Abstract
This trial aims to evaluate the effectiveness of adding melatonin to the treatment protocol of hospitalized coronavirus disease 2019 (COVID‐19) patients. This was an open‐label, randomized controlled clinical trial in hospitalized COVID‐19 patients. Patients were randomized into a treatment arm receiving melatonin plus standard care or a control arm receiving standard care alone. The trial's primary endpoint was sleep quality examined by the Leeds Sleep Evaluation Questionnaire (LSEQ). The trial's secondary endpoints were symptoms alleviation by Day 7, intensive care unit admission, 10‐day mortality, white blood cell count, lymphocyte count, C‐reactive protein status, and peripheral capillary oxygen saturation. Ninety‐six patients were recruited and allocated to either the melatonin arm (n = 48) or control arm (n = 48). Baseline characteristics were similar across treatment arms. There was no significant difference in symptoms on Day 7. The mean of the LSEQ scores was significantly higher in the melatonin group (p < 0.001). There was no significant difference in laboratory data, except for blood oxygen saturation, which has improved significantly in the melatonin group compared with the control group (95.81% vs. 93.65% respectively, p = 0.003). This clinical trial study showed that the combination of oral melatonin tablets and standard treatment could substantially improve sleep quality and blood oxygen saturation in hospitalized COVID‐19 patients.
Keywords: 2019‐nCoV, COVID‐19, melatonin, N‐acetyl‐5‐methoxytryptamine, SARS‐CoV‐2, sleep quality
Highlights
Melatonin can provide significant improvement in COVID‐19 patients' sleep quality.
Melatonin can be helpful for increasing blood oxygen saturation in COVID‐19 patients in combination with the standard treatment.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and resulting continue to cause a serious public health issue. Currently, there are millions of confirmed cases, including more than three million deaths. 1 The key symptoms of COVID‐19 are cough, fever, and dyspnea from 2 to 14 days after infection. Infected patients can be asymptomatic carriers that can transmit the virus to the community without being affected and presenting symptoms or progress to mild, moderate, and severe stages. 2 , 3 , 4
Several therapeutic agents with different mechanisms have been used against COVID‐19 and studied in clinical trials after months of effort. One of the principal trials, in this case, is the SOLIDARITY trial, 5 , 6 which the World Health Organization (WHO) launched to study the effectiveness of interferon‐beta, chloroquine/hydroxychloroquine, lopinavir‐ritonavir, and remdesivir. The other large trial is the RECOVERY study, 7 , 8 which includes several repurposed drugs (e.g., lopinavir‐ritonavir, azithromycin, hydroxychloroquine, tocilizumab, REGN‐COV2, and dexamethasone) and convalescent plasma administrated on hospitalized COVID‐19 patients in the UK. Repurposing existing pharmaceuticals in such situations could be the best strategy to find an effective and safe agent. However, except the recommendation for steroid use in Severe and Critical stages of disease (https://www.covid19treatmentguidelines.nih.gov), there are no fully approved treatment approaches yet.
Melatonin is the main neurohormone released by the pineal gland and a sleep‐wake cycle regulator. 9 It is also a multifunctional hormone that affects most organ metabolism and plays a positive role in healthiness and aging. This chronobiotic agent could be effective against viral infections due to its antiapoptotic, immunomodulatory, anti‐inflammatory, and antioxidative features. 10 Although an immunomodulatory effect has been hypothesized, the major known effect of melatonin is to recalibrate sleep‐wake rhythms, 11 thereby possibly reducing the need for sedation in severely compromised hospitalized patients. 12
Previous studies have reported the promising performance of melatonin in improving acute respiratory stress caused by bacteria, radiation, virus, and so forth. 13 , 14 , 15 Different reviews have been published in recent months, suggesting the promising effects of melatonin against the COVID‐19 pandemic. 10 , 16 , 17 , 18 , 19 Although there is inadequate experimental/clinical evidence regarding the effectiveness of melatonin against viral infections, especially SAS‐CoV‐2 infection, since there is an urgent need for an economical, viable, and widely available treatment for the current pandemic, it would be prudent to invest in a therapeutic agent that suggested by many scientists with reasonable evidence. In addition, sleep disorder is one of the main complaints among hospitalized COVID‐19 patients, which develop during isolation treatment. 20 Thus, sleep disturbances in such individuals could be regulated with melatonin. Therefore, we conducted a randomized, active‐controlled trial to evaluate the effectiveness of adding melatonin to hospitalized COVID‐19 patients' routine treatment protocol.
