Introduction
REGEN‐COV, a neutralizing antibody cocktail of casirivimab plus imdevimab, has been shown to reduce viral load [1] and prevent symptomatic disease in household contacts of infected people [2]. However, the effectiveness of this cocktail against the currently surging and highly infectious delta variant of Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is unknown. Therefore, the objective of this study was to assess the effectiveness of cocktail antibody therapy in high‐risk SARS‐CoV‐2‐positive patients.
Methods
This is a single‐center prospective observational cohort study, approved by the Institutional Ethics Committee of AIG Hospitals. A total of 285 high‐risk, RT‐PCR‐positive SARS‐CoV‐2 patients presenting within 7 days from the onset of symptoms between July 15 and 29, 2021, were recruited from the fever clinics of AIG Hospitals, Hyderabad, India. Patients were assigned in a nonrandomized manner to either the cocktail group (monoclonal antibody [mAb]; n = 208), which received casirivimab and imdevimab (600 mg each/100 ml saline), or the standard‐of‐care (SOC; n = 78) group, which received remdesivir (200 mg on Day 1 and 100 mg/day from Days 2–5), based on their choice of therapy. All the patients in the SOC group were admitted to the hospital and were discharged. Patients (15%) with multiple comorbidities treated with mAb above 65 years of age were hospitalized as a precautionary measure. Patients treated with mAb did not receive remdesivir. The primary aim was to assess the amount of time taken for resolution of symptoms and change in viral load from baseline through Day 7 (interpreted by Ct values). Complete blood counts, inflammatory markers, and nasopharyngeal swab testing (TaqPath, ThermoScientific, USA) were performed at baseline and Day 7, and clinical symptoms were monitored. Viral genome sequencing was performed in 115 mAb and 25 SOC patients (COVIDseq; Illumina, USA) [3, 4, 5]. Neutralizing activity of the cocktail against the delta variant was assessed by a pseudovirus neutralization assay in vitro [6]. Continuous variables were expressed as means, and categorical variables as proportions. Student's t‐test for continuous variables and proportion Z‐test for categorical variables were used. Data were analysed using the R statistical environment and GraphPad Prism 9.
Results
The mean age (56.46 ± 15.77 vs. 53.51 ± 15.00 years; p = 0.15), sex (male; 56.52% vs. 57.69%), and comorbidity profiles (Table S1) of the two groups were similar. The vast majority (>98.0%) of patients in both groups harbored the delta variant (B.1.617.2) (Fig. 1a), comparable to the prevalence in the community. Assessing all the symptoms, the number of symptomatic individuals on Day 7 was significantly lower in the cocktail group than in the SOC group (23/108 [21.30%] vs. 39/78 [50.0%]; p = 0.0001) while the remaining patients in each of the groups recovered completely. Similarly, fewer patients tested positive by RT‐PCR on Day 7 in the cocktail group than in the SOC group (28/108 [25.0%] vs. 26/50 [52.0%]; p = 0.0001) (Fig. 1b,c). Among the inflammatory markers, serum ferritin levels (p = 0.027) and absolute neutrophil counts (p = 0.020) were significantly increased on Day 7 in the SOC group, while these were not significantly different in the cocktail group in comparison to levels on Day 0. A greater increase (1.5‐ vs. 1.27‐fold) in absolute lymphocyte counts was also noted in the cocktail group (Table S2). No mortality/progression to severe disease was seen in either of the groups on Day 28. There was no loss to follow‐ups in the present study. The neutralizing activity of the cocktail against the delta variant was comparable to its activity against the original Wuhan‐Hu‐1 strain (Fig. 1d). Although the mean Ct values in those who tested positive were not significantly different between the groups on Day 7, the increase in Ct values was significantly higher in the cocktail group (p = 0.003 [RDRP gene]; p = 0.006 [N gene]) (Fig. 1e).
Fig. 1.
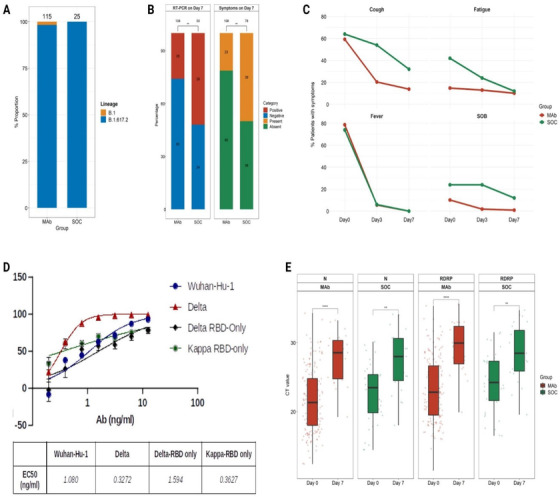
(a) A stacked bar plot representing the proportions of various SARS‐CoV‐2 lineages in antibody‐treated (mAb) and standard of care‐treated groups (SOC). The total number of genomes in a group is indicated on the top of the bar. (b) Percentage of individuals with positive RT‐PCR for SARS‐CoV‐2 and symptoms on Day 7 after the treatment. (c) Resolution of symptoms from Days 0 to 7 of treatment. (d) In vitro neutralization of pseudoviruses, including the total spike protein of SARS‐CoV‐2. (e) Comparison of Ct values of RdRp and N gene within the two groups. The rate of change in Ct values between the mAb and SOC groups was fitted using a linear model, and statistical significance was calculated using paired t‐test. **p = 0.0006; ****p = 0.0003.
