Abstract
Two messenger RNA (mRNA) vaccines developed by Pfizer‐BioNTech and Moderna are being rolled out. Despite the high volume of emerging evidence regarding adverse events (AEs) associated with the COVID‐19 mRNA vaccines, previous studies have thus far been largely based on the comparison between vaccinated and unvaccinated control, possibly highlighting the AE risks with COVID‐19 mRNA vaccination. Comparing the safety profile of mRNA vaccinated individuals with otherwise vaccinated individuals would enable a more relevant assessment for the safety of mRNA vaccination. We designed a comparative safety study between 18 755 and 27 895 individuals who reported to VigiBase for adverse events following immunization (AEFI) with mRNA COVID‐19 and influenza vaccines, respectively, from January 1, 2020, to January 17, 2021. We employed disproportionality analysis to rapidly detect relevant safety signals and compared comparative risks of a diverse span of AEFIs for the vaccines. The safety profile of novel mRNA vaccines was divergent from that of influenza vaccines. The overall pattern suggested that systematic reactions like chill, myalgia, fatigue were more noticeable with the mRNA COVID‐19 vaccine, while injection site reactogenicity events were more prevalent with the influenza vaccine. Compared to the influenza vaccine, mRNA COVID‐19 vaccines demonstrated a significantly higher risk for a few manageable cardiovascular complications, such as hypertensive crisis (adjusted reporting odds ratio [ROR], 12.72; 95% confidence interval [CI], 2.47–65.54), and supraventricular tachycardia (adjusted ROR, 7.94; 95% CI, 2.62–24.00), but lower risk of neurological complications such as syncope, neuralgia, loss of consciousness, Guillain‐Barre syndrome, gait disturbance, visual impairment, and dyskinesia. This study has not identified significant safety concerns regarding mRNA vaccination in real‐world settings. The overall safety profile patterned a lower risk of serious AEFI following mRNA vaccines compared to influenza vaccines.
Keywords: COVID‐19, influenza vaccine, mRNA vaccine, post‐implementation surveillance, safety, VigiBase
1. INTRODUCTION
In May 2020, the 42nd Global Advisory Committee on Vaccine Safety (GACVS) addressed pharmacovigilance preparedness for the launch of the future COVID‐19 vaccines 1 ; experts have voiced that achieving herd immunity at the population level through mass vaccination is a potential strategy to control coronavirus disease (COVID‐19). 2 Two vaccines, the Pfizer‐BioNTech messenger RNA (mRNA) and the Moderna mRNA vaccine, have completed phase 3 trials, 2 , 3 , 4 , 5 and are being actively rolled out. These mRNA vaccines are based on new technologies that have not been deployed to the general population, and as such, concerns about their safety in real‐world settings intersect with optimism for their extraordinarily encouraging efficacy in clinical trials. 2 , 3 , 6
Although the safety profiles of mRNA vaccines have been evaluated in serial clinical trials, 4 , 5 , 7 concerns remain as the safety evaluations in clinical trials were limited to relatively healthy people, excluding vulnerable populations such as children, pregnant women, and individuals with severe underlying illnesses. 2 , 3 , 7 However, due to vaccine shortages, 3 , 8 , 9 vulnerable patients at high risk for severe courses of COVID‐19 are prioritized for vaccination. 10 Therefore, the safety results from these trials may be unrepresentative of the populations that are prioritized to receive them. 11 This discrepancy between the trial settings and real‐world roll‐out strategy warrants urgent interim post‐implementation surveillance. 3
Despite the high volume of emerging evidence regarding adverse events (AEs) associated with the COVID‐19 mRNA vaccines, the previous studies have thus far been largely based on the comparison between vaccinated and unvaccinated control, possibly standing out the AE risks with COVID‐19 mRNA vaccination. Comparing the safety profile of mRNA vaccinated individuals with otherwise vaccinated individuals would enable a more relevant assessment for the safety of mRNA vaccination.
This study aimed to conduct post‐implementation pharmacovigilance analysis for the Pfizer‐BioNTech and Moderna mRNA vaccines by investigating vaccinated individuals who were reported for AEFIs to VigiBase, the global database of individual case safety reports (ICSRs) provided by the WHO. To the best of our knowledge, this study is the first to report the comparative safety of the mRNA COVID‐19 vaccine against conventional influenza vaccines.
2. METHODS
2.1. Study design and data source
The large post‐implementation pharmacovigilance study was conducted using VigiBase, a WHO global deduplicated individual case safety reports (ICSR) database, 12 which has collected adverse event (AE) reports from over 130 countries and 23 million ICSRs since inception in 1967. VigiBase is managed by the Uppsala Monitoring Center (UMC, Sweden). For the database, reported adverse reactions were coded into the Medical Dictionary for Regulatory Activities (MedDRA) Preferred Terms (PTs). 13
AE following immunization (AEFI) is defined as any untoward medical event that follows immunization and that does not necessarily have a causal relationship with the usage of the vaccine. 14 AEFIs were reported from various sources, including healthcare professionals, pharmaceutical companies, and patients, and the sources are generally provided with post‐market notifications. We extracted AEFI cases from VigiBase reported with two novel mRNA COVID‐19 vaccines, Pfizer‐BioNTech and Moderna mRNA vaccines, and influenza vaccines from the beginning of 2020 to January 17, 2021. AEFI were reported from America, Europe, and Asia with COVID‐19 vaccines and America, Europe, Asia, Africa, and Australia with influenza vaccines. The Ethics Committee of Yonsei University Severance Hospital, Seoul, Republic of Korea, approved this study and granted a waiver of review from the formal Institutional Review Board (no. 4‐2020‐1379) for the use of deidentified data.
2.2. Baseline characteristics
The baseline characteristics of individuals reported to VigiBase for any AEFI after mRNA COVID‐19 and influenza vaccination are described in Table 1. The VigiBase provides data on demographics (age, sex, and regions), drug history (components, dosage, regimen, indications, and duration of administration), AEs (MedDRA PT classification terms, time to onset, seriousness of AEs, fetal outcomes, and death), and general administrative information (date of report, reports from clinical trials, and reporter type).
Table 1.
Baseline characteristics of participants vaccinated against COVID‐19 and influenza reported to VigiBase for any adverse event following immunization (AEFI)
| COVID‐19 vaccine (n = 18 755) | Influenza vaccine (n = 27 895) | |
|---|---|---|
| Regions reporting | ||
| Americas | 6947/18 755 (37.0) | 17 730/27 895 (63.6) |
| Europe | 11 787/18 755 (62.9) | 8380/27 895 (30.0) |
| Australia | 0/18 755 (0.0) | 1377/27 895 (4.8) |
| Asia | 21/18 755 (0.1) | 327/27 895 (1.2) |
| Africa | 0/18 755 (0.0) | 81/27 895 (0.4) |
| Report from clinical trials | 94/18 755 (0.5) | 1326/28 750 (4.8) |
| Reporting months | ||
| 2020.01–2020.10 | 0/18 755 (0.0) | 16 338/27 895 (58.6) |
| 2020.11 | 1/18 755 (0.0) | 2302/27 895 (8.2) |
| 2020.12 | 2087/18 755 (11.1) | 9217/27 895 (32.0) |
| 2021.01 | 16 667/18 755 (88.9) | 898/27 895 (3.2) |
| Reporter | ||
| Health care professional | 8459/18 755 (45.1) | 4054/27 895 (14.5) |
| Non‐health care professional | 3364/18 755 (17.9) | 6009/27 895 (21.5) |
| Unreported | 6942/18 755 (37.0) | 17 832/27 895 (64.0) |
| Age groups | ||
| <45 years | 9389/18 755 (50.1) | 10 703/27 895 (38.3) |
| 45–64 years | 6422/18 755 (34.2) | 6504/27 895 (23.3) |
| 65–74 years | 449/18 755 (2.4) | 5132/27 895 (18.4) |
| ≥75 years | 1282/18 755 (6.8) | 2777/27 895 (10.0) |
| Unreported | 1213/18 755 (6.5) | 2779/27 895 (10.0) |
| Sex | ||
| Male | 3838/18 755 (20.5) | 9263/27 895 (33.2) |
| Female | 14 514/18 755 (77.4) | 18 262/27 895 (65.5) |
| Unreported | 403/18 755 (2.1) | 370/27 895 (1.3) |
| Serious AEFIs | 3737/18 755 (19.9) | 3343/27 573 (12.1) |
| Outcomes | n = 13 058 | n = 14 317 |
| Deathsa | 119/13 058 | 113/14371 |
| Time to AEFIs onset | n = 10 876 | n = 14 925 |
| Median days (IQR) | 1.0 (0.0–1.0) | 0.0 (0.0–0.0) |
Abbreviations: AEFIs, adverse events following immunization; IQR, interquartile range.
As denominator, all vaccinated participants with AEFIs reported rather than all vaccinated persons were used; we did not present percentile estimations given that they must be larger than those observed in real‐world settings. The AEFIs for the COVID‐19 and influenza vaccine were extracted from January 2020 to January 17, 2021. Values are presented as n (%) or n/N (%), unless otherwise indicated. Severe AEFI was defined as AEFI that is life‐threatening, causes persistent or significant disability, requires hospitalization (first or prolonged), or results in death.
Common AEFI was defined as AEFI with a frequency ≥1% of all COVID‐19 vaccinated individuals reported for any adverse reaction to VigiBase. A serious AEFI is defined as an AEFI that is associated with death, is life‐threatening, involves hospitalization or its prolongation, results in chronic damage/disability, and requires interventions to prevent permanent impairment. 14 The selection process of common and serious AEFI is presented in Figures S1–2.
2.3. Removal of potentially false reports
Potentially false reports are partially prevented at an early data collection stage as most national centers review case reports before they are sent to UMC, and incoming reports to the VigiBase are systematically checked according to pre‐defined quality criteria; unmet reports are flagged and subsequently inspected by UMC for reprocessing. 12 Despite the effort, the noise safety signals may still exist, and we triaged to select validated safety signals using two approaches. First, we incorporated information component (IC), an indicator value for disproportionate reporting, that has been proven to be effective in avoiding false positive 15 and thus suitable for conducting pharmacovigilance studies using spontaneous adverse reaction reporting databases. 16 , 17 , 18 Second, we triaged to remove potentially false reports of adverse drug reactions (ADRs) using disproportionality analysis and clinical appraisal. Given that false reports by chance are less likely to survive in stringent association tests, we ran disproportionality analyses for 1980 ADRs and excluded nonsignificant ADRs that were deemed clinically irrelevant with vaccines or potentially containing false reports, leaving 49 ADRs subjected to comparative analysis of mRNA COVID‐19 and influenza vaccines. We further excluded ADRs that were unlikely to be associated with vaccination (i.e., chronic diseases) by manual review. Death, anaphylactic reactions, and selected 49 reported ADRs out of 1980 MedDRA PTs are summarized in Table 2 and analyzed for the comparative safety between the vaccines (Figure 1). Our careful approach to using those reports deemed genuine and clinically meaningful for our comparative analyses minimized the risk of false reports driving the misleading results. The detailed triage process for AEFI is demonstrated in Figures S1–2.
