Abstract
The global disruption of the COVID‐19 pandemic has impacted the life of every child either directly or indirectly. This review explores the pathophysiology, immune response, clinical presentation and treatment of COVID‐19 in children, summarising the most up‐to‐date data including recent developments regarding variants of concern. The acute infection with SARS‐CoV‐2 is generally mild in children, whilst the post‐infectious manifestations, including paediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS) and ‘long COVID’ in children, are more complex. Given that most research on COVID‐19 has focused on adult cohorts and that clinical manifestations, treatment availability and impacts differ markedly in children, research that specifically examines COVID‐19 in children needs to be prioritised.
Keywords: COVID‐19, management, PIMS‐TS, SARS‐CoV‐2, virology
Key Points.
The mild nature of COVID‐19 in most children points to important age‐related factors in the pathogenesis of disease. Emerging evidence suggests differences in innate and adaptive immunity.
COVID‐19 in children consists of acute COVID‐19 ‐ both complicated and uncomplicated ‐, post‐infectious multi‐system inflammatory syndromes and post‐acute sequelae of COVID‐19 (PASC) also known as ‘Long COVID’. ‘Long COVID’ in children is poorly characterised, but likely less frequent than in adults.
The management of acute COVID‐19 in children is based at present on data from adult trials, and management of multi‐system inflammatory syndromes on observational data. In both cases, anti‐inflammatory treatments appear to show greatest benefit.
The World Health Organisation (WHO) declared coronavirus disease 2019 (COVID‐19) a global pandemic on 11 March 2020, and spread of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has brought unprecedented health, social and economic disruption world‐wide. 1 World‐wide, the research community has mobilised to generate an astounding volume of data regarding disease pathogenesis as well as approaches to diagnosis and management of COVID‐19. This review (Part II of II) aims to summarise the current understanding of COVID‐19 disease in children. The authorship group have together been active across research, clinical care, treatment guidelines, policy development and advocacy with respect to COVID‐19 in children.
Pathogenesis of COVID‐19
Viral cell entry and replication
SARS‐CoV‐2 is from the Coronaviridae family of enveloped, positive sense, single‐stranded ribonucleic acid (RNA) viruses, with relatedness to SARS‐CoV (the virus that caused the SARS pandemic of 2002) and other bat‐origin betacoronaviruses. 2 The virus spike (S) protein is both key to human infection and the major antigen for humoral immunity and as such is the antigen used in licenced vaccines employed in most jurisdictions. 3 The primary human cell receptor for the S protein receptor binding domain (RBD) is Angiotensin‐Converting Enzyme 2 (ACE2), with a binding affinity much higher in SARS‐CoV‐2 compared to SARS‐CoV. 4 Mutations in the SARS‐CoV‐2 RBD are associated with enhanced ACE2 affinity and are considered to underpin key characteristics of variants such as Delta (B.1.617.2), which show increased transmissibility and immune evasion. 5
The ACE2 protein is present on multiple human epithelial surfaces including the upper and lower respiratory tract, gastrointestinal tract and endovascular epithelia. Limited evidence suggests ACE2 expression in the upper respiratory epithelium increases across the age‐spectrum. 6 SARS‐CoV‐2 downregulates ACE2 expression following infection and this may contribute in the lung pathology, and more widely to dysregulation of angiotensin‐related physiology in particular with respect to endothelial function and inflammation. Paradoxically, children show increased ACE2 protein density on pneumocytes, which may confer protection against dysregulation of the angiotensin system during acute COVID‐19. 7
Immunological response in children
The immune response in children to SARS‐CoV‐2 is of particular interest for two reasons 1 : the apparent mild acute spectrum of disease may yield important lessons for management of severe disease in adults, and 2 the phenomenon of post‐infectious systemic inflammation – namely paediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS) – which occurs mostly in children may reveal new insights into aberrant adaptive immune responses.