2. METHODS
2.1. Study design and participants
We performed an investigator‐initiated, open‐label, randomized parallel‐group, and active‐controlled clinical trial. Patients were recruited from April 14, 2020, to June 15, 2020, from Imam Khomeini Hospital, Mazandaran University of Medical Sciences, Sari, Iran. The trial protocol was approved by the Ethics Committee of Mazandaran University of Medical Sciences (approval number IR.MAZUMS.REC.1399.056) on April 12, 2020, and registered with the Iranian Registry of Clinical Trials (IRCT20200411047030N1).
2.2. Patients
Hospitalized patients with COVID‐19 typical symptoms such as fever, fatigue, cough, and so forth, as well as elevated C‐reactive protein (CRP), were evaluated for inclusion if they were confirmed with chest computed tomography scan findings or reverse transcription‐polymerase chain reaction nasopharyngeal swab for SARS‐CoV‐2 infection. Exclusion criteria were history of epilepsy, coagulation disorders, taking anticoagulants such as warfarin, and uncontrolled diabetes and blood pressure. All patients gave written informed consent for participation in the study.
2.3. Randomization
Patients were allocated to either the melatonin or control group alternately. Physicians, patients, and individuals who assessed the outcomes were not blinded for the assigned treatment.
2.4. Trial procedures
Eligible patients received standard treatment according to the national Iranian treatment guidelines, including hydroxychloroquine, atazanavir, methylprednisolone, azithromycin, naproxen, and Lopinavir/Ritonavir. The intervention group (melatonin group) received a single nightly 3 mg oral melatonin tablet (Norm Life Vanatonin Melatonin) 1 h before bedtime plus standard care for 7 days or until death. Trial protocol adherence was evaluated daily up to Day 10.
2.5. Outcome and data collection
The primary outcome was sleep quality of patients (examined daily by Leeds Sleep Evaluation Questionnaire until Day 7). Secondary endpoints included: (1) symptoms alleviation by Day 7 in the morning in comparison with last Day; (2) intensive care unit (ICU) admission (examined within 10 days of hospitalization); (3) 10‐day mortality; (4) white blood cell (WBC) count; (5) lymphocyte count (tested on Day 1 and Day 7); (6) CRP status (tested on Day 1 and Day 7); and (7) SPO2 (recorded in Day 1 and 7)
2.6. Leeds Sleep Evaluation Questionnaire tool
The Leeds Sleep Evaluation Questionnaire (LSEQ) 21 is a 10‐item, subjective, self‐report tool for evaluating sleep quality during psychopharmacological therapy. The LSEQ scale measures four domains of sleep and wakefulness behavior: (1) ease of getting into sleep; (2) sleep quality; (3) awakening status; and 4) feeling following wakefulness. This scale has been validated through various studies. 22
2.7. Statistical analysis
Categorical variables were analyzed using χ 2 and Fisher's exact test, and continuous variables were compared using the t‐test and the Mann–Whitney U Test. The GLM univariate analysis was done for sex and age adjustments in treatment effect. A p value was considered statistically significant at the p < 0.05 threshold. Statistical analysis was performed using SPSS version 16.0.
3. RESULTS
To conduct this trial, between April 14 and June 15, 2020, 105 patients were assessed for enrollment, of which 96 patients were eligible for entering the study. Out of 96 participants, 48 patients were randomized to the melatonin group and 48 patients to the control group (Figure 1). The mean age of cases and controls was 51.06 (±15.86) and 54.77 (±15.34), respectively, and 55.2% of all patients were females (Table 1). The mean duration of symptoms onset to hospital admission was about eight days in both groups, and treatment duration (admission to discharge/death) was about 6 days (Table 1).
Figure 1.
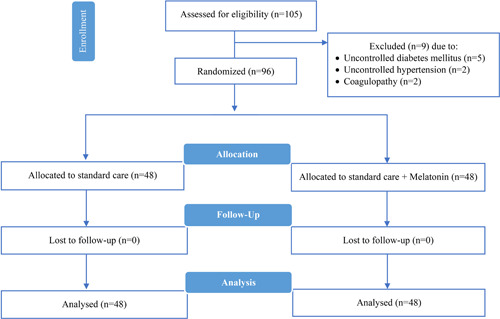
CONSORT 2010 flow diagram
Table 1.
