Abstract
Chronic liver disease increased the risk of severe coronavirus disease 2019 (COVID‐19). Trials to assess efficacy/safety of COVID‐19 vaccines in liver disease are underway. We evaluated the humoral immune response and safety of anti–COVID‐19 vaccination among patients waiting liver transplantation (LT). We enrolled all pre‐LT adults who completed anti‐COVID‐19 vaccination between January 2021‐August 2021 as study group. Patients with histories of COVID‐19 received 1 vaccine dose, and all others received 2 doses. Patients were tested for COVID‐19 immunoglobulin G (IgG) within 1 and 2 months after vaccination. Safety was evaluated with telephone interviews/outpatient visits. A control group of 30 healthcare workers who underwent vaccination in January 2021 and tested for IgG after 4 months was included. In the 89 pre‐LT patients, at T1 (23 days after vaccination), seroconversion rate was 94.4%, and median IgG value was 1980 binding antibody units/mL (interquartile range 646‐2080), and at T2 (68 days after vaccination) was 92.0%, with IgG value of 1450 (577‐2080); (T1 versus T2, P = 0.38). In the 10/89 patients who received 1 vaccine dose, the median IgG value was 274 (68‐548) before vaccine (T0), 2080 (1165‐2080) at T1, and 2030 (964‐2080) at T2 (T0 versus T1, P = 0.03; T1 versus T2, P = 0.99). All controls tested positive at 4 months after vaccination, with a median value of 847 (509‐1165; P < 0.001 versus T1 and P = 0.04 versus T2 in the study group). No serious adverse event was reported in both groups. Our data from 89 pre‐LT patients suggest a high rate of immunization (94.4%) after a median time of 23 days from safe COVID‐19 vaccine. None of the patients developed COVID‐19.
Abbreviations
- BAU
binding antibody units
- CLD
chronic liver disease
- COVID‐19
coronavirus disease 2019
- eGFR
estimated glomerular filtration rate
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IgG
immunoglobulin G
- IgG4
Immunoglobulin G4
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- mRNA
messenger RNA
- NASH
nonalcoholic steatohepatitis
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- T0
before vaccination
Patients affected by chronic liver disease (CLD) have well‐recognized deficiencies in innate and humoral immunity, the so‐called immune dysfunction, which predisposes to infections.( 1 , 2 ) With the rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), significant concerns have been raised regarding patients with liver impairment. Coronavirus disease 2019 (COVID‐19) is a multisystem condition caused by SARS‐CoV‐2, and the pathogenesis of liver involvement includes viral cytotoxicity, ischemic failure attributed to vascular endotheliitis and secondary to immune dysregulation or drug‐induced injury.( 3 , 4 ) A total of 2 meta‐analyses( 5 , 6 ) described an increased risk of severe COVID‐19 infection, decompensation, and mortality in patients with CLD, and according to a UK survey( 7 ) of more than 17 million patients, COVID‐19 infection was associated with twice an increased risk of mortality with CLD. In particular, the presence of cirrhosis was found to be an independent predictor of mortality in COVID‐19 infection, and large registries reported a fatality rate up to 38.0%, which may be as high as 70.0% in decompensated cirrhosis.( 8 , 9 ) In patients with CLD, the deficiency of innate and adaptive immune system and comorbidities (ie, diabetes mellitus, obesity, steatohepatitis, chronic kidney disease) predispose to the impaired immunological responses seen with existing vaccination.( 10 ) According to current literature, there is no confirmed information on the immunogenicity and safety of novel COVID‐19 vaccines in patients with CLD with or without hepatocellular carcinoma (HCC).( 11 , 12 ) Nevertheless, without data suggesting harm, the American Association for the Study of Liver and the European Association for the Study of the Liver currently recommend the vaccination against SARS‐CoV‐2.( 13 , 14 ) Trials to assess the efficacy and safety of COVID‐19 vaccines are underway, but data on COVID‐19 vaccination in patients with liver disease are eagerly awaited.
Our working hypothesis is that patients affected by CLD will have an attenuated immune response to SARS‐CoV‐2 vaccination and a third vaccination dose might be necessary to improve the seroconversion rate, as has been reported in solid organ transplant recipients.( 15 , 16 ) In this study, we evaluated the humoral immune response and safety of messenger RNA (mRNA) anti‐SARS‐CoV‐2 vaccination among patients listed for liver transplantation (LT) in our center.