Recent efforts have begun to differentiate the SARS‐CoV‐2 immune response between adults and children. One Australian study of a family with SARS‐CoV‐2 infection showed that both adults and children mounted similar cellular, antibody and mucosal adaptive immune responses to SARS‐CoV‐2. 8 However, some of the children did not have detectable SARS‐CoV‐2 nucleic acid by polymerase chain reaction (PCR) testing and had minimal or mild symptoms, in contrast to the parents who were symptomatic and PCR positive. 8 The authors suggested that mucosal immunity in children may prevent the establishment of SARS‐CoV‐2 infection. This finding is now supported by detailed research showing an increased innate anti‐viral response in the upper airways of children compared to adults. 9
Interestingly, there appear to be differences in circulating innate immune cells in children compared to adults during SARS‐CoV‐2 infection. 10 Additionally, children with SARS‐CoV‐2 infection have a more targeted antibody response compared to adults. Furthermore, anti‐spike antibodies from children appear to have less neutralising activity. 11 Whilst this does not explain the milder infections in children, the authors argue that the more restricted and less functional antibody response may be secondary to better control of the virus by innate or T cell immune responses in children. 11 Somewhat paradoxically healthy elderly individuals have higher titres of cross‐reactive SARS‐CoV‐2 immunoglobulins (active against a range of human coronaviruses) compared to children, 12 and severe COVID‐19 in adults is associated with cross‐reactive low avidity SARS‐CoV‐2‐specific CD4+ T cells. 13
Taken together, it appears likely that innate immune responses in the upper respiratory tract may be more effective in children and that prior and more frequent common coronavirus infections in adults may result in immunological memory that hampers, rather than enhances, the antigen‐specific immune response to a neoantigen such as SARS‐CoV‐2. 12 Furthermore, senescence of the immune system with impaired thymic output and T‐cell receptor repertoire in the elderly, 14 and possible impacts on innate immune function of obesity and the metabolic syndrome, 15 may further impair immune responses to SARS‐CoV‐2.
Studies of human leukocyte antigen (HLA) genotype associations with disease severity are few. 16 Limited evidence suggests particular HLA genotypes may confer vulnerability to (or protection from) severe disease, and differences may underly variability in disease spectrum among ethnic groups, 17 but further validation is required in larger patient groups. A full understanding of the immunology in adults versus children is still evolving with many aspects not yet fully elucidated.
Clinical Presentation of COVID‐19 Disease in Children
Mild disease not requiring hospitalisation
The majority of children with COVID‐19 have mild disease, with asymptomatic infection reported in 15–42% of children (Fig. 1). 18 , 22 However, accuracy of prevalence estimates in countries with widespread and high daily case numbers of COVID‐19 is limited by low case ascertainment of paucisymptomatic children. 23
Fig 1.
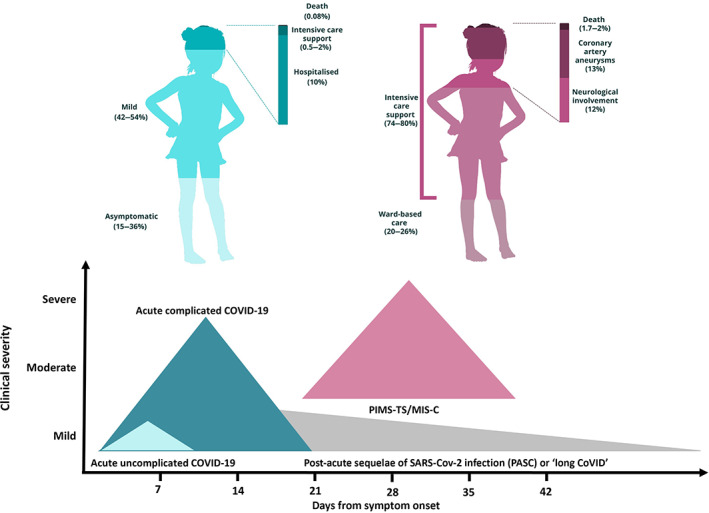
Overall severity of COVID‐19 disease in children. 18 , 19 , 20 , 21 The left‐hand panel depicts outcomes from acute COVID‐19 infection; the right‐hand panel represents outcomes from PIMS‐TS in children. Estimates of the proportion hospitalised and those requiring intensive care shown here are higher than are being observed in Australia in 2021 (Hospitalised ~1% for medical reasons and ICU admitted ~0.1% of symptomatic cases; unpublished data, PN Britton).
Children with symptomatic COVID‐19 infection usually present with one or more respiratory symptoms, which are indistinguishable from seasonal respiratory viral infections, most frequently fever and cough (Table 1). 22 Non‐specific presentations are common and it is possible that these have been under‐recognised and under‐reported in many studies. 24 Illness duration increases with age in children with a median duration of 6 days in school‐aged cohorts. 24
Table 1.
Frequency of symptoms in children diagnosed with COVID‐19 infection
| Symptom | Frequency in children with COVID‐19 infection | Reference |
|---|---|---|
| Fever | 46–64% | 22 |
| Cough | 32–56% | 22 |
| Rhinorrhoea | <10–20% | 23 |
| Sore throat | <10–20% | 23 |
| Dyspnoea | <10–20% | 23 |
| Headache and malaise | Up to 60%† | 24 |
| Gastrointestinal symptoms (diarrhoea, nausea, vomiting and/or abdominal pain) | 10–20% | 22, 23 |
| Other: fatigue, myalgia, arthralgia, rash, conjunctivitis, disturbances of smell or taste | Up to 20% | 22 |
Most common in adolescents.