Baseline patient characteristics
| Demographical history | Melatonin (n = 48) | Control (n = 48) | p value |
|---|---|---|---|
| Age, mean (±SD) | 51.06 (±15.86) | 54.77 (±15.34) | 0.247 |
| Sex, n (%) | |||
| Male | 25 (52.1) | 18 (37.5) | 0.218 |
| Female | 23 (47.9) | 30 (62.5) | |
| BMI, mean (±SD) | 28.10 (±5.06) | 28.46 (±3.88) | 0.695 |
| Education, n (%) | |||
| Illiterate | 9 (19.1) | 16 (33.3) | 0.306 |
| Primary | 7 (14.9) | 3 (6.2) | |
| Secondary | 15 (31.9) | 14 (29.2) | |
| Higher | 16 (34.0) | 15 (31.2) | |
| Onset of symptoms to admit (day) mean (±SD) | 8.10 (±3.85) | 8.50 (±4.41) | 0.679 |
| Admission to discharge/death (day), mean (±SD) | 6.12 (±2.01) n = 47 | 6.58 (±2.13) | 0.121 |
| ICU admission, n (%) | 6 (12.5) | 10 (20.8) | 0.412 |
| Comorbidities, n (%) | |||
| Diabetes mellitus | 17 (35.4) | 10 (20.8) | 0.173 |
| Asthma | 3 (6.2) | 2 (4.2) | 1.000 |
| Renal failure | 0 (0.0) | 5 (10.4) | 0.056 |
| Cardiovascular disease | 7 (14.6) | 8 (16.7) | 1.000 |
| Hypertension | 17 (35.4) | 12 (25.0) | 0.374 |
| Thalassemia | 2 (4.2) | 1 (2.1) | 1.000 |
| Thyroid disorders | 2 (4.2) | 6 (12.5) | 0.268 |
| Chronic obstructive pulmonary disease | 1 (2.1) | 2 (4.2) | 1.000 |
| Clinical data at inclusion (Day 1) | |||
| Lung involvement in CT‐scan (%), mean (±SD) | 29.04 (±12.27) n = 47 | 31.56 (15.88) | 0.191 |
| Fever, n (%) | 48 (100.0) | 40 (83.3) | 0.006 |
| Shaking, n (%) | 41 (85.4) | 31 (64.6) | 0.033 |
| Headache, n (%) | 31 (64.6) | 22 (45.8) | 0.100 |
| Pharyngitis, n (%) | 27 (56.2) | 20 (41.7) | 0.220 |
| Chest pain, n (%) | 26 (54.2) | 22 (45.8) | 0.541 |
| Dyspnea, n (%) | 43 (89.6) | 37 (77.1) | 0.170 |
| Olfactory disorder, n (%) | 20 (41.7) | 12 (25.0) | 0.129 |
| Taste disorder, n (%) | 18 (37.5) | 12 (25.0) | 0.271 |
| Nausea, n (%) | 22 (45.8) | 19 (39.6) | 0.680 |
| Vomiting, n (%) | 11 (22.9) | 7 (14.6) | 0.433 |
| Diarrheal, n (%) | 11 (22.9) | 4 (8.3) | 0.089 |
| Abdominal pain, n (%) | 9 (18.8) | 5 (10.4) | 0.386 |
| Anorexia, n (%) | 44 (91.7) | 33 (68.8) | 0.009 |
| Myalgia, n (%) | 39 (81.2) | 28 (58.3) | 0.025 |
| Laboratory data at inclusion (Day 1) | |||
| WBC, ×109/L | 6.45 (±2.93) n = 39 | 5.84 (±1.87) n = 41 | 0.607 |
| Lymphocyte count, ×109/L | 0.97 (±0.38) n = 47 | 0.98 (±0.39) | 0.803 |
| C‐reactive protein, mg/L | 50.94 (±29.34) | 51.15 (±24.61) | 0.111 |
| SpO2, % | 92.85 (±5.29) | 92.19 (±3.92) | 0.188 |
| Treatment regimen, n (%) | |||
| Chloroquine/hydroxychloroquine | 46 (95.8) | 48 (100.0) | 0.495 |
| Lopinavir/ritonavir | 2 (4.2) | 0 (0.0) | 0.495 |
| Atazanavir | 46 (95.8) | 48 (100.0) | 0.495 |
| Naproxen | 6 (12.5) | 1 (2.1) | 0.111 |
| Azithromycin | 48 (100.0) | 48 (100.0) | – |
| Methylprednisolone | 33 (68.8) | 46 (95.8) | 0.001 |
Abbreviations: BMI, body mass index; CT, computed tomography; ICU, intensive care unit; SD, standard deviation; WBC, white blood cells.
3.1. Primary outcomes
The mean of the LSEQ score was significantly higher in the melatonin group for all four domains versus the control group (Figure 2).
Figure 2.