Table 2.
Adverse events following immunization (AEFIs) associated with the COVID‐19 and the influenza vaccine in the full database of the VigiBase from January, 2020
| COVID‐19 vaccinea | IC/IC0.25 | ROR (95% CI) | Influenza vaccinea | IC/IC0.25 | ROR (95% CI) | Full databasea (since 2020.01) | |
|---|---|---|---|---|---|---|---|
| Total individuals with AEFIs | 18 755 | 27 895 | 2 720 221 | ||||
| Common AEFIs | |||||||
| Vaccination site pain | 824 | 5.04/4.94 | 45.20 (41.75–48.92) | 868 | 4.55/4.45 | 31.99 (29.61–34.57) | 3568 |
| Lymphadenopathy | 685 | 4.66/4.55 | 32.27 (29.67–35.09) | 287 | 2.84/2.67 | 7.83 (6.94–8.84) | 3855 |
| Oral paraesthesia | 472 | 4.68/4.54 | 32.62 (29.50–36.08) | 150 | 2.47/2.23 | 5.92 (5.02–6.98) | 2608 |
| Myalgia | 2137 | 3.58/3.52 | 14.54 (13.87–15.24) | 1443 | 2.44/2.37 | 5.97 (5.65–6.30) | 25 821 |
| Heart rate increased | 357 | 3.00/2.85 | 8.67 (7.78–9.65) | 175 | 1.41/1.19 | 2.73 (2.35–3.17) | 6393 |
| Pain in extremities | 1524 | 2.92/2.84 | 8.55 (8.10–9.02) | 2664 | 3.15/3.10 | 10.61 (10.17–11.06) | 29 187 |
| Headache | 4974 | 2.84/2.79 | 9.68 (9.36–10.01) | 2474 | 1.31/1.25 | 2.66 (2.56–2.78) | 97 345 |
| Fatigue | 3123 | 2.84/2.79 | 8.79 (8.45–9.14) | 1498 | 1.21/1.14 | 2.42 (2.30–2.55) | 63 151 |
| Lethargy | 242 | 2.95/2.76 | 8.30 (7.28–9.45) | 292 | 2.65/2.48 | 6.77 (6.01–7.63) | 4491 |
| Pyrexia | 3577 | 2.73/2.68 | 8.30 (7.99–8.61) | 3324 | 2.05/2.00 | 4.73 (4.56–4.91) | 78 189 |
| Chills | 2476 | 2.59/2.54 | 7.06 (6.76–7.37) | 1484 | 1.28/1.21 | 2,.55 (2.42–2.69) | 59 451 |
| Arthralgia | 1338 | 2.58/2.50 | 6.59 (6.22–6.97) | 1190 | 1.84/1.75 | 3.79 (3.57–4.02) | 32 482 |
| Influenza‐like illness | 359 | 2.30/2.15 | 5.19 (4.66–5.77) | 494 | 2.19/2.06 | 4.84 (4.42–5.30) | 10 486 |
| Chest discomfort | 398 | 2.01/1.87 | 4.21 (3.80–4.65) | 231 | 0.66/0.47 | 1.60 (1.40–1.82) | 14 247 |
| Dizziness | 2022 | 1.37/1.31 | 2.81 (2.68–2.95) | 1480 | 0.35/0.27 | 1.29 (1.23–1.36) | 113 320 |
| Flushing | 543 | 1.43/1.30 | 2.77 (2.55–3.02) | 192 | −0.64/−0.85 | 0.63 (0.55–0.73) | 29 262 |
| Blood pressure increased | 240 | 1.37/1.18 | 2.64 (2.30–3.00) | 100 | −0.46/−0.76 | 0.72 (0.59–0.88) | 13 442 |
| Cough | 546 | 1.14/1.02 | 2.27 (2.08–2.47) | 702 | 0.93/0.83 | 1.96 (1.81–2.11) | 35 788 |
| Palpitations | 511 | 1.04/0.92 | 2.10 (1.92–2.30) | 183 | −1.01/−1.23 | 0.49 (0.42–0.57) | 36 033 |
| Nausea | 2515 | 0.94/0.88 | 2.07 (1.99–2.16) | 1578 | −0.31/−0.38 | 0.80 (0.76–0.84) | 190 359 |
| Diarrhea | 748 | 0.43/0.32 | 1.36 (1.27–1.47) | 671 | −0.30/−0.41 | 0.80 (0.75–0.87) | 80 681 |
| Dyspnea | 774 | 0.38/0.27 | 1.31 (1.22–1.41) | 791 | −0.16/−0.27 | 0.89 (0.83–0.95) | 86 465 |
| Death, anaphylactic reaction, and uncommon but serious AEFI b | |||||||
| Death | 103 | −1.37/−1.66 | 0.38 (0.31–0.46) | 104 | −1.93/−2.22 | 0.26 (0.21–0.31) | 38 799 |
| Anaphylactic reaction | 149 | −0.12/−0.36 | 0.92 (0.78–1.08) | 147 | −0.71/−0.95 | 0.61 (0.52–0.71) | 23 415 |