Children with mild or asymptomatic COVID‐19, representing the majority of paediatric cases, can be managed safely without hospitalisation. Adequate hydration and supportive care are the primary management priorities in these children, in common with other respiratory viruses. In well‐resourced settings, hospital in the home services provides additional support, including a medical escalation pathway, often operating predominantly via telehealth.
Pulmonary disease in hospitalised children
Hospitalisation and critical care support are required in only a small proportion of SARS‐CoV‐2 positive children (Fig. 1). 25 Risk factors for severe disease in children include young age 26 and pre‐existing medical conditions such as obesity, asthma, diabetes mellitus and cancer 25 ; infection in the neonatal period is a particular risk factor. 27 Children who develop severe disease requiring intensive care level support are more likely to have had lower respiratory tract signs and symptoms at time of presentation. 25 The timing of respiration deterioration is not well characterised in children with severe disease due to low case numbers. Case series suggest that the natural history is similar to that in adult cohorts 28 with hospitalisation occurring approximately 1 week after symptom onset and acute lung injury, if it evolves, manifesting in the second week.
Radiological investigations in hospitalised children have shown patchy opacities on plain radiographs and ground‐glass opacities on chest computed tomography (CT). 23 Given the radiation load of CT imaging, this modality is not justified in a paucisymptomatic child. Laboratory features include elevated inflammatory markers such as C‐reactive protein in approximately 50%. 23 Serum ferritin and lactate dehydrogenase may also be raised, less frequently procalcitonin, erythrocyte sedimentation rate and interleukin‐6. 23 Most children with COVID‐19 have a normal blood count, 19 with lymphopenia (16%) and leukocytosis (10%) in a minority, 29 in contrast to adults in whom lymphopenia is common. Coagulopathy markers such as D‐dimer may be elevated, and less frequently biomarkers for organ injury such as troponin, liver function tests, pro B‐type natriuretic peptide (proBNP) and creatinine kinase‐MB. 23
Extrapulmonary manifestations in hospitalised children
In children, extrapulmonary involvement is rare but can be severe. Such non‐pulmonary findings, including neurological manifestations and cardiac dysfunction of varying severity, are seen in under 5% of hospitalised children and often coexist with pulmonary disease. 23 In contrast to adult infection with SARS‐CoV‐2, clinically significant acute hepatitis is rare in children with COVID‐19 though occasional case reports exist. 30
Neurological findings in acute COVID‐19 include status epilepticus, encephalopathy, encephalitis, Guillain‐Barré syndrome and acute demyelinating syndromes. 31 These occur rarely, in approximately 4% of hospitalised children, and are most commonly seen in children with pre‐existing neurological conditions. 31 A significant proportion (37%) may have ongoing neurological deficits at the time of discharge from hospital. 31
Acute COVID‐19 can rarely cause cardiac dysfunction, manifesting as acute myocardial injury, myocarditis, arrhythmias and cardiomyopathy. The proposed pathophysiology 32 is comparable to our understanding of myocardial injury in adult populations with COVID‐19.
Post‐infectious inflammatory syndrome (PIMS‐TS/MIS‐C)
PIMS‐TS is a hyper‐inflammatory syndrome related to COVID‐19, variously referred to as PIMS‐TS in the UK and multi‐system inflammatory syndrome in children (MIS‐C) in the USA and by the WHO. Initially reported in April 2020, PIMS‐TS occurs approximately 4–6 weeks following infection with SARS‐CoV‐2. 33 This condition has caused as much if not greater morbidity and mortality in children as the direct impact of the acute infection itself. 34
The peak age for PIMS‐TS is 9–10 years and may follow a clinically insignificant acute infection as paediatric COVID‐19 is usually mild. 35 It is estimated that PIMS‐TS occurs in approximately one in 3000 children infected with SARS‐CoV‐2, 36 a figure supported by Australian registry data (case notification rate <1 per 1000 cases of COVID‐19 in children and adolescents). 37 PIMS‐TS has also been described in younger children and in adults. It is more common in Black, Hispanic and South Asian populations. 20 The reasons for these racial differences are unclear and may partly reflect socio‐economic differences, such as health‐care access and populations with higher transmission of SARS‐CoV‐2.
PIMS‐TS shares some features with Kawasaki disease (KD), but it is a distinct syndrome in terms of epidemiology, clinical symptoms, signs and laboratory features. 21 It is most commonly characterised by fever, rash, conjunctival injection, gastrointestinal symptoms (particularly pain) and shock due to myocardial dysfunction. Laboratory features include lymphopenia, marked inflammation (neutrophilia, increased C‐reactive protein, procalcitonin and ferritin), coagulopathy (increased D‐dimer) and myocardial dysfunction (elevated troponin and proBNP). 35 In more severe cases, echocardiography may demonstrate myocardial dysfunction leading to shock; extracorporeal membrane oxygenation (ECMO) may be required (in 4%). 38 As in KD, coronary artery dilatation or aneurysms occurs in 15–25% of cases. 20 , 21 Anecdotally, the coronary artery lesions appear less severe than in KD and resolve more quickly. 39 Milder cases are increasingly being recognised in settings with high COVID‐19 incidence.