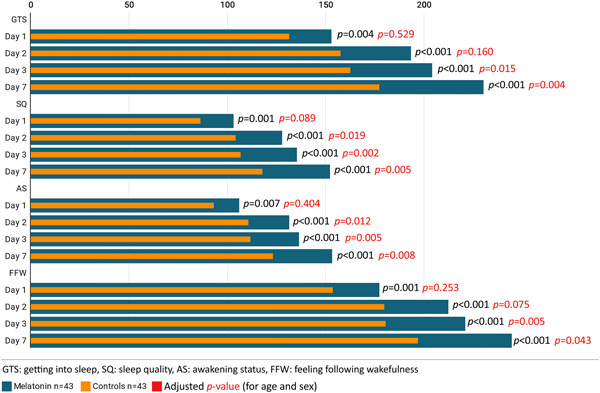
Leeds sleep evaluation questionnaire score of melatonin arm versus control arm
3.2. Secondary outcomes
Regarding symptom alleviation, there was no significant difference between the melatonin group and controls for all the considered symptoms on Day 7 (Table 2). Six patients from the melatonin group (12.5%) and 10 patients from the control group (20.8%) were admitted to ICU; however, it was nonsignificant (p = 0.412) (Table 1). As presented in Table 2, laboratory data evaluation in Day 7 indicated no significant differences between the two groups regarding WBC count, lymphocyte count, and CRP levels. However, blood oxygen saturation has improved significantly in the melatonin group compared with the control group (95.81% vs. 93.65%, respectively, p = 0.003). On the 10th day of follow‐up, one patient from the melatonin group (2.1%) and three patients from the control group (6.2%) died, but it was not significant (p = 0.617) (Table 2).
Table 2.
Treatment response monitoring
| Melatonin (n = 48) | Control (n = 48) | p value | |
|---|---|---|---|
| Symptoms duration (day), mean (±SD) | |||
| Fever | 7.45 (±3.50) n = 47 | 6.50 (±3.80) n = 40 | 0.160 |
| Shaking | 6.81 (±3.66) n = 36 | 5.81 (±4.15) n = 31 | 0.218 |
| Headache | 5.65 (±4.11) n = 26 | 5.52 (±3.29) n = 21 | 0.804 |
| Pharyngitis | 7.40 (±4.04) n = 20 | 6.39 (±3.98) n = 18 | 0.433 |
| Chest pain | 4.44 (±2.23) n = 25 | 4.24 (±2.11) n = 21 | 0.718 |
| Dyspnea | 3.68 (±2.27) n = 41 | 3.38 (±1.86) n = 37 | 0.414 |
| Olfactory disorder | 6.20 (±3.64) n = 15 | 5.82 (±2.52) n = 11 | 0.852 |
| Taste disorder | 5.33 (±3.42) n = 12 | 6.17 (±3.40) n = 12 | 0.613 |
| Nausea | 5.00 (±2.92) n = 19 | 3.58 (±2.38) n = 19 | 0.127 |
| Vomiting | 3.43 (±2.99) n = 7 | 3.00 (±1.89) n = 6 | 0.882 |
| Diarrheal | 3.78 (±2.94) n = 9 | 3.75 (±3.59) n = 4 | 0.753 |
| Abdominal pain | 2.60 (±1.14) n = 5 | 4.75 (±2.63) n = 4 | 0.174 |
| Anorexia | 5.73 (±3.37) n = 41 | 5.90 (±3.33) n = 31 | 0.764 |
| Myalgia | 6.24 (±3.54) n = 37 | 6.07 (±3.57) n = 27 | 0.815 |
| Laboratory data in Day 7 | |||
| WBC count, ×109/L | 8.68 (±4.31) n = 43 | 9.75 (±3.68) n = 34 | 0.158 |
| Lymphocyte count, ×109/L | 1.18 (±0.56) n = 47 | 1.03 (±0.46) | 0.177 |
| C‐reactive protein, mg/L | 17.89 (±15.72) | 18.54 (±14.96) | 0.703 |
| SpO2, % | 95.81 (±3.61) | 93.65 (±4.22) | 0.003 |
| Patient's outcome, n (%) | |||
| Recovered and discharged | 47 (97.9) | 45 (93.8) | 0.617 |
| Died | 1 (2.1) | 3 (6.2) |
Abbreviations: SD, standard deviation; WBC, white blood cells.
4. DISCUSSION
In this randomized clinical trial, including 96 individuals hospitalized due to SARS‐CoV‐2 infection, melatonin plus standard treatment compared with standard treatment alone substantially improved the sleep quality and SpO2 of COVID‐19 patients in 7 days of treatment. However, melatonin was not associated with the alleviation of clinical symptoms, ICU admission, and death.