| Intensive care | 36 | 3.71/3.20 | 17.49 (12.37–24.73) | 54 | 3.80/3.39 | 18.71 (13.98–25.05) | 333 |
| Facial paralysis | 76 | 3.27/2.92 | 10.93 (8.66–13.81) | 99 | 3.10/2.80 | 9.76 (7.94–12.01) | 1081 |
| Resuscitation | 8 | 2.82/1.65 | 12.26 (5.96–25.24) | 12 | 3.02/2.09 | 12.87 (7.05–23.52) | 102 |
| Syncope | 180 | 1.64/1.42 | 3.20 (2.76–3.71) | 735 | 3.10/2.99 | 9.55 (8.85–10.31) | 8341 |
| Unresponsive to stimuli | 41 | 1.88/1.41 | 3.89 (2.85–5.31) | 117 | 2.83/2.56 | 7.85 (6.50–9.49) | 1560 |
| Endotracheal intubation | 9 | 2.41/1.32 | 7.33 (3.75–14.32) | 25 | 3.40/2.78 | 15.00 (9.84–22.86) | 186 |
| Hypertensive crisis | 14 | 1.95/1.10 | 4.42 (2.59–7.52) | 2 | −1.09/−3.68 | 0.41 (0.10–1.65) | 471 |
| Obstructive airway disorder | 10 | 2.13/1.10 | 5.42 (2.88–10.19) | 9 | 1.51/0.42 | 3.25 (1.67–6.32) | 276 |
| Supraventricular tachycardia | 22 | 2.66/2.00 | 7.53 (4.91–11.57) | 4 | −0.16/−1.90 | 0.88 (0.33–2.35) | 443 |
| Sensory loss | 14 | 1.89/1.04 | 4.19 (2.47–7.14) | 26 | 2.25/1.64 | 5.35 (3.61–7.95) | 495 |
| Neuralgia | 33 | 1.16/0.63 | 2.30 (1.63–3.25) | 107 | 2.29/2.00 | 5.20 (4.28–6.31) | 2101 |
| Aphonia | 14 | 1.17/0.32 | 2.38 (1.41–4.04) | 18 | 0.99/0.25 | 2.06 (1.29–3.29) | 860 |
| Lacunar infarction | 3 | 2.31/0.26 | 16.01 (4.86–52.77) | 1 | 0.89/−2.90 | 3.33 (0.45–24.43) | 30 |
| Vestibular neuronitis | 3 | 2.17/0.12 | 11.68 (3.60–37.89) | 7 | 3.04/1.78 | 20.48 (9.06–46.30) | 40 |
| Loss of consciousness | 72 | 0.46/0.11 | 1.39 (1.10–1.75) | 488 | 2.65/2.51 | 6.76 (6.16–7.41) | 7565 |
| Visual impairment | 58 | −0.37/−0.77 | 0.77 (0.59–1.00) | 141 | 0.33/0.09 | 1.27 (1.07–1.50) | 10 905 |
| Aphasia | 13 | 0.01/−0.88 | 1.01 (0.58–1.74) | 31 | 0.68/0.13 | 1.63 (1.14–2.32) | 1869 |
| Neuralgic amyotrophy | 2 | 1.46/−1.13 | 5.05 (1.23–20.71) | 16 | 3.90/3.11 | 35.93 (20.24–63.80) | 59 |
| Gait disturbance | 43 | −0.77/−1.23 | 0.58 (0.43–0.78) | 198 | 0.85/0.64 | 1.83 (1.59–2.11) | 10 681 |
| Seizure | 35 | −0.94/−1.46 | 0.52 (0.37–0.72) | 213 | 1.08/0.88 | 2.15 (1.88–2.47) | 9795 |
| Dyskinesia | 13 | −0.87/−1.76 | 0.54 (0.31–0.92) | 64 | 0.82/0.45 | 1.80 (1.40–2.30) | 3506 |
| Sudden hearing loss | 2 | 0.53/−2.06 | 1.63 (0.40–6.56) | 7 | 1.68/0.42 | 3.93 (1.85–8.37) | 179 |
| Pericarditis | 2 | −0.19/−2.78 | 0.85 (0.21–3.41) | 12 | 1.64/0.71 | 3.52 (1.98–6.27) | 341 |
| Myelitis | 1 | 0.28/−3.52 | 1.36 (0.19–9.74) | 12 | 2.97/2.04 | 12.20 (6.69–22.24) | 107 |
| Myocarditis | 2 | −1.19/−3.78 | 0.38 (0.10–1.54) | 17 | 1.09/0.32 | 2.23 (1.38–3.61) | 753 |
| Neuritis | 1 | −0.11/−3.91 | 0.89 (0.12–6.35) | 14 | 2.74/1.89 | 9.07 (5.24–15.69) | 163 |
| Guillain‐Barre syndrome | 1 | −1.84/−5.63 | 0.20 (0.03–1.46) | 233 | 4.92/4.73 | 48.14 (41.13–56.35) | 704 |
Abbreviations: AEFI, adverse event following immunization; IC, information component; NA, not applicable; ROR, reporting odds ratio.
