Abstract
Early evidence from China suggested that blood groups may be involved in susceptibility to COVID‐19. Several subsequent studies reported controversial results. We conducted a retrospective matched case‐control study that aims to investigate the association between blood groups and the risk and/or severity of COVID‐19. We compared the blood groups distribution of 474 patients admitted to the hospital for COVID‐19 between March 2020 and March 2021, to that of a positive control group of outpatients infected with COVID‐19 and matched them for sex and age, as well as to the distribution in the general population. Three hundred and eighteen HC+ pairs with available blood group information were matched. The proportion of group A Rh+ in hospitalized patients (HC+) was 39.9% (CI 35.2%–44.7%), compared to 44.8% (CI 39.8%–49.9%) and 32.3% in the positive outpatient controls (C+) and the general population (C−), respectively. Both COVID‐19‐positive groups (HC+ and C+) had significantly higher proportions of group A Rh+ compared to the general population (p = 0.0019 and p < 0.001, respectively), indicating that group A Rh+ increases susceptibility to COVID‐19. Although blood group A Rh+ was more frequent in the outpatients C+ compared to the hospitalized group HC+, the association did not reach statistical significance, indicating that blood group A Rh+ is not associated with severity. There was no significant relationship between COVID‐19 and other blood groups. Our findings indicate that blood group A Rh+ increases the susceptibility for COVID‐19 but is not associated with higher disease severity.
Keywords: ABO, blood groups, COVID‐19, pandemic, Rhesus, severity, susceptibility
1. INTRODUCTION
Ever since the appearance of the novel coronavirus SARS‐CoV‐2, its rapid spread and overwhelming impact on healthcare systems made it a priority to identify potential risk factors associated with susceptibility to infection and/or disease severity. Apart from the SARS‐CoV‐2 variants per se, multiple medical and sociodemographic risk factors were quickly identified, such as diabetes, hypertension, 1 , 2 obesity, 3 , 4 male sex, 5 , 6 , 7 , 8 increasing age, 9 , 10 , 11 and ethnicity. 12 , 13 , 14 , 15 Early evidence from China suggested that ABO blood groups may also potentially be involved in susceptibility to COVID‐19, hypothesizing that blood group A increases susceptibility to SARS‐CoV‐2 infection while blood group O confers a reduced susceptibility to infection. 16 The growing interest in the relationship between ABO blood groups and COVID‐19 led to several subsequent studies which reported controversial results. 17 , 18 , 19 , 20 , 21 , 22 These studies had some methodological limitations, including a small number of subjects and unmatched retrospective designs. The aim of our study is to assess the relationship between ABO‐Rhesus blood groups with COVID‐19 infection in terms of susceptibility and severity using well‐matched populations.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a retrospective matched case‐control study that aims to investigate the association between ABO‐Rhesus blood groups and the susceptibility and/or severity of COVID‐19 infection. This single‐center, hospital‐based study was conducted between March 2020 and March 2021 at Hôtel‐Dieu de France (HDF) hospital, Beirut, Lebanon, one of the largest tertiary care hospitals in the capital, Beirut.
2.2. Cases (HC+)
A case (HC+) was defined as any patient who tested positive for SARS‐CoV‐2 and was admitted to HDF between March 2020 and March 2021 for a SARS‐CoV‐2‐related diagnosis. These patients were hospitalized in three divisions: the division of internal medicine, infectious diseases, and pulmonary and critical care. COVID‐19 infection was determined by SARS‐CoV‐2‐specific polymerase chain reaction (PCR) testing of nasal swabs.
2.3. Controls (C+)
A control (C+) was defined as any patient who had a positive PCR test obtained at HDF between March 2020 and March 2021 but whose SARS‐CoV‐2‐related symptoms did not warrant hospitalization. The controls were recruited from a variety of settings, including the HDF emergency department, flu clinic, or drive‐thru testing center.
2.4. Matching method
The pool of controls contained 4234 unique subjects and was used to create 1:1 matches for the hospitalized cases (HC+). When drawing matches, the case order was randomized to minimize the bias resulting from systematic ordering. For each case in the case group (HC+), a match was attempted with patients in the control group (C+). The matching relied on the FUZZY algorithm in Python, which matches cases and controls contained in a single data set. The cases and controls were matched for age and sex. For age, priority was given to exact matches, and when not feasible, it used approximate matching (with tolerance of up to 7 years). Exact equality was used for gender.
2.5. Ethical considerations
The study was approved by the ethics committee of Saint Joseph University with a waiver of consent because the study represented no more than minimal privacy risk to individuals.