Current evidence shows that various antiviral, anti‐inflammatory, anti‐fungal, immunomodulatory, and other therapeutic agents, which were specified and approved for different purposes such as hepatitis, malaria, influenza, human immunodeficiency virus (HIV), immunodeficiency disorders, and so forth, have been administered off label globally for the treatment of COVID‐19 patients since the rise of this infection in December 2019.
For example, one of the most controversial agents was hydroxychloroquine (HCQ), which has attracted scientists' attention at the early onset of the pandemic. However, after conducting numerous studies on the subject, HCQ showed no clinical benefits in COVID‐19 patients, and this arm has been removed from SOLIDARITY and RECOVERY trials. 23 Two other potential therapeutic candidates were lopinavir‐ritonavir 24 , 25 and favipiravir, 26 , 27 , 28 which have not shown clear clinical efficacy. Moreover, although findings regarding remdesivir—the other debated antiviral—were different, promising effects weighed more, 29 , 30 , 31 , 32 and the United States Food and Drug Administration (FDA) has authorized emergency use of remdesivir for hospitalized COVID‐19 patients. 33 In addition to potential antivirals RECOVERY study, revealed that there is a significant survival effect given by to the dexamethasone administration in critical COVID‐19 patients during the hypoxic phase of the infection. 34 Nevertheless, no medications have been proven to be a certain effective agent against COVID‐19.
In addition to the ongoing treatment approaches, multifunctional molecule melatonin has attracted scientists' attention for months, mainly due to its anti‐inflammatory, antioxidative, and immunomodulatory functions. 35 In this case, previous studies demonstrate the successful performance of melatonin against sleep disorders, respiratory and atherosclerosis diseases, and viral infections (e.g., respiratory syncytial virus, Venezuelan equine encephalitis virus, hepatitis, and Ebola). 35 , 36 , 37 Although the role of melatonin in bat antiviral immunity is unclear, it seems that this agent has a potential role against SARS‐CoV‐2 through various pathways. 36 , 38 Investigations showed that melatonin levels in different bats species range from 60 to 500 pg/ml and 20–90 pg/ml during night and day, respectively. 39 , 40 However, melatonin concentration in humans is substantially lower, especially in older people. It has been reported that the melatonin night peak in the elderly is 11.2 (±1.6) pg/ml, which is significantly higher in young individuals (83 ± 20 pg/ml). Interestingly, the highest level of melatonin has been assessed in children between 1 and 3 years old with 329.5 (±42) pg/ml, 41 , 42 those who seem to be much less likely to get COVID‐19 in comparison with older ages. 43 This is one of the leading hypotheses toward less deterioration in adolescents than in elderly COVID‐19 patients.
Mortality in the present study was not a primary outcome, however, results show that the rate of mortality in the intervention group was less than the control group, but this difference was not significant. Ramlall et al. 44 study show that melatonin exposure after intubation was significantly associated with a positive outcome in COVID‐19 and non‐COVID‐19 patients.
Unfortunately, after months of study and many calls for clinical trials on melatonin effectiveness, there are still no published results in this case. Also, searching terms of “COVID‐19” AND “melatonin” resulted in eight registered trials in ClinicalTrials.gov, two trials in the EU Clinical Trials Register system, and four trials in the Iranian Registry of Clinical Trials on the efficacy and safety of oral or intravenous melatonin administration in COVID‐19 patients. In this regard, only Jehi et al., 45 in their statistical model study on predicting SARS‐CoV‐2 infection through various aspects, found that the risk of being positive for the COVID‐19 test was reduced in individuals who were on melatonin intake.
To the best of our knowledge, the present study is the first clinical trial on the subject that indicated a very significant effect of melatonin on the sleep quality of COVID‐19 patients, which was expected according to previous studies in different populations. 46 , 47 , 48 Besides, different people, regardless of being infected or not, might be affected during the pandemic and experience degrees of sleep disorder. 49 , 50 , 51 , 52 In one case series in 10 patients, clinical stabilization and/or improvement was noted within 4–5 days after initiation of high‐dose melatonin in all COVID‐19 patients. 53
Hence, given the available evidence and our findings, melatonin could be a useful supplement for both the healthy population and COVID‐19 patients. Moreover, significant improvement of blood oxygen saturation in the melatonin group compared to the controls could be due to melatonin's influence on oxygen delivery and its utilization in tissues as one of the functions of melatonin, which studied experimentally and clinically. 54 , 55 , 56 , 57 Decrease vascular permeability, therefore decreased pulmonary infiltration could be another explanation, albeit the present study did not evaluate pulmonary infiltration resolution during the hospitalization period between the two groups, which could be evaluated in further studies. Hence, it seems that it would be prudent to invest in more extensive clinical trials to find more about the melatonin associations with COVID‐19 outcomes. In this case, a study on prophylactic effects of melatonin against COVID‐19 is highly recommended by the authors.
