétien, M. (2016). Prophylactic efficacy of quercetin 3‐β‐O‐D‐glucoside against Ebola virus infection. Antimicrobial Agents and Chemotherapy, 60, 5182–5188. 10.1128/AAC.00307-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj, U. , & Varadwaj, P. K. (2016). Flavonoids as multi‐target inhibitors for proteins associated with Ebola virus: In silico discovery using virtual screening and molecular docking studies. Interdisciplinary Sciences: Computational Life Sciences, 8, 132–141. 10.1007/s12539-015-0109-8 [DOI] [PubMed] [Google Scholar]
- Rauf, A. , Imran, M. , Khan, I. A. , ur‐Rehman, M. , Gilani, S. A. , Mehmood, Z. , & Mubarak, M. S. (2018). Anticancer potential of quercetin: A comprehensive review. Phytotherapy Research, 32, 2109–2130. 10.1002/ptr.6155 [DOI] [PubMed] [Google Scholar]
- Rima, B. , Collins, P. , Easton, A. , Fouchier, R. , Kurath, G. , Lamb, R. A. , … Wang, L. (2017). ICTV virus taxonomy profile: Pneumoviridae. Journal of General Virology, 98, 2912–2913. 10.1099/jgv.0.000959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, N. , del Campo, J. A. , Clement, S. , Lemasson, M. , García‐Valdecasas, M. , Gil‐Gómez, A. , … Romero‐Gómez, M. (2016). Effect of quercetin on Hepatitis C virus life cycle: From viral to host targets. Scientific Reports, 6, 1–9. 10.1038/srep31777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudot‐Thoraval . (2021). Epidemiology of hepatitis C virus infection. Clinics and Research in Hepatology and Gastroenterology, 45, 101596. 10.1016/J.CLINRE.2020.101596 [DOI] [PubMed] [Google Scholar]
- Ryu, W.‐S. (2017). Retroviruses. Molecular Virology of Human Pathogenic Viruses (pp. 227–246). Academic Press. 10.1016/B978-0-12-800838-6.00017-5 [DOI] [Google Scholar]
- Sadati, S. M. , Gheibi, N. , Ranjbar, S. , & Hashemzadeh, M. S. (2019). Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidas. Biomedical Reports, 10, 33–38. 10.3892/br.2018.1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi‐Boroujeni, A. , & Mahmoudian‐Sani, M. R. (2021). Anti‐inflammatory potential of quercetin in COVID‐19 treatment. Journal of Inflammation (United Kingdom), 18, 1–9. 10.1186/s12950-021-00268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajitha Lulu, S. , Thabitha, A. , Vino, S. , Mohana Priya, A. , & Rout, M. (2016). Naringenin and quercetin—Potential anti‐HCV agents for NS2 protease targets. Natural Product Research, 30, 464–468. 10.1080/14786419.2015.1020490 [DOI] [PubMed] [Google Scholar]
- Schaefer, S. (2007). Hepatitis B virus genotypes in Europe. Hepatology Research, 37, S20–S26. 10.1111/j.1872-034X.2007.00099.x [DOI] [PubMed] [Google Scholar]
- Sheu, T. G. , Fry, A. M. , Garten, R. J. , Deyde, V. M. , Shwe, T. , Bullion, L. , … Gubareva, L. v. (2011). Dual resistance to adamantanes and oseltamivir among seasonal influenza A(H1N1) viruses: 2008‐2010. Journal of Infectious Diseases, 203, 13–17. 10.1093/infdis/jiq005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozuka, K. , Kikuchi, Y. , Nishino, C. , Mori, A. , & Tawata, S. (1988). Inhibitory effect of flavonoids on DNA‐dependent DNA and RNA polymerases. Experientia, 44, 882–885. 10.1007/BF01941188 [DOI] [PubMed] [Google Scholar]
- Singh, C. K. , Chhabra, G. , Ndiaye, M. A. , Siddiqui, I. A. , Panackal, J. E. , Mintie, C. A. , & Ahmad, N. (2020). Quercetin–resveratrol combination for prostate cancer management in TRAMP mice. Cancers, 12, 1–18. 10.3390/cancers12082141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. D. , & Smith, J. C. (2020). Repurposing therapeutics for COVID‐19: Supercomputer‐based docking to the SARS‐CoV‐2 viral spike protein and viral spike protein‐human ACE2 interface. ChemRxiv. 10.26434/chemrxiv.11871402.v4 [DOI] [Google Scholar]
- Spanakis, N. , Pitiriga, V. , Gennimata, V. , & Tsakris, A. (2014). A review of neuraminidase inhibitor susceptibility in influenza strains. Expert Review of Anti‐Infective Therapy, 12, 1325–1336. 10.1586/14787210.2014.966083 [DOI] [PubMed] [Google Scholar]