Discussion
As the effectiveness of REGEN‐COV on the delta variant of SARS‐CoV‐2 is unknown in the Indian context, we compared outcomes of the cocktail group with those of the group treated by the SOC. Faster resolution of symptoms by Day 3 in the cocktail group indicates an enhanced benefit against worsening in high‐risk patients receiving the cocktail early in the disease course. This result supports earlier observations reported in outpatients [1]. Significantly lower RT‐PCR positivity on Day 7 along with higher increase in Ct values in patients receiving REGEN‐COV reveals enhanced viral clearance. Lower serum ferritin and neutrophil counts but higher lymphocyte counts on Day 7 suggest an improved inflammatory profile in the cocktail group. The neutralizing efficacy of the antibody cocktail against pseudoviral variants also confirms its ability to block the entry of the delta variant, similar to a previous report [7], thus reducing the viral load. Nonrandomization with a small sample size is the major limitation of this study; however, the findings are clinically relevant.
In conclusion, REGEN‐COV antibody cocktail‐treated high‐risk SARS‐CoV‐2 patients infected with the delta variant showed faster resolution of symptoms along with reduced viral loads.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Jagadeesh Kumar V: Conceptualization, Investigation, Writing‐review and editing; Sofia Banu: Methodology, Data curation, Formal analysis; Sasikala M, Conceptualization, Project Administration, Supervision, Writing original draft, Writing‐review and editing; Kishore VL Parsa: Methodology, Formal analysis; Divya Tej Sowpati: Methodology, Data curation, Formal analysis, Writing‐review and editing; Rupali Yadav: Methodology; Karthik Bharadwaj Tallapaka: Methodology, Data curation, Formal analysis, Writing‐review and editing; Archana Bharadwaj Siva: Project Administration, Writing‐review and editing; Ravikanth Vishnubhotla: Data Curation, Writing original draft, Writing‐review and editing; G.V. Rao: Investigation, Writing‐review and editing; D Nageshwar Reddy: Conceptualization, Supervision, Funding acquisition, Writing original draft, Writing‐review and editing.
Supporting information
Supporting Information
Acknowledgement
The authors are thankful to all the patients for consenting to participate in the study. The authors are grateful for the grants received from Asian Healthcare Foundation (AHF‐AIG 22/2021) and SBI foundation (GAP570) to conduct this study.
Kumar V J, Banu S, Sasikala M, Parsa KVL, Sowpati DT, Yadav R, et al. Effectiveness of REGEN‐COV antibody cocktail against the B.1.617.2 (delta) variant of SARS‐CoV‐2: A cohort study. J Intern Med. 2021;00:1‐4. 10.1111/joim.13408
References
- 1. Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN‐COV2, a neutralizing antibody cocktail, in outpatients with Covid‐19. N Engl J Med. 2021;384(3):238‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Brien MP, Forleo‐Neto E, Musser BJ, Isa F, Chan K‐C, Sarkar N, et al. Subcutaneous REGEN‐COV antibody combination to prevent Covid‐19. N Engl J Med. 2021;385:1184–95. 10.1056/NEJMoa2109682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014;30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. Graph‐based genome alignment and genotyping with HISAT2 and HISAT‐genotype. Nat Biotechnol. 2019;37:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hadfield J, Megill C, Bell SM, Huddleston J, Potter B, Callender C, et al. Nextstrain: real‐time tracking of pathogen evolution. Bioinformatics 2018;34:4121–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt F, Weisblum Y, Muecksch F, Hoffmann H‐H, Michailidis E, Lorenzi JCC, et al. Measuring SARS‐CoV‐2 neutralizing antibody activity using pseudotyped and chimeric viruses. J Exp Med. 2020;217(11):e20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Copin R, Baum A, Wloga E, Pascal KE, Giordano S, Fulton BO, et al. The monoclonal antibody combination REGEN‐COV protects against SARS‐CoV‐2 mutational escape in preclinical and human studies. Cell. 2021;184(15):3949–61.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