In the present study, the intervention group received daily 3 mg oral melatonin tablets. The best dose of melatonin for older adults has not been determined yet. 58 Albeit the reports regarding the accepted safety profile of higher doses of melatonin in some animal/human studies, 59 at the time of the current study design and period, some of them have not been issued. Moreover, paucity of data for melatonin administration logic and efficacy in COVID‐19 patients with some concerns about the adverse effects of a higher dose of the drug in patients with underling considerable prevalence of nausea/vomiting and headache 60 which could be seen as the melatonin adverse effect either‐ have all played roles for the 3 mg dose selection of the melatonin for the current study. However, with some promising results of this study, It would be prudent to invest in more studies with larger sample sizes and a higher dose of melatonin.
This study has several limitations as follows: (1) the study was open‐label due to time restriction for placebo production in the current situation, (2) study was performed among hospitalized patients and hospital conditions may affect the sleep quality; and (3) the small sample size would lead to type II error. Future larger clinical trials are needed to robust the findings of this study with prolonged period and more detailed information.
5. CONCLUSION
This clinical trial study showed that the combination of oral melatonin tablets and standard treatment could substantially improve sleep quality and blood oxygen saturation after 7 days of treatment in hospitalized COVID‐19 patients.
CONFLICT INTERESTS
The authors declare that there are no conflict interests.
ETHICS STATEMENT
The trial protocol was approved by the Ethics Committee of Mazandaran University of Medical Sciences (approval number IR.MAZUMS.REC.1399.056) on April 12, 2020, and registered with the Iranian Registry of Clinical Trials (IRCT20200411047030N1).
AUTHOR CONTRIBUTIONS
Seyed Abbas Mousavi, Reza Alizadeh‐Navaei, Majid Saeedi, and Amir Shamshirianh designed the study. Reza Alizadeh‐Navaei worked on the statistical analysis. Hossein Mehravaran, Keyvan Heydari, and Amir Shamshirianh performed the research. Amir Shamshirian wrote the first draft of the manuscript. Akbar Hedayatizadeh‐Omran, Seyed Abbas Mousavi, Reza Alizadeh‐Navaei, Majid Saeedi, and Hossein Mehravaran helped with the preparation of the manuscript. All authors have read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors appreciate the Vice‐Chancellor for Research at Mazandaran University of Medical Sciences for the current study's approval and support. The study has funded by the Vice‐Chancellor for Research at Mazandaran University of Medical Sciences.
Mousavi SA, Heydari K, Mehravaran H, et al. Melatonin effects on sleep quality and outcomes of COVID‐19 patients: an open‐label, randomized, controlled trial. J Med Virol. 2021;94:263‐271. 10.1002/jmv.27312
Contributor Information
Hossein Mehravaran, Email: lab2002b@yahoo.com.
Amir Shamshirian, Email: shamshirian.amir@gmail.com, Email: a.shamshirian@uq.net.au.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. WHO . Coronavirus disease (COVID‐19). 2020.
- 2. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guan W‐j, Ni Z‐y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss SL, Peters MJ, Alhazzani W, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID‐19). Intensive Care Med. 2020;46:1‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahase E. Covid‐19: what treatments are being investigated? BMJ. 2020;368:m1252. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization (WHO) . Solidarity” clinical trial for COVID‐19 treatments. World Health Organization (WHO) Situation reports Geneva: WHO. Accessed April 5, 2020. https://www.whoint/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments 2020
- 7. RECOVERY . A randomised trial of treatments to prevent death in patients hospitalised with COVID‐19 (coronavirus). 2020. http://www.isrctn.com/ISRCTN50189673
- 8. Normand S‐LT. The RECOVERY platform. N Engl J Med. 2020;384:757‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017;34:10‐22. [DOI] [PubMed] [Google Scholar]
- 10. Zhang R, Wang X, Ni L, et al. COVID‐19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zambrelli E, Canevini M, Gambini O, D'Agostino A. Delirium and sleep disturbances in COVID‐19: a possible role for melatonin in hospitalized patients? Sleep Med. 2020;70:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mistraletti G, Umbrello M, Sabbatini G, et al. Melatonin reduces the need for sedation in ICU patients: a randomized controlled trial. Minerva Anestesiol. 2015;81(12):1298‐1310. [PubMed] [Google Scholar]
- 13. Yip HK, Chang YC, Wallace CG, et al. Melatonin treatment improves adipose‐derived mesenchymal stem cell therapy for acute lung ischemia–reperfusion injury. J Pineal Res. 2013;54(2):207‐221. [DOI] [PubMed] [Google Scholar]
- 14. Wu X, Ji H, Wang Y, et al. Melatonin alleviates radiation‐induced lung injury via regulation of miR‐30e/NLRP3 Axis. Oxid Med Cell Longevity. 2019;2019:4087298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang SH, Cao XJ, Liu W, Shi XY, Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res. 2010;48(2):109‐116. [DOI] [PubMed] [Google Scholar]