- Spedding, G. , Ratty, A. , & Middleton, E. (1989). Inhibition of reverse transcriptases by flavonoids. Antiviral Research, 12, 99–110. 10.1016/0166-3542(89)90073-9 [DOI] [PubMed] [Google Scholar]
- Teixeira, T. S. P. , Caruso, Í. P. , Lopes, B. R. P. , Regasini, L. O. , de Toledo, K. A. , Fossey, M. A. , & de Souza, F. P. (2017). Biophysical characterization of the interaction between M2‐1 protein of hRSV and quercetin. International Journal of Biological Macromolecules, 95, 63–71. 10.1016/j.ijbiomac.2016.11.033 [DOI] [PubMed] [Google Scholar]
- Thapa, M. , Kim, Y. , Desper, J. , Chang, K. O. , & Hua, D. H. (2012). Synthesis and antiviral activity of substituted quercetins. Bioorganic and Medicinal Chemistry Letters, 22, 353–356. 10.1016/j.bmcl.2011.10.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand, J. M. A. , Smits, S. L. , & Haagmans, B. L. (2015). Pathogenesis of Middle East respiratory syndrome coronavirus. Journal of Pathology, 235, 175–184. 10.1002/path.4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. Y. , Huang, S. C. , Zhang, Y. , Lai, Z. R. , Kung, S. H. , Chang, Y. S. , & Lin, C. W. (2012). Antiviral ability of Kalanchoe gracilis leaf extract against enterovirus 71 and coxsackievirus A16. Evidence‐Based Complementary and Alternative Medicine 2012, 2012, 1–13. 10.1155/2012/503165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, A. , Bertoni, M. , Bienert, S. , Studer, G. , Tauriello, G. , Gumienny, R. , … Schwede, T. (2018). SWISS‐MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Research, 46, W296–W303. 10.1093/nar/gky427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, D. P. , Klenerman, P. , & Dusheiko, G. M. (2015). Hepatitis C. In The Lancet (pp. 1124–1135). United Kingdom: Lancet Publishing Group. 10.1016/S0140-6736(14)62401-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley, R. J. (1996). Herpesviruses. Diseases of Swine, 548–575. [Google Scholar]
- Wu, X. , Wu, X. , Sun, Q. , Zhang, C. , Yang, S. , Li, L. , & Jia, Z. (2017). Progress of small molecular inhibitors in the development of anti‐influenza virus agents. Theranostics, 7, 826–845. 10.7150/THNO.17071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, D. , Hu, M. J. , Wang, Y. Q. , & Cui, Y. L. (2019). Antioxidant activities of quercetin and its complexes for medicinal application. Molecules, 24(6), 1123. 10.3390/molecules24061123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Zhu, X. , Ji, H. , Deng, J. , Lu, P. , Jiang, Z. , … Zhu, H. (2018). Quercetin synergistically reactivates human immunodeficiency virus type 1 latency by activating nuclear factor‐κB. Molecular Medicine Reports, 17, 2501–2508. 10.3892/mmr.2017.8188 [DOI] [PubMed] [Google Scholar]
- Yao, C. , Xi, C. , Hu, K. , Gao, W. , Cai, X. , Qin, J. , … Wei, Y. (2018). Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virology Journal, 15, 116. 10.1186/s12985-018-1023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zell, R. (2018). Picornaviridae—The ever‐growing virus family. Archives of Virology, 163, 299–317. 10.1007/s00705-017-3614-8 [DOI] [PubMed] [Google Scholar]
- Zeng, Y. , Pu, X. , Du, J. , Yang, X. , Li, X. , Mandal, M. S. N. , … Yang, J. (2020). Molecular mechanism of functional ingredients in barley to combat human chronic diseases. Oxidative Medicine and Cellular Longevity, 2020, 1–26. 10.1155/2020/3836172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, D. , Liu, M. , Cao, Y. , Zhu, Y. , Bian, S. , Zhou, J. , … Ye, D. (2015). Discovery of metal ions chelator quercetin derivatives with potent anti‐HCV activities. Molecules, 20, 6978–6999. 10.3390/molecules20046978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoulim, F. , & Locarnini, S. (2009). Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology, 137, 1593–1608.e2. 10.1053/j.gastro.2009.08.063 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in [repository name] at http://doi.org/[doi], reference number [reference number].