2.6. Data collection and statistical methods
We reviewed the electronic files of 474 patients infected with COVID‐19 admitted to the hospital (H) between March 2020 and March 2021. The accuracy of the matching process was checked using the paired T‐test for age (the expected mean difference had to be less than the previously defined tolerance); and that of gender was not tested due to the exact matching algorithm.
Blood groups distribution in these hospitalized patients (HC+) was compared to that of a positive control group (C+) of outpatients infected with COVID‐19 and matched for sex and age, using the McNemar‐Bowker test. The comparison with the distribution of blood groups in the general population (C−) relied on χ 2 tests and expressed as 95% confidence intervals calculated by the exact binomial method. All the analyses were run using SPSS software (IBM Corp. Released 2019, SPSS Statistics for Windows Version 26.0).
This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines. 23
3. RESULTS
The study sample consisted of a total of 474 hospitalized patients with COVID‐19 (HC+), of which 404 (85.2%) had a known blood group. Blood group distribution was as follows: blood group A+ was the predominant group as it was present in 161 patients (39.9%), followed by O+ which was present in 144 patients (35.6%), B+ in 45 patients (11.1%), AB+ in 20 patients (5.0%), O− and A− in 11 patients each (2.7%), B− in 8 patients (2.0%), and AB− in 4 patients (1.0%).
465 controls who tested positive for COVID‐19 but were not hospitalized (C+) could be matched by the algorithm to the hospitalized cases and were enrolled. 368 of them had a known blood group, with the following distribution: 137 had blood group O (37.2%), 177 A (48.1%), 34 B (9.2%), and 20 AB (4.2%).
318 HC+ pairs with available blood group information were matched. The mean age was 64 in the hospitalized group (HC+) and 59 in the outpatients group (C+), and females accounted for 32.5% and 33.1% of HC+ and C+ groups, respectively (Table 1). ABO‐Rhesus blood group distribution for cases, controls, and the general population are shown in Table 2 and Chart 1.
Table 1.
Characteristics of case patients (HC+) and control patients (C+)
| Characteristic | Cases (HC+) (N = 474) | Controls (C+) (N = 465) |
|---|---|---|
| Female sex, n (%) | 154 (32.5 ± 4.1) | 154 (33.1 ± 4.2) |
| Mean age (SD) (year) | 64 (16) | 59 (17) |
Table 2.
ABO‐Rh blood group distribution among COVID‐19‐positive hospitalized patients (HC+), COVID‐19‐positive outpatients (C+), and the general population (C−)
| Cases (HC+) | Controls (C+) | General population (C−) | |||
|---|---|---|---|---|---|
| Value | Percentage | Value | Percentage | Percentage | |
| O+ | 144 | 35.6 | 124 | 33.7 | 38.4 |
| O− | 11 | 2.7 | 13 | 3.5 | 7.7 |
| A+ | 161 | 39.9 | 165 | 44.8 | 32.3 |
| A− | 11 | 2.7 | 12 | 3.3 | 6.5 |
| B+ | 45 | 11.1 | 30 | 8.2 | 9.4 |
| B− | 8 | 2.0 | 4 | 1.1 | 1.7 |
| AB+ | 20 | 5.0 | 18 | 4.9 | 3.2 |
| AB− | 4 | 1.0 | 2 | 0.5 | 0.8 |
| Total | 404 | 100 | 368 | 100 | 100 |
Chart 1.
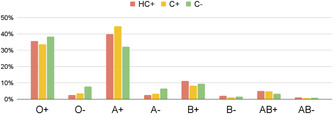
ABO‐Rh blood group distribution among COVID‐19‐positive hospitalized patients (HC+), COVID‐19‐positive outpatients (C+), and the general population (C−)
The proportion of blood group A Rh+ in hospitalized patients with COVID‐19 was 39.9% (CI 35.2%–44.7%), compared to 44.8% (CI 39.8%–49.9%) and 32.3% in the positive outpatient controls (C+) and the general population (C−), respectively. Our analysis shows that both COVID‐19‐positive groups (HC+ and C+), whether inpatients or outpatients, had significantly higher proportions of blood group A‐Rh+ compared to the general population (p = 0.0019 and p < 0.001, respectively). This indicates that blood group A Rh+ is associated with an increased risk of COVID‐19. Although blood group A‐Rh+ was more frequent in the outpatients (C+) compared to the hospitalized group (HC+), the association did not reach statistical significance, indicating that blood group A‐Rh+ is not associated with severity. There was no significant relationship between COVID‐19 and other blood groups.
4. DISCUSSION
This retrospective study was conducted at HDF hospital, one of the largest tertiary care centers and one of the leading centers for COVID‐19 treatment in Lebanon. Herein, we evaluated the relationship between blood groups and COVID‐19 susceptibility and severity and found that blood group A‐Rh+ increased the susceptibility for COVID‐19, but was not associated with increased disease severity.