Considerations in immunocompromised children
The highest relative risk of severe COVID‐19 disease undoubtedly occurs in patients with defects of innate immunity, many of whom were undiagnosed before the pandemic. 40 , 41 In older adults, this is most commonly associated with autoantibodies against type I interferons, which block the immune response to the virus. 42 These autoantibodies are rare in children, other than those who have autoimmune polyendocrine syndrome type I (APS1). 43 Genetic defects in the antiviral type I interferon response 40 and X‐linked Toll‐like receptor‐7 deficiency 41 also confer a more than 50‐fold increased relative risk for COVID‐19. By contrast, adaptive primary immunodeficiency or immunosuppression that impacts T cells, B cells, and antibodies may be less important than expected in terms of COVID‐19 disease severity. Primary immunodeficiency patients 44 and patients with combined immunodeficiency or HIV, 45 who might have been expected to have difficulties with viral control, are not clearly defined as carrying additional risk from SARS‐CoV‐2 infection. The assessment of iatrogenic immunocompromise is harder to quantify, though outcomes appear similar to immunocompetent children. 46 Children with cancer have similar disease severity, 47 though all‐cause mortality appears to be higher than the general population. 48
Considerations in neonates and pregnancy
Newborn babies can be affected by COVID‐19 either indirectly or directly. SARS‐CoV‐2 infection may be severe during pregnancy and is associated with increased risk of preterm delivery, 49 often initiated for maternal indications such as hypoxia or pre‐eclampsia. 50 Perinatal acquisition of SARS‐CoV‐2 has been demonstrated in 1.8–10% of tested neonates born to mothers with COVID‐19, 50 with antepartum (12%), intrapartum (17%) and postpartum (71%) transmission well recognised. 51 Detection of SARS‐CoV‐2 RNA in placental samples and amniotic fluid is uncommon. 50 SARS‐CoV‐2 RNA is rarely found in breastmilk; however, SARS‐CoV‐2 antibodies are detectable in breastmilk from 83% of COVID‐19 positive mothers; hence, breastfeeding is encouraged for women with COVID‐19. 52 Contact with the infected mother increases the risk of late onset SARS‐CoV‐2 infection (>72 h of age), presumably via respiratory droplets as breast feeding is not a risk factor. 51
Disease severity data in neonatal COVID‐19 are variable. Active surveillance in a hospitalised UK cohort revealed 42% of neonates with COVID‐19 had severe disease. 27 A quarter of these babies were born prematurely and one‐third received one or more forms of respiratory support. Other studies report mostly mild illness, with asymptomatic infections in 11–48%. 27 , 50 In symptomatic neonates, fever, poor feeding or vomiting are common, as well as coryza, cough, respiratory distress, diarrhoea and lethargy. 27 , 50 Apnoea and cardiovascular features such as tachycardia and hypotension, as well as rash and conjunctivitis, have been reported. 51 Investigations typically reveal elevated lactate, with C‐reactive protein and procalcitonin raised in only the minority.
Published guidance for management of newborn infants at risk of SARS‐CoV‐2 infection has been varied. Australian guidelines have largely promoted rooming‐in and breastfeeding unless separation is necessitated by the degree of maternal or neonatal illness. 53
Management of COVID‐19 in Children
Recommendations on therapy for COVID‐19 in children are predominantly extrapolated from adult data, reflecting exclusion of children from these therapeutic clinical trials. At time of writing, over 300 randomised controlled COVID‐19 trials have been published and over 3000 registered, but almost none included persons <18 years for therapy of acute COVID‐19 among trial participants (as opposed to trials of vaccines). 54 Consideration of repurposed or novel therapeutic agents in children must take into account regulatory requirements, extrapolation of efficacy data from adult studies and paediatric‐specific safety and dosing information, which may be limited.