As denominator, all vaccinated participants with AEFIs reported rather than all vaccinated persons were used; we did not present percentile estimations given that they must be larger than those observed in real‐world settings.
Due to the volume, only serious AEFIs that are significantly associated with either COVID‐19 or influenza vaccine are listed in this table, while serious AEFIs that were not associated with the vaccines are presented in the supplementary material. The first AEFI associated with the COVID‐19 vaccine was reported on December 15, 2020. The IC/IC0.25 and ROR of AEFIs associated with COVID‐19 and influenza vaccines were compared with the entire database of VigiBase from January 01, 2020, to January 17, 2021. A positive IC0.25 value (>0) in bold is the traditional threshold used for statistical signal detection.
Figure 1.
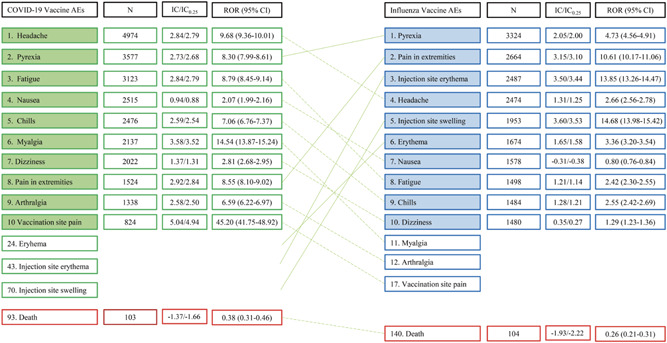
Comparative safety of mRNA vaccines to conventional influenza vaccines: Common adverse events following immunization (AEFIs). The numbers in the first column represent the ranking of AEFIs. Values >0 for the lower 95% credibility interval endpoint of the information component (IC0.25) and >1 for the lower confidence interval (CI) of ROR indicate statistical significance. AEFI, adverse event following immunization; IC, information component; mRNA, messenger RNA; N, number; ROR, reporting odds ratio
2.4. Comparative safety between COVID‐19 and influenza vaccines
We have set influenza vaccines as a control given that they have endured iterative and thorough safety evaluations in the form of continued population‐based post‐market surveillance, 19 which have deemed them acceptably safe. 19 , 20 The most frequently reported AEFIs and death after COVID‐19 and influenza vaccination were compared in overall individuals reported to the database for AEFI. For uncommon but serious AEFIs that were identified to be potentially associated with the COVID‐19 and influenza vaccine (IC0.25 > 0), the variable adjusted reporting odds ratio (ROR) between mRNA COVID‐19 and influenza vaccines for specific AEFI was calculated as described in a previous study 18 to identify comparative safety. The adjusted ROR was used to quantify the degree of difference in odds of specific AEFI between the COVID‐19 and influenza vaccine; since the odds of specific AEFI in the influenza vaccine were used as a control, ROR > 1 indicates the higher risk of the AEFI in COVID‐19 vaccines compared to influenza vaccines.
2.5. Statistical analysis
Given that VigiBase is composed of an extensive sample size (23 880 736 reports from inception), the data are eligible for disproportionality analysis (also known as case–non‐case analysis), for which large sample size is essential to guarantee applicable power and resolution. 21 When individuals exposed to a particular drug or vaccine (cases) have higher odds of reporting for certain adverse reactions than those not exposed to the drug or vaccine (non‐cases), the association between the intervention and the adverse reaction suggests a possible safety signal. The IC and ROR are indicator parameters used to detect signals from the disproportionate analysis developed by the UMC;>0 for lower 95% credibility interval endpoint of information component (IC0.25) and >1 for lower confidence interval (CI) of ROR are deemed significant, respectively. The formula for the calculation of the IC is presented in Table S3.
The IC was calculated by comparing observed and expected adverse reaction values using the Bayesian neural network method developed by the UMC, 15 and AEFIs associated with vaccines were detected. Probabilistic logic in intelligent systems (information theory) has been proven to be useful in controlling both big data and missing data. 15 This sensitive algorithm allowed the detection of early signals of mRNA vaccines and identified any potential risks. Of note, VigiBase was not designed to verify the causal relationship between the vaccine and health problems; instead, they were established to detect uncommon or unexpected patterns of AEFIs that imply possible safety concerns with vaccines.
We used a multivariable logistic regression model to produce age and sex‐adjusted ROR to compare ADR reporting between mRNA COVID‐19 and influenza vaccines. Categorical variables are described as number count (%), and continuous variables are reported as the median and interquartile range (IQR). The cases reported from COVID‐19 and influenza vaccination and full database reports were compared using the χ 2 test or Fisher's exact test. Statistical significance was defined as two‐tailed p < 0.05. Comparative analyses were conducted using the IBM statistical package for the social sciences (SPSS) version 25.0 (SPSS Inc.).