- 16. Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID‐19 pandemic? Int Rev Immunol. 2020;39:1‐10. [DOI] [PubMed] [Google Scholar]
- 17. Reiter RJ, Abreu‐Gonzalez P, Marik PE, Dominguez‐Rodriguez A. Therapeutic algorithm for use of melatonin in patients with COVID‐19. Front Med. 2020;7:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El‐Missiry MA, El‐Missiry ZMA, Othman AI. Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of COVID‐19. Eur J Pharmacol. 2020;882:173329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Acuña‐Castroviejo D, Escames G, Figueira JC, de la Oliva P, Borobia AM, Acuña‐Fernández C. Clinical trial to test the efficacy of melatonin in COVID‐19. J Pineal Res. 2020;69(3):e12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu K, Chen Y, Wu D, Lin R, Wang Z, Pan L. Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID‐19. Complement Ther Clin Pract. 2020;39:101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parrott AC, Hindmarch I. The Leeds Sleep Evaluation Questionnaire in psychopharmacological investigations: a review. Psychopharmacology. 1980;71(2):173‐179. [DOI] [PubMed] [Google Scholar]
- 22. Tarrasch R, Laudon M, Zisapel N. Cross‐cultural validation of the Leeds Sleep Evaluation Questionnaire (LSEQ) in insomnia patients. Hum Psychopharmacol. 2003;18(8):603‐610. [DOI] [PubMed] [Google Scholar]
- 23. Pathak DSK, Salunke DAA, Thivari DP, et al. No benefit of hydroxychloroquine in COVID‐19: results of systematic review and meta‐analysis of randomized controlled trials. Diabetes Metab Syndrome. 2020;14(6):1673‐1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osborne V, Davies M, Lane S, et al. Lopinavir‐ritonavir in the treatment of COVID‐19: a dynamic systematic benefit‐risk assessment. Drug Saf. 2020;43(8):809‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID‐19. N Engl J Med. 2020;382(19):1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lou Y, Liu L, Yao H, et al. Clinical outcomes and plasma concentrations of baloxavir marboxil and favipiravir in COVID‐19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 2021;157:105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID‐19: a randomized clinical trial. MedRxiv. 2020. [Google Scholar]
- 28. Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID‐19: an open‐label control study. Engineering. 2020;6:1192‐1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2020;395(10236):1569‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe COVID‐19. N Engl J Med. 2020;382(24):2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Glaus MJ, Von Ruden S. Remdesivir and COVID‐19. Lancet. 2020;396(10256):952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frediansyah A, Nainu F, Dhama K, Mudatsir M, Harapan H. Remdesivir and its antiviral activity against COVID‐19: A systematic review. Clin Epidemiol Glob Health. 2020;9:123‐127. 10.1016/j.cegh.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. US Food and Drug Administration . Emergency Use Authorization (EUA) information, and list of all current EUAs. 2020.
- 34. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID‐19: preliminary report. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kleszczyński K, Slominski AT, Steinbrink K, Reiter RJ. Clinical trials for use of melatonin to fight against COVID‐19 are urgently needed. Nutrients. 2020;12(9):2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bahrampour Juybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S. Melatonin potentials against viral infections including COVID‐19: current evidence and new findings. Virus Res. 2020;287:198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson G, Reiter RJ. Melatonin: roles in influenza, COVID‐19, and other viral infections. Rev Med Virol. 2020;30(3):e2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID‐19 pandemic? Int Rev Immunol. 2020;39(4):153‐162. [DOI] [PubMed] [Google Scholar]
- 39. Tresguerres M, Parks SK, Goss GG. V‐H(+)‐ATPase, Na(+)/K(+)‐ATPase and NHE2 immunoreactivity in the gill epithelium of the Pacific hagfish (Epatretus stoutii). Comp Biochem Physiol A Mol Integr Physiol. 2006;145(3):312‐321. [DOI] [PubMed] [Google Scholar]
- 40. Heideman PD, Bhatnagar KP, Hilton FK, Bronson FH. Melatonin rhythms and pineal structure in a tropical bat, Anoura geoffroyi, that does not use photoperiod to regulate seasonal reproduction. J Pineal Res. 1996;20(2):90‐97. [DOI] [PubMed] [Google Scholar]
- 41. Waldhauser F, Weiszenbacher G, Tatzer E, et al. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab. 1988;66(3):648‐652. [DOI] [PubMed] [Google Scholar]
- 42. Iguchi H, Kato K‐I, Ibayashi H. Age‐dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endocrinol Metab. 1982;55(1):27‐29. [DOI] [PubMed] [Google Scholar]
- 43. CDC COVID‐19 Response Team . Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramlall V, Zucker J, Tatonetti N. Melatonin is significantly associated with survival of intubated COVID‐19 patients. medRxiv [Preprint]. Published online October 18, 2020.
- 45. Jehi L, Ji X, Milinovich A, et al. Individualizing risk prediction for positive coronavirus disease 2019 testing: results from 11,672 patients. Chest. 2020;158(4):1364‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sletten TL, Magee M, Murray JM, et al. Efficacy of melatonin with behavioural sleep‐wake scheduling for delayed sleep‐wake phase disorder: a double‐blind, randomised clinical trial. PLoS Med. 2018;15(6):e1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Serfaty MA, Osborne D, Buszewicz MJ, Blizard R, Raven PW. A randomized double‐blind placebo‐controlled trial of treatment as usual plus exogenous slow‐release melatonin (6 mg) or placebo for sleep disturbance and depressed mood. Int Clin Psychopharmacol. 2010;25(3):132‐142. [DOI] [PubMed] [Google Scholar]
- 48. Rondanelli M, Opizzi A, Monteferrario F, Antoniello N, Manni R, Klersy C. The effect of melatonin, magnesium, and zinc on primary insomnia in long‐term care facility residents in Italy: a double‐blind, placebo‐controlled clinical trial. J Am Geriatr Soc. 2011;59(1):82‐90. [DOI] [PubMed] [Google Scholar]
- 49. Simpson N, Manber R. Treating Insomnia during the COVID‐19 pandemic: observations and perspectives from a behavioral sleep medicine clinic. Behav Sleep Med. 2020;18(4):573‐575. [DOI] [PubMed] [Google Scholar]
- 50. Seghi F, Barbini B, Franchini L, Colombo C. The challenge of mental health during COVID‐19 outbreak: experience from metropolitan area of Milan. Eur Arch Psychiatry Clin Neurosci. 2020;271:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Morin CM, Carrier J. The acute effects of the COVID‐19 pandemic on insomnia and psychological symptoms. Sleep Med. 2021;77:346‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Altena E, Baglioni C, Espie CA, et al. Dealing with sleep problems during home confinement due to the COVID‐19 outbreak: practical recommendations from a task force of the European CBT‐I Academy. J Sleep Res. 2020;29(4):e13052. [DOI] [PubMed] [Google Scholar]
- 53. Castillo RR, Quizon GRA, Juco MJM, et al. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC‐19 PRO): a case series. Melatonin Res. 2020;3(3):297‐310. [Google Scholar]
- 54. Zinchuk VV, Poluyan IA, Hlutkin SV. Effects of melatonin on the oxygen transport in blood, gas transmitters, and prooxidant–antioxidant balance in the exercise. Hum Physiol. 2019;45(6):693‐700. [Google Scholar]
- 55. Zinchuk VV, Firago ME. Participation of melatonin in regulation of blood oxygen‐transport function in oxidative stress induced by injection of lipopolisaccharide. Biomed Khim. 2017;63(6):520‐526. [DOI] [PubMed] [Google Scholar]
- 56. Manchester LC, Coto‐Montes A, Boga JA, et al. Melatonin: an ancient molecule that makes oxygen metabolically tolerable. J Pineal Res. 2015;59(4):403‐419. [DOI] [PubMed] [Google Scholar]
- 57. Hlutkin S, Zinchuk V. Effect of melatonin on the blood oxygen transport during hypothermia and rewarming in rats. Adv Med Sci. 2008;53(2):234‐239. [DOI] [PubMed] [Google Scholar]
- 58. Reiter RJ, Abreu‐Gonzalez P, Marik PE, Dominguez‐Rodriguez A. Therapeutic algorithm for use of melatonin in patients with COVID‐19. Front Med. 2020;7(226):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tan D‐X, Hardeland R. Estimated doses of melatonin for treating deadly virus infections: focus on COVID‐19. Melatonin Res. 2020;3(3):276‐296. [Google Scholar]
- 60. Centers for Disease Control and Prevention . Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID‐19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