Clinician confidence is strong in recommending supportive care only for the vast majority of children with mild disease and those not requiring supplemental oxygen. There is insufficient evidence for definitive recommendations on use of other agents in children with more severe disease (Fig. 2). Several guidelines for treatment of children with acute COVID‐19 suggest consideration of antiviral therapy with remdesivir and/or immunomodulatory therapy with corticosteroids (dexamethasone) or biologic agents (tocilizumab) based on efficacy data from adult studies (Fig. 2). 55 Dexamethasone and other steroids are widely used in children for other conditions and have a well‐established safety and toxicity profile, whilst fewer data are available for remdesivir and tocilizumab. The Therapeutic Goods Administration has approved the monoclonal antibody therapy Sotrovimab for use in children aged 12 years and above with risk factors for progression to severe COVID‐19 disease. 56 Given the absence of safety data for this agent in children and the mild course of COVID‐19 infection in most children, use of Sotrovimab should be considered on a case‐by‐case basis. The use of other emerging therapies, for which safety and efficacy data are lacking in children, should be carefully considered and preferably administered in a clinical trial only.
Fig 2.
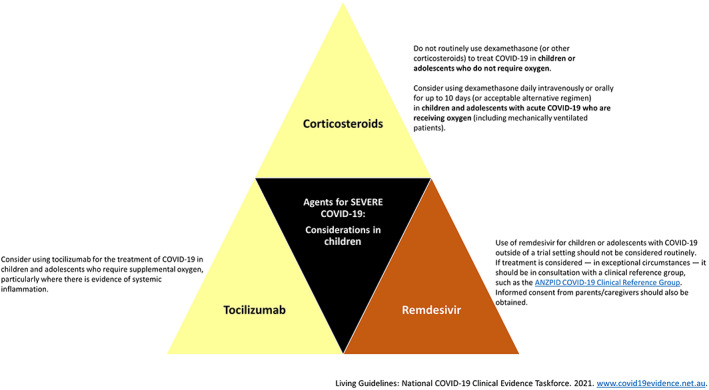
Pharmaceutical agents available for use in severe COVID‐19 disease and important considerations for use in children,
55
based on the principles of Grading of Recommendations, Assessment, Development and Evaluations (GRADE). These considerations are reflective of advice current on 5 October 2021, which is updated regularly. The reader is directed to the most up‐to‐date recommendations at ( ), Recommended; (
), Recommended; ( ), conditional recommendation; (
), conditional recommendation; ( ), conditional recommendation against; (
), conditional recommendation against; ( ), not recommended.
), not recommended.
A number of other agents have proven ineffective in the treatment of COVID‐19 infection and hence should not be used. These include hydroxychloroquine, azithromycin, colchichine, aspirin and convalescent plasma. 55
The RECOVERY trial in the UK is currently recruiting children and adolescents with COVID‐19 to some of these interventions. 57 We await these results to inform future treatment approaches and recommend the inclusion of children in future planned clinical trials on the prevention and treatment of COVID‐19. Evidence is rapidly evolving and clinicians are advised to consult the paediatric‐specific aspects of their local guidelines in real time (e.g. https://covid19evidence.net.au/). Consultation with an infectious diseases specialist is recommended to consider optimal treatment on a case‐by‐case basis.
Treatment approaches for PIMS‐TS are based on similarity to KD, a condition with which paediatricians are familiar. 58 In addition to supportive care, both intravenous immunoglobulin and corticosteroids – alone or in combination – are effective in reducing myocardial dysfunction and inflammation. 59
Outcomes of COVID‐19 in Children Including ‘Long COVID’
Mortality from COVID‐19 in children is extremely low, reportedly between 0.005% and 0.01%. 60 Whilst children with obesity or pre‐existing conditions carry a relatively higher risk of death compared to those without co‐morbidities, the additional absolute risk is very small. In neonates, data on COVID‐19 outcomes are still emerging. All‐cause mortality rates of 1.7–2.0% are described; however, most deaths were deemed not related to COVID‐19 so should be interpreted with caution. 27 , 50
Mortality rates vary widely between countries, likely contributed to by factors such as malnutrition, health‐care access, delayed diagnosis and reduced ascertainment of paucisymptomatic patients. 61 Such factors are key determinants of COVID‐19 disease outcome in low income countries. Specific groups subject to adverse social determinants of health in Australia, including those in remote settings and Aboriginal and Torres Strait Islander communities, need targeted risk mitigation to prevent worse outcomes.
‘Long COVID’, characterised by persistence of symptoms for over 3 months, 62 occurs mostly in those 12 years or over. 24 This condition, with a wide constellation of symptoms including fatigue, breathlessness, ‘brain fog’ and depression, hinders the patient's ability to re‐engage with normal activities, and hence carries significant long‐term morbidity. 63 There is marked heterogeneity in existing data, leading to variability in prevalence estimates (between 0% and 27% of children diagnosed with COVID‐19). Reassuringly, a recent systematic review suggests that symptoms of ‘long COVID’ in children rarely persist beyond 8 weeks following the acute diagnosis. 64 Establishing a clear definition for ‘long COVID’ in children and identifying objective methods for surveillance are urgent priorities, alongside further research on associated risk factors, prevalence and natural history. 65 The inclusion of control groups in such studies will be important to account for the confounding impacts of the pandemic.
Outcomes from PIMS‐TS appear promising in the short to medium term with low rates of coronary artery aneurysms and even lower mortality. 58 There have been approximately 37 reported deaths from PIMS‐TS (MIS‐C) across the USA during a period of high COVID‐19 case numbers, and these occurred mostly early in the pandemic. 66 Studies of longer‐term outcomes are ongoing.
Outstanding Questions Regarding Emerging Variants of Concern, Including the Delta Variant
Much of the early data on COVID‐19 clinical phenotypes, diagnosis and management were based on the ancestral SARS‐CoV‐2 lineage. Emergence of novel variants of concern, including the Delta variant, is forcing re‐evaluation of the applicability of this earlier information to the evolving features of newer SARS‐CoV‐2 variants. In one study, the emergent Delta variant has shown a viral load in the upper respiratory tract of adults three orders of magnitude higher than the ancestral strain. 67 For Delta as well as future variants of concern, ongoing validation of current diagnostic and treatment frameworks is warranted, including in children. Reassuringly, severity of COVID‐19 disease in children due to the Delta variant appears largely unchanged with low hospitalisation and case fatality rates. 68
Conclusions
The COVID‐19 pandemic has brought monumental disruption spanning from global economic alliances to the lives of each individual child. Current understanding of immune response, diagnosis and treatment of COVID‐19 is heavily skewed towards adult data. Whilst the acute pneumonitis of COVID‐19 is typically mild in children, the complexities of its downstream manifestations, including PIMS‐TS and ‘long COVID’, are incompletely elucidated. Children need to be prioritised in future research efforts given the clinical manifestations and impacts are so distinct in the paediatric setting compared to adult populations. The additional indirect impacts of the pandemic on the mental health, wellbeing and educational attainment of children are marked and must be considered alongside the clinical aspects discussed in this review.
Conflict of interest: None declared.
References
- 1. World Health Organization . Weekly Epidemiological Update on COVID‐19: Edition 50. Geneva: WHO; 2021. [Google Scholar]
- 2. Wu F, Zhao S, Yu B et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579: 265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sharma K, Koirala A, Nicolopoulos K, Chiu C, Wood N, Britton PN. Vaccines for COVID‐19: Where do we stand in 2021? Paediatr. Respir. Rev. 2021; 39: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nguyen HL, Lan PD, Thai NQ, Nissley DA, O'Brien EP, Li MS. Does SARS‐CoV‐2 bind to human ACE2 more strongly than does SARS‐CoV? J. Phys. Chem. B 2020; 124: 7336–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khateeb J, Li Y, Zhang H. Emerging SARS‐CoV‐2 variants of concern and potential intervention approaches. Critical Care (London, England) 2021; 25: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. JAMA 2020; 323: 2427–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID‐19? J Microbiol Immunol Infect 2020; 53: 371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tosif S, Neeland MR, Sutton P et al. Immune responses to SARS‐CoV‐2 in three children of parents with symptomatic COVID‐19. Nat. Commun. 2020; 11: 5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loske J, Röhmel J, Lukassen S et al. Pre‐activated antiviral innate immunity in the upper airways controls early SARS‐CoV‐2 infection in children. Nat. Biotechnol. 2021. 10.1038/s41587-021-01037-9. [DOI] [PubMed] [Google Scholar]
- 10. Neeland MR, Bannister S, Clifford V et al. Innate cell profiles during the acute and convalescent phase of SARS‐CoV‐2 infection in children. Nat. Commun. 2021; 12: 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weisberg SP, Connors TJ, Zhu Y et al. Distinct antibody responses to SARS‐CoV‐2 in children and adults across the COVID‐19 clinical spectrum. Nat. Immunol. 2021; 22: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selva KJ, van de Sandt CE, Lemke MM et al. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 2021; 12: 2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bacher P, Rosati E, Esser D et al. Low‐avidity CD4(+) T cell responses to SARS‐CoV‐2 in unexposed individuals and humans with severe COVID‐19. Immunity 2020; 53: 1258–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmer DB. The effect of age on thymic function. Front. Immunol. 2013; 4: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korakas E, Ikonomidis I, Kousathana F et al. Obesity and COVID‐19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am. J. Physiol. Endocrinol. Metab. 2020; 319: E105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Migliorini F, Torsiello E, Spiezia F, Oliva F, Tingart M, Maffulli N. Association between HLA genotypes and COVID‐19 susceptibility, severity and progression: A comprehensive review of the literature. Eur. J. Med. Res. 2021; 26: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langton DJ, Bourke SC, Lie BA et al. The influence of HLA genotype on the severity of COVID‐19 infection. HLA 2021; 98: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wurzel D et al. COVID‐19 and its multi‐system inflammatory complications in children ‐ a multi‐centre nationwide Australian study. BMJ Open. 2021; in press
- 19. Liguoro I, Pilotto C, Bonanni M et al. SARS‐COV‐2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020; 179: 1029–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feldstein LR, Rose EB, Horwitz SM et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020; 383: 334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whittaker E, Bamford A, Kenny J et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA 2020; 324: 259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viner RM, Ward JL, Hudson LD et al. Systematic review of reviews of symptoms and signs of COVID‐19 in children and adolescents. Arch. Dis. Child. 2020; 106: 802–7. [DOI] [PubMed] [Google Scholar]
- 23. Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID‐19: A systematic review and meta‐analysis. Arch. Dis. Child. 2021; 106: 440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molteni E, Sudre CH, Canas LS et al. Illness duration and symptom profile in symptomatic UKschool‐aged children tested for SARS‐CoV‐2. Lancet Child Adolesc Health 2021; 5: 708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Götzinger F, Santiago‐García B, Noguera‐Julián A et al. COVID‐19 in children and adolescents in Europe: A multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4: 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parcha V, Booker KS, Kalra R et al. A retrospective cohort study of 12,306 pediatric COVID‐19 patients in the United States. Sci. Rep. 2021; 11: 10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gale C, Quigley MA, Placzek A et al. Characteristics and outcomes of neonatal SARS‐CoV‐2 infection in the UK: A prospective national cohort study using active surveillance. Lancet Child Adolesc Health 2021; 5: 113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang D, Hu B, Hu C et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cui X, Zhao Z, Zhang T et al. A systematic review and meta‐analysis of children with coronavirus disease 2019 (COVID‐19). J. Med. Virol. 2021; 93: 1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brisca G, Mallamaci M, Tardini G et al. SARS‐CoV‐2 infection may present as acute hepatitis in children. Pediatr. Infect. Dis. J. 2021; 40: e214–5. [DOI] [PubMed] [Google Scholar]
- 31. Ray STJ, Abdel‐Mannan O, Sa M et al. Neurological manifestations of SARS‐CoV‐2 infection in hospitalised children and adolescents in the UK: A prospective national cohort study. Lancet Child Adolesc Health 2021; 5: 631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abi Nassif T, Fakhri G, Younis NK et al. Cardiac manifestations in COVID‐19 patients: A focus on the pediatric population. Can. J. Infect. Dis. Med. Microbiol. 2021; 2021: 5518979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toubiana J, Poirault C, Corsia A et al. Kawasaki‐like multisystem inflammatory syndrome in children during the covid‐19 pandemic in Paris, France: Prospective observational study. BMJ (Clin Res ed) 2020; 369: m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams PCM, Howard‐Jones AR, Hsu P et al. SARS‐CoV‐2 in children: Spectrum of disease, transmission and immunopathological underpinnings. Pathology 2020; 52: 801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feldstein LR, Tenforde MW, Friedman KG et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS‐C) compared with severe acute COVID‐19. JAMA 2021; 325: 1074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Payne AB, Gilani Z, Godfred‐Cato S et al. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS‐CoV‐2. JAMA Netw. Open 2021; 4: e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Australian Government Department of Health . COVID‐19 Australia: Epidemiology Report 25. 23 September 2020.
- 38. Rodriguez‐Gonzalez M, Castellano‐Martinez A, Cascales‐Poyatos HM, Perez‐Reviriego AA. Cardiovascular impact of COVID‐19 with a focus on children: A systematic review. World J. Clin. Cases 2020; 8: 5250–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singh‐Grewal D, Lucas R, McCarthy K et al. Update on the COVID‐19‐associated inflammatory syndrome in children and adolescents; paediatric inflammatory multisystem syndrome‐temporally associated with SARS‐CoV‐2. J. Paediatr. Child Health 2020; 56: 1173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Q, Bastard P, Liu Z et al. Inborn errors of type I IFN immunity in patients with life‐threatening COVID‐19. Science 2020; 370: 4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fallerini C, Daga S, Mantovani S et al. Association of Toll‐like receptor 7 variants with life‐threatening COVID‐19 disease in males: Findings from a nested case‐control study. Elife 2021; 10: 67569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bastard P, Rosen LB, Zhang Q et al. Autoantibodies against type I IFNs in patients with life‐threatening COVID‐19. Science 2020; 370: eabd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bastard P, Orlova E, Sozaeva L et al. Preexisting autoantibodies to type I IFNs underlie critical COVID‐19 pneumonia in patients with APS‐1. J. Exp. Med. 2021; 218: e20210554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marcus N, Frizinsky S, Hagin D et al. Minor clinical impact of COVID‐19 pandemic on patients with primary immunodeficiency in Israel. Front. Immunol. 2020; 11: 614086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meyts I, Bucciol G, Quinti I et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J. Allergy Clin. Immunol. 2020; 147: 520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goss MB, Galvan NTN, Ruan W et al. The pediatric solid organ transplant experience with COVID‐19: An initial multi‐center, multi‐organ case series. Pediatr. Transplant. 2021; 25: e13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Millen GC, Arnold R, Cazier JB et al. Severity of COVID‐19 in children with cancer: Report from the United Kingdom Paediatric Coronavirus Cancer Monitoring Project. Br. J. Cancer 2021; 124: 754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mukkada S, Bhakta N, Chantada GL et al. Global characteristics and outcomes of SARS‐CoV‐2 infection in children and adolescents with cancer (GRCCC): A cohort study. Lancet Oncol. 2021; 22: 1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ciapponi A, Bardach A, Comandé D et al. COVID‐19 and pregnancy: An umbrella review of clinical presentation, vertical transmission, and maternal and perinatal outcomes. PLoS One 2021; 16: e0253974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Neef V, Buxmann H, Rabenau HF, Zacharowski K, Raimann FJ. Characterization of neonates born to mothers with SARS‐CoV‐2 infection: Review and meta‐analysis. Pediatr. Neonatol. 2021; 62: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Raschetti R, Vivanti AJ, Vauloup‐Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS‐CoV‐2 infections. Nat. Commun. 2020; 11: 5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu F, Zozaya C, Zhou Q, De Castro C, Shah PS. SARS‐CoV‐2 genome and antibodies in breastmilk: A systematic review and meta‐analysis. Arch. Dis. Child 2021; 106: 514–21. [DOI] [PubMed] [Google Scholar]
- 53. Vogel JP, Tendal B, Giles M et al. Clinical care of pregnant and postpartum women with COVID‐19: Living recommendations from the national COVID‐19 clinical evidence taskforce. Aust N Z J Obstet Gynaecol 2020; 60: 840–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. The COVID NMA Initiative 2021. Available from: https://covid-nma.com.
- 55. Living Guidelines: National COVID‐19 Clinical Evidence Taskforce 2021. Available from: www.covid19evidence.net.au. [DOI] [PMC free article] [PubMed]
- 56. Australian Government Department of Health . Australian prescription medicine decision summaries: Xevudy. 20 August 2021.
- 57. RECOVERY Trial . A randomised trial of treatments to prevent death in patients hospitalised with COVID‐19 (coronavirus). 2021. Contract No.: ISRCTN50189673.
- 58. Harwood R, Allin B, Jones CE et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID‐19 (PIMS‐TS): Results of a national Delphi process. Lancet Child Adoles Health 2021; 5: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McArdle AJ, Vito O, Patel H et al. Treatment of multisystem inflammatory syndrome in children. N. Engl. J. Med. 2021; 385: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith C, Odd D, Harwood R et al. Deaths in children and Young people in England following SARS‐CoV‐2 infection during the first pandemic year: A national study using linked mandatory child death reporting data. Res. Sq. 2021. 10.21203/rs.3.rs-689684/v1. [DOI] [Google Scholar]
- 61. Kurtz A, Grant K, Marano R et al. Long‐term effects of malnutrition on severity of COVID‐19. Sci. Rep. 2021; 11: 14974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buonsenso D, Munblit D, De Rose C et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021; 110: 2208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. The Lancet . Understanding long COVID: A modern medical challenge. Lancet 2021; 398: 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr. Infect. Dis. J. 2021. 10.1097/inf.0000000000003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Amin‐Chowdhury Z, Ladhani SN. Causation or confounding: Why controls are critical for characterizing long COVID. Nat. Med. 2021; 27: 1129–30. [DOI] [PubMed] [Google Scholar]
- 66. Centers for Disease Control and Prevention . Health Department‐Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS‐C) in the United States. Atlanta, GA: CDC; 2021. Available from: https://www.cdc.gov/mis/cases/index.html [accessed 21 August 2021]. [Google Scholar]
- 67. Li B, Deng A, Li K et al. Viral infection and transmission in a large well‐traced outbreak caused by the Delta SARS‐CoV‐2 variant. medRxiv: the preprint server for health sciences 2021: 2021.07.07.21260122. [Google Scholar]
- 68. Herlihy R, Bamberg W, Burakoff A et al. Rapid increase in circulation of the SARS‐CoV‐2 B.1.617.2 (Delta) variant ‐ Mesa County, Colorado, April‐June 2021. MMWR Morb. Mortal. Wkly Rep. 2021; 70: 1084–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