3. RESULTS
From January 1, 2020, to January 17, 2021, 18 755 and 27 895 AEFIs for the COVID‐19 and influenza vaccines were reported to VigiBase. The AEFIs were most frequently reported from individuals under 64 years of age for COVID‐19 and influenza vaccine (Table 1). Ninety‐four individuals out of 18755 (0.5%) and 1326 individuals out of 28 750 (4.8%) were reported from clinical trials for COVID‐19 and influenza vaccines, respectively; the remaining reports were collected from spontaneous, nonclinical trial settings. A total of 23 880 736 and 2 720 221 ICSRs have been reported to VigiBase since the inception of the database (1967) and since 2020, respectively; and these reports were used as non‐case. We identified safety signals associated with the vaccines, which are statistically significant (defined as IC0.25 > 0) compared to non‐cases (Tables 2 and S1).
3.1. Common adverse events
COVID‐19 and influenza vaccines showed numerous statistically significant AEFIs, of which many were related to systemic reaction and injection site reactogenicity (Table 2). The 10 most common AEFIs and deaths for the entire population are shown in Figure 1. A more detailed list of total AEFIs after COVID‐19 vaccination and the selection process of common AEFIs are presented in the Supplementary material. In Figure 1, the cross‐over pattern suggested that COVID‐19‐vaccinated individuals are more likely to experience systemic symptoms such as headache, myalgia, pyrexia, and fatigue, while influenza‐vaccinated individuals were more likely to experience injection site reactogenicity events.
3.2. Uncommon but serious adverse events
Our analysis detected uncommon but serious AEFIs that were significantly associated with COVID‐19 vaccines (Table 2). We assessed the comparative safety between COVID‐19 and influenza vaccines for serious AEFIs by calculating the adjusted ROR; cardiovascular AEFIs were more prevalent with COVID‐19 vaccines: hypertensive crisis (adjusted ROR, 12.72; 95% CI, 2.47–65.54) and supraventricular tachycardia (adjusted ROR, 7.94; 95% CI, 2.62–24.00). In contrast, neurologic AEFIs, such as syncope, neuralgia, loss of consciousness, Guillain‐Barre syndrome, gait disturbance, visual impairment, and dyskinesia were more prevalent with influenza vaccines (Figure 2).
Figure 2.
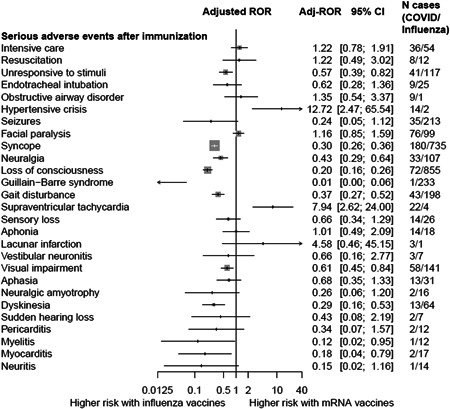
Comparative safety of mRNA COVID‐19 vaccines versus influenza vaccines with respect to serious adverse events after immunization (AEFIs). Adj‐ROR, adjusted reported odd ratios; 95% CI, 95% confidential interval; mRNA, messenger RNA
3.3. Death
COVID‐19‐vaccinated individuals experienced fewer deaths compared to those not exposed to the vaccines, possibly indicating a protective effect of the vaccine (IC0.25, −1.66; ROR, 0.38; 95% CI, 0.31–0.46, Table 2). Influenza‐vaccinated individuals also experienced fewer deaths compared to those not exposed to the vaccines (IC0.25, −2.22; ROR, 0.26; 95% CI, 0.21–0.31, Table 2).
4. DISCUSSION
To the best of our knowledge, this is the first post‐implementation pharmacovigilance study to investigate a diverse range of adverse reactions and provide comparative views for the COVID‐19 mRNA vaccine and influenza vaccine. This study has not identified significant safety concerns regarding mRNA vaccination in real‐world settings. We have set influenza vaccines as a control given that they have undergone iterative and thorough safety evaluations in the form of continued population‐based post‐market surveillance, 19 which have deemed them acceptably safe. 19 , 20 This interim safety surveillance data revealed that the safety profiles of novel mRNA vaccines may be divergent from those of influenza vaccines; the overall pattern suggested that systematic reactions like chill, myalgia, fatigue were more noticeable with the mRNA COVID‐19 vaccine, while injection site reactogenicity events were more prevalent with the influenza vaccine (Figure 1). The overall safety profile patterned a lower risk of serious AEFI following mRNA vaccines compared to influenza vaccines (Figure 2).
The two novel vaccines contain mRNAs that encode spike proteins of SARS‐CoV‐2 formulated in a lipid nanoparticle. In principle, mRNA vaccines have a unique mechanism compared to conventional vaccines in terms of immunogenicity. Exogenously administered mRNA can strongly stimulate the innate immune system through RNA‐sensing pattern recognition receptors. 22 Although mRNA has been structurally modified to reduce innate immune responses in current mRNA vaccines, 23 the safety of mRNA vaccines needs to be carefully evaluated. Further safety concerns were raised from the fact that the safety evaluations in clinical trials were limited to relatively healthy people while vulnerable patients at high risk for severe courses of COVID‐19 were prioritized to the vaccination in real‐world settings. 3 , 8 , 9 This study was designed to investigate this gap and promptly detect safety signals undiscovered at the trial level but could snowball as vaccine coverage spans across the billions of people worldwide. Of note, this analysis aims to raise hypotheses for further, more definitive studies, not to test hypotheses and inform recommendations.
Our data revealed that COVID‐19‐vaccinated individuals experienced significantly fewer deaths compared to those not exposed to the vaccines, possibly indicating a protective effect of the vaccine (Table 2). When stratifying death risk by age group, the proportion of death among all AEFI‐reported vaccinated individuals in the age group was higher in the >65 years age groups, and the tendency was more prominent for those ≥75 years old (Table S2). This observation could be explained, in part, by the selective roll‐out of mRNA vaccines to particularly vulnerable elderly populations, such as those receiving care in long‐term care facilities (LTCF), who are frail and at a higher risk of severe courses. Therefore, it is difficult to attribute the higher odds of death in the elderly, especially those >75 years, to mRNA vaccination per se without more data that may help extricate a causal relationship. Further studies should be conducted to elucidate the causal relationship and underlying mechanisms for this association.
It is noteworthy that mRNA vaccines demonstrated a significantly higher risk for a few cardiovascular complications, such as hypertensive crisis and supraventricular tachycardia (SVT) compared with influenza vaccines; however, risks for most other cardiovascular adverse events such as atrial fibrillation, myocardial infarction, cardiogenic shock, and cardiac failure were not increased with mRNA vaccination (Supporting Information Data). Considering hypertensive crisis and SVT are mostly manageable and rarely cause permanent or chronic damages, these cardiovascular signals are less likely to pose a burden to a large population. Moreover, lower risks of other serious complications, especially neurological complications (i.e., neuralgia, Guillain‐Barre syndrome, dyskinesia, and gait disturbance), with mRNA vaccines compared to influenza vaccines may further support the comparative safety of mRNA vaccines in real‐world settings (Figure 2). The findings and hypotheses raised from this first postimplementation surveillance data may support evidence‐based discussions and risk‐benefit assessments for ongoing mass vaccination.
The results of this study should be interpreted in the context of known limitations. First, VigiBase relies on spontaneous reports, and therefore the data are subject to reporting biases. To address this, we triaged to remove potentially false reports using disproportionality analysis and clinical appraisal, as demonstrated in the methods. Second, VigiBase was primarily designed to identify unusual or unexpected safety signals that might be associated with vaccines rather than to determine a causal relationship. Therefore, our analysis should be interpreted as confined to the associations, and be aware that this analysis is intended to raise hypotheses for further, more definitive studies. Last, we employed disproportionality analyses between the AEFIs reported with mRNA COVID‐19/influenza vaccines and the total number of individual case safety reports for the entire VigiBase database. However, the advantages of VigiBase and the methods used in this study (disproportionate analysis) have been well established through numerous studies 16 , 24 , 25 , 26 and may provide sufficient evidence to bring the potential safety signals to the attention of public health professionals and decision‐makers.
CONCLUSION
This pharmacovigilance study has not identified significant safety concerns regarding mRNA vaccination in real‐world settings. The overall safety profile patterned a lower risk of serious AEFI following mRNA vaccines compared to influenza vaccines.
CONFLICT OF INTERESTS
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the World Health Organization (WHO) and the International Vaccine Institute (IVI). The authors declare that they have no known competing financial interests or personal relationships with companies that could have influenced the work reported in this paper. Dr. Jean‐Louis Excler reports nonfinancial support from Brighton Collaboration, personal fees from US Military HIV Research Program, personal fees from Johnson & Johnson, outside the submitted work; Dr. Jerome H. Kim reports personal fees from SK bioscience, personal fees from educational companies during the period covered by this manuscript; Min Seo Kim, Se Yong Jung, Jong Gyun Ahn, Se Jin Park, Yehuda Shoenfeld, Andreas Kronbichler, Ai Koyanagi, Elena Dragioti, Kalthoum Tizaoui, Sung Hwi Hong, Louis Jacob, Joe‐Elie Salem, Dong Keon Yon, Seung Won Lee, Shuji Ogino, Hanna Kim, Florian Marks, John D. Clemens, Michael Eisenhut, Yvonne Barnett, Laurie Butler, Cristian Petre Ilie, Eui‐Cheol Shin, Jae Il Shin, and Lee Smith have no commercial associations that may present a conflict of interest regarding this manuscript.
AUTHOR CONTRIBUTIONS
Min Seo Kim, Se Yong Jung, and Jae Il Shin contributed to the study concept and design. Se Yong Jung and Jae Il Shin acquired data. Min Seo Kim and Se Yong Jung analyzed the data. Min Seo Kim and Se Yong Jung wrote the first draft of the manuscript. Min Seo Kim finalized the manuscript. Hanna Kim supported organizing influenza vaccine data and supplementary materials. Joe‐Elie Salem, Jerome Kim, Jean‐Louis Excler, Florian Marks, John D. Clemens supervised the interpretation of vaccine pharmacovigilance results. Jong Gyun Ahn, Se Jin Park, Yehuda Shoenfeld, Andreas Kronbichler, Ai Koyanagi, Elena Dragioti, Kalthoum Tizaoui, Sung Hwi Hong, Louis Jacob, Dong Keon Yon, Seung Won Lee, Shuji Ogino, Michael Eisenhut, Yvonne Barnett, Laurie Butler, Cristian Petre Ilie, Eui‐Cheol Shin, and Lee Smith contributed to the intellectual discussion, organization of contents, and critical revision of the manuscript. JI Shin and JE Salem provided statistical advice or supervised the statistical interpretations. All authors saw and approved the final submitted version.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
We appreciate the members of the custom search team at the Uppsala Monitoring Centre (Uppsala, Sweden) research section, who were invaluable to the successful performance of this study. This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2021 (2021‐32‐0049). The funders had no role in the design, analyses, or interpretation of the study. Hanna Kim is a spouse of Minseo Kim. However, Hanna Kim contributed to this work indepentdently.
Kim MS, Jung SY, Ahn JG, et al. Comparative safety of mRNA COVID‐19 vaccines to influenza vaccines: A pharmacovigilance analysis using WHO international database. J Med Virol. 2022;94:1085‐1095. 10.1002/jmv.27424
Min Seo Kim and Se Yong Jung contributed equally to this study.
DATA AVAILABILITY STATEMENT
Study protocol and Statistical code are available from Prof. Shin (e‐mail, shinji@yuhs.ac). Data set is available from the WHO Program for International Drug Monitoring through a data use agreement.
REFERENCES
- 1.World Health Orgnaization. Global Advisory Committee on Vaccine Safety; 2020. Accessed May 28, 2020. https://www.who.int/vaccine_safety/committee/reports/May_2020/en/
- 2. Connors M, Graham BS, Lane HC, Fauci AS. SARS‐CoV‐2 vaccines: much accomplished, much to learn. Ann Intern Med. 2021;174:687‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim JH, Marks F, Clemens JD. Looking beyond COVID‐19 vaccine phase 3 trials. Nature Med. 2021:27(2):1‐7. [DOI] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2020;384:403‐416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castells MC, Phillips EJ. Maintaining safety with SARS‐CoV‐2 vaccines. N Engl J Med. 2021.384(7):643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh EE, Frenck RW Jr., Falsey AR, et al. Safety and immunogenicity of two RNA‐based Covid‐19 vaccine candidates. N Engl J Med. 2020;383(25):2439‐2450. 10.1056/NEJMoa2027906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnabas RV, Wald A. A Public Health COVID‐19 Vaccination Strategy to Maximize the Health Gains for Every Single Vaccine Dose. American College of Physicians. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matrajt L, Eaton J, Leung T, Brown ER. Vaccine optimization for COVID‐19: who to vaccinate first? Sci Adv. 2020;7(6):eabf1374. 10.1126/sciadv.abf1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Omer S, Faden R, Kochhar S, et al. WHO SAGE Roadmap for Prioritizing Uses of COVID‐19 Vaccines in the Context of Limited Supply. World Health Organization; 2020. [Google Scholar]
- 11. Doshi P. Will covid‐19 vaccines save lives? Current trials aren't designed to tell us. BMJ. 2020;371:m4037. [DOI] [PubMed] [Google Scholar]
- 12. Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Drug Inf J. 2008;42(5):409‐419. [Google Scholar]
- 13. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33(36):4398‐4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . COVID‐19 Vaccines: Safety Surveillance Manual. World Health Organization; 2020. [Google Scholar]
- 15. Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315‐321. [DOI] [PubMed] [Google Scholar]
- 16. Salem J‐E, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19(12):1579‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salem JE, Ederhy S, Lebrun‐Vignes B, Moslehi JJ. Cardiac events associated with chimeric antigen receptor T‐cells (CAR‐T): a VigiBase perspective. J Am Coll Cardiol. 2020;75(19):2521‐2523. 10.1016/j.jacc.2020.02.070 [DOI] [PubMed] [Google Scholar]
- 18. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang X‐F, Li L, Liu D‐W, et al. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. N Engl J Med. 2011;364(7):638‐647. [DOI] [PubMed] [Google Scholar]
- 20. Yamayoshi S, Kawaoka Y. Current and future influenza vaccines. Nature Med. 2019;25(2):212‐220. [DOI] [PubMed] [Google Scholar]
- 21. Caster O, Aoki Y, Gattepaille LM, Grundmark B. Disproportionality analysis for pharmacovigilance signal detection in small databases or subsets: recommendations for limiting false‐positive associations. Drug Saf. 2020;43(5):479‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chow KT, Gale M Jr., Loo Y‐M. RIG‐I and other RNA sensors in antiviral immunity. Annu Rev Immunol. 2018;36:667‐694. [DOI] [PubMed] [Google Scholar]
- 23. Wang Y, Zhang Z, Luo J, Han X, Wei Y, Wei X. mRNA vaccine: a potential therapeutic strategy. Mol Cancer. 2021;20(1):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302(7):750‐757. [DOI] [PubMed] [Google Scholar]
- 25. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune‐related adverse events in patients with cancer. JAMA Oncol. 2020;6:865‐871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salem JE, Yang T, Moslehi JJ, et al. Androgenic effects on ventricular repolarization: a translational study from the international pharmacovigilance database to iPSC‐cardiomyocytes. Circulation. 2019;140(13):1070‐1080. 10.1161/circulationaha.119.040162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Study protocol and Statistical code are available from Prof. Shin (e‐mail, shinji@yuhs.ac). Data set is available from the WHO Program for International Drug Monitoring through a data use agreement.