Patients and Methods
Study Period
The enrollment period was from January 1, 2021, to August 5, 2021. The follow‐up was closed on August 31, 2021.
Study Protocol
According to the National Health Institute, since January 2021, health care workers in Italy were allowed to undergo anti‐SARS‐CoV‐2 vaccination, and starting from March 2021, prioritized vaccination was launched for patients on the transplant waiting list. Mask wearing indoors and physical distancing measures were recommended for all participants, irrespective of their vaccination status.
The participants with a previous documented history of COVID‐19 infection received a single intramuscular dose of 30 mcg of the mRNA vaccine Comirnaty (Pfizer‐BioNTech, New York, NY) within 6 months after infection. All other participants received 2 intramuscular doses of 30 mcg Comirnaty or 100 mcg of the Moderna (Cambridge, MA) COVID‐19 mRNA vaccine, administered 21 and 28 days apart, respectively.
In our tertiary hospital, occupational medicine in collaboration with the microbiology unit offered the opportunity to health care workers to test anti‐SARS‐CoV‐2 IgG antibodies at 4 months after vaccination. We collected, as a control group, 30 healthy medical doctors of our hospital who underwent vaccination in January 2021 and tested antibodies 4 months later, without personal or family members' history of COVID‐19, and without major comorbidities (arterial hypertension, renal insufficiency, cancers, autoimmune disease, etc.).
We enrolled all of the patients awaiting LT in our center who completed anti‐SARS‐CoV‐2 vaccination during the enrollment period and reached at least the 1‐week follow‐up as a study group. All of the characteristics of the LT candidates were collected as reported in Table 1. Estimated glomerular filtration rate (eGFR) was calculated with the Cockcroft‐Gault equation.
TABLE 1.
Characteristics of Pretransplant Patients (n = 89)
| Male | 62 (69.7) |
| Age, years | 56 (50‐62) |
| Body mass index, kg/m2 | 24.6 (22.3‐27.8) |
| Creatinine clearance, mL/minute* | 95 (71‐126) |
| Dialysis | 4 (4.5) |
| Blood group | |
| A | 16 (18.0) |
| B | 25 (28.1) |
| AB | 5 (5.6) |
| 0 | 43 (48.3) |
| Etiology of liver disease | |
| Viral cirrhosis | 29 (32.6) |
| Alcohol‐related cirrhosis | 18 (20.2) |
| Biliary cirrhosis | 10 (11.2) |
| Dysmetabolic cirrhosis | 10 (11.2) |
| Liver‐kidney polycystic disease † | 7 (7.9) |
| Liver polycystic disease | 6 (6.7) |
| Cryptogenic cirrhosis | 3 (3.4) |
| Autoimmune cirrhosis | 2 (2.2) |
| Budd‐Chiari cirrhosis | 2 (2.2) |
| Hepatic adenomatosis | 2 (2.2) |
| Hepatocellular carcinoma | 37 (41.6) |
| Severe portal hypertension | 69 (77.5) |
| MELD score at the time of LT waitlist registration | 12 (8‐15) |
| mRNA vaccine | |
| Comirnaty (Pfizer‐BioNTech, New York, NY) | 83 (93.3) |
| Moderna (Cambridge, MA) | 6 (6.7) |
Categorical variables are presented as number (%), and quantitative variables are presented as median (IQR).
Cockcroft‐Gault equation.
Patients listed for simultaneous liver‐kidney transplantation.
Patients with previous COVID‐19 infection were tested for SARS‐CoV‐2 IgG antibodies before vaccination. All participants were tested within 1 month (T1) and 2 months (T2) after the completion of the vaccine schedule.
Safety in pre‐LT patients was evaluated with weekly telephone interviews and outpatient visits, if necessary, until 21 days after the second dose and at least 1 planned outpatient visit at 1 month after the second dose.
No organs from executed prisoners were used. According to Italian law, regional transplantation centers are the custodians of recipient/donor biomedical data for research purposes. All study procedures comply with the ethical standards of the 2000 Declaration of Helsinki and the Declaration of Istanbul 2008.