In March 2020, Zhao et al. were among the first to report a potential association between ABO blood groups and the susceptibility to SARS‐CoV‐2. 24 Since then, numerous studies have reported heterogeneous results, and most have identified a higher proportion of blood group A individuals among COVID‐19 patients compared to healthy controls. 19 , 20 A recent meta‐analysis even classified the risk of infection related to blood groups as follows: A > O > B > AB. 25
To our knowledge, there is only one other Lebanese study that tackles the association between blood groups and COVID‐19 severity. 26 Similar to our findings, Khalil et al. were not able to detect any relationship between blood groups and COVID‐19 severity, but our results bear higher validity because of the matching design and the bigger sample size (n = 404 vs. n = 146). Furthermore, their study does not evaluate susceptibility and does not include Rhesus status.
The relationship between ABO blood groups and infectious diseases is not new. Previous studies have reported an association between blood groups and numerous viruses such as hepatitis B, 27 Dengue virus, 28 Rotavirus, 29 Norovirus, 30 , 31 , 32 and even bacteria such as Helicobacter pylori 33 and Neisseria gonorrhoeae 34 and parasitic microorganisms like Plasmodium falciparum. 35 These studies have shown that ABO blood groups affect the host's genetic susceptibility to various viral diseases and this may also apply to SARS‐Cov‐2.
Exploring this relationship became especially crucial after the beginning of the pandemic, and several hypotheses pertaining to the possible physiopathology of group A sensitivity in COVID‐19 have been proposed. One possibility implicates the presence of an extra N‐acetyl‐ galactosamine sugar on the surface of blood group A cells which could translate into more effective pathogen–host contact. 36 Alternatively, the presence of anti‐A antibodies in the serum of O blood type carriers could inhibit the virus‐cell adhesion process, thus functioning as viral neutralizing antibodies. 37 In other words, the SARS‐CoV‐2 receptor‐binding domains could be specifically bound by human anti‐A antibodies. This would block the interaction with the angiotensin‐converting enzyme 2 receptors (ACE2R), thereby preventing entry into the lung epithelial cells. 38 This would help explain differences in initial susceptibility for SARS‐CoV‐2 infection between different blood groups. Nonetheless, the exact mechanism related to ABO blood groups' different susceptibility to infection remains mostly unclear.
There are some limitations to consider when interpreting ABO and COVID‐19 studies, including the current study. Although this is the largest study regarding ABO and COVID‐19 in Lebanon to date, it is retrospective and involves a single urban academic hospital in the capital, Beirut. These limitations may hinder the generalizability of the results.
Furthermore, our study does not take into account the effect of other risk factors, such as comorbidity and obesity. Comorbid conditions are unevenly distributed among cases and controls, and also among different blood groups. As these established risk factors normally worsen the outcomes of SARS‐CoV‐2 infections, they may play a role of confounders in our analysis. Prospective studies with bigger sample sizes and more rigorous designs that adjust for these confounders are needed to better evaluate the independent effect of blood groups on COVID‐19 outcomes.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
The research in this paper was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
AUTHOR CONTRIBUTIONS
All authors listed have significantly contributed to the investigation, development, drafting of the article, revising it critically for important intellectual content, and the final approval of the version to be submitted.
ACKNOWLEDGMENT
This study did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Kerbage A, Haddad SF, Nasr L, et al. Impact of ABO and Rhesus blood groups on COVID‐19 susceptibility and severity: a case‐control study. J Med Virol. 2022;94:1162‐1166. 10.1002/jmv.27444
Anthony Kerbage and Sara F. Haddad contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data sets generated during and/or analyzed during the current study are available from the corresponding author on request.
REFERENCES
- 1. O'Hearn M, Liu J, Cudhea F, Micha R, Mozaffarian D. Coronavirus disease 2019 hospitalizations attributable to cardiometabolic conditions in the United States: a comparative risk assessment analysis. J Am Heart Assoc. 2021;10(5):e019259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tartof SY, Qian L, Hong V, et al. Obesity and mortality among patients diagnosed with COVID‐19: results from an integrated health care organization. Ann Intern Med. 2020;173(10):773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for COVID‐19 hospital admission. Clin Infect Dis. 2020;71(15):896‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kragholm K, Andersen MP, Gerds TA, et al. Association between male sex and outcomes of Coronavirus Disease 2019 (Covid‐19)—a Danish nationwide, register‐based study. Clin Infect Dis. 2020; 13:924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID‐19‐related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2021 Mar;174(3):362‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID‐19—United States, May‐August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Escobar GJ, Adams AS, Liu VX, et al. Racial disparities in COVID‐19 testing and outcomes: retrospective cohort study in an integrated health system. Ann Intern Med. 2021;174(6):786‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore JT, Ricaldi JN, Rose CE, et al. Disparities in incidence of COVID‐19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020—22 states, February–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(33):1122‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li J, Wang X, Chen J, Cai Y, Deng A, Yang M. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020;190(1):24‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID‐19 infection, intubation, and death. Nat Commun. 2020;11(1):5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latz CA, DeCarlo C, Boitano L, et al. Blood type and outcomes in patients with COVID‐19. Ann Hematol. 2020;99(9):2113‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnkob MB, Pottegård A, Støvring H, et al. Reduced prevalence of SARS‐CoV‐2 infection in ABO blood group O. Blood Adv. 2020;4(20):4990‐4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Severe Covid‐19 GWAS Group, Ellinghaus D, Degenhardt F, et al. Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383(16):1522‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Franchini M, Cruciani M, Mengoli C, et al. ABO blood group and COVID‐19: an updated systematic literature review and meta‐analysis. Blood Transfus. 2021;19(4):317‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ray JG, Schull MJ, Vermeulen MJ, Park AL. Association between ABO and Rh blood groups and SARS‐CoV‐2 infection or severe COVID‐19 illness: a population‐based cohort study. Ann Intern Med. 2021;174(3):308‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. STROBE_checklist_v4_case‐control.pdf [Internet] Accessed: October 23, 2021. https://www.equator-network.org/wp-content/uploads/2015/10/STROBE_checklist_v4_case-control.pdf
- 24. Zhao J, Yang Y, Huang H, et al. Relationship between the ABO blood group and the coronavirus disease 2019 (COVID‐19) susceptibility. Clin Infect Dis. 2021;73(2):328‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kabrah SM, Kabrah AM, Flemban AF, Abuzerr S. Systematic review and meta‐analysis of the susceptibility of ABO blood group to COVID‐19 infection. Transfus Apher Sci. 2021;60(4):103169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khalil A, Feghali R, Hassoun M. The Lebanese COVID‐19 cohort; a challenge for the ABO blood group system. Front Med. 2020;7:585341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang D‐S, Chen D‐L, Ren C, et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer. 2012;131(2):461‐468. [DOI] [PubMed] [Google Scholar]
- 28. Kalayanarooj S, Gibbons RV, Vaughn D, et al. Blood group AB is associated with increased risk for severe dengue disease in secondary infections. J Infect Dis. 2007;195(7):1014‐1017. [DOI] [PubMed] [Google Scholar]
- 29. Sharma S, Hagbom M, Svensson L, Nordgren J. The impact of human genetic polymorphisms on rotavirus susceptibility, epidemiology, and vaccine take. Viruses. 2020;12(3):E324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9(5):548‐553. [DOI] [PubMed] [Google Scholar]
- 31. Hennessy EP, Green AD, Connor MP, Darby R, MacDonald P. Norwalk virus infection and disease is associated with ABO histo‐blood group type. J Infect Dis. 2003;188(1):176‐177. [DOI] [PubMed] [Google Scholar]
- 32. Tan M, Jiang X. Histo‐blood group antigens: a common niche for norovirus and rotavirus. Expert Rev Mol Med. 2014;16:e5. [DOI] [PubMed] [Google Scholar]
- 33. Brandão de Mattos CC, de Mattos LC. Histo‐blood group carbohydrates as facilitators for infection by Helicobacter pylori . Infect Genet Evol. 2017;53:167‐174. [DOI] [PubMed] [Google Scholar]
- 34. Foster MT, Labrum AH. Relation of infection with Neisseria gonorrhoeae to ABO blood groups. J Infect Dis. 1976;133(3):329‐330. [DOI] [PubMed] [Google Scholar]
- 35. Loscertales M‐P, Owens S, O'Donnell J, Bunn J, Bosch‐Capblanch X, Brabin BJ. ABO blood group phenotypes and Plasmodium falciparum malaria: unlocking a pivotal mechanism. Adv Parasitol. 2007;65:1‐50. [DOI] [PubMed] [Google Scholar]
- 36. Silva‐Filho JC, de Melo CGF, de Oliveira JL. The influence of ABO blood groups on COVID‐19 susceptibility and severity: a molecular hypothesis based on carbohydrate–carbohydrate interactions. Med Hypotheses. 2020;144:110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gérard C, Maggipinto G, Minon J‐M. COVID‐19 and ABO blood group: another viewpoint. Br J Haematol. 2020;190(2):e93‐e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goel R, Bloch EM, Pirenne F, et al. ABO blood group and COVID‐19: a review on behalf of the ISBT COVID‐19 Working Group. Vox Sang. 2021;116(8):849‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on request.