Tests
Antibodies were tested with Liaison SARS‐CoV‐2 TrimericS IgG assay (DiaSorin, Saluggia, Italy), which uses a recombinant trimeric spike glycoprotein as capture antigen with a cutoff value for positivity of 34 binding antibody units (BAU) per mL; the assay range is 5 to 2080 BAU/mL. According to the manufacturer, sensitivity and specificity of the test are 98.7% and 99.5%, respectively, and the positive agreement with the plaque reduction neutralization test is 100.0%. Currently, the plaque reduction neutralization test is considered the gold standard for detecting and measuring antibodies that can neutralize viruses.
Statistical Analysis
Categorical variables are represented as number (percentage) and compared using Fisher’s exact test or chi‐square test. Quantitative variables are shown as median (interquartile range [IQR]; 25th–75th percentiles), and their distribution was evaluated with the D’Agostino and Pearson test. Parametric data were evaluated by t test, and nonparametric data were evaluated by Mann‐Whitney U test and Wilcoxon matched‐pairs signed rank test, as appropriate. Spearman rank correlation was used to test correlations between variables. P < 0.05 in a 2‐tailed test was considered statistically significant.
Results
Humoral Immunity of mRNA Vaccination
At the beginning and at the end of the enrollment period, in our center, 76 and 69 adult patients were, respectively, on the LT waiting list, and 100 patients received transplantations. We enrolled 89 patients who completed the anti‐SARS‐CoV‐2 vaccination (93.3% Comirnaty, 6.7% Moderna COVID‐19). A total of 79 patients received 2 vaccine doses, and 10 patients received only 1 dose, as per protocol. The median follow‐up after completion of the vaccine schedule was 110 days (IQR, 98‐122 days). Of the patients, 70% lived in Piedmont, and 30.0% in other Italian regions. The median age of the pre‐LT patients and healthy controls was similar: 56 years (IQR, 50‐62 years) versus 55 years (IQR, 46‐59 years), respectively (P = 0.17), and men were equally represented in the 2 groups (69.7% versus 70.0%, respectively; P = 0.97). In the study group, the median body mass index (BMI) was 24.6 kg/m2, and 48.3% of the patients had blood type 0. Of the patients, 32.6% were affected by viral cirrhosis, 41.6% by HCC, and 77.5% by severe portal hypertension. The 2 patients listed for autoimmune cirrhosis were treated with low‐dose steroids. The median Model for End‐Stage Liver Disease (MELD) score at the time of waitlist registration was 12 (IQR, 8‐15). A total of 7 patients were affected by polycystic liver‐kidney disease (4 of them on dialysis, and all were listed for simultaneous liver‐kidney transplantation), and 6 patients had polycystic liver disease with massive hepatomegaly, malnutrition, cholestasis, and portal hypertension (Table 1).
The median elapsed time between vaccination and IgG test in the pre‐LT patients was 23 days (IQR, 14‐42 days) at T1 and 68 days (IQR, 55‐85 days) at T2, whereas the control group underwent an anti‐SARS‐CoV‐2 test 4 months after vaccination (P < 0.01 versus T1 and versus T2).
In the study group, the rate of seroconversion at T1 was 94.4% (84/89), with a median IgG value of 1980 BAU/mL (IQR, 646‐2080 BAU/mL). The 5 patients who tested negative received the Comirnaty vaccine. A total of 3 patients were affected by decompensated liver disease, with median MELD scores of 19 (range, 18‐22), 1 patient was treated with steroids because of IgG4 cholangitis, none of the patients had diabetes mellitus, renal failure, or HIV infection (Table 2). Among the 50 patients who reached T2 of follow‐up, 4 remained negative, as reported in Table 2, and 46 retested positive, with a seroconversion rate of 92.0% (46/50) and a median IgG titer of 1450 BAU/mL (IQR, 577‐2080 BAU/mL; T1 versus T2, P = 0.38).
TABLE 2.
Characteristics of Pretransplant Patients With Undetectable IgG Anti‐SARS‐CoV‐2 After Vaccination
| Patients' characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Sex | Male | Female | Male | Male | Male |
| Age, years | 62 | 57 | 38 | 50 | 42 |
| BMI, kg/m2 | 28.4 | 23.0 | 23.3 | 21.5 | 23.7 |
| eGFR, mL/minute | 102 | 76 | 103 | 102 | 105 |
| Etiology of cirrhosis | Alcoholic NASH | HCV | Cryptogenic | IgG4 cholangitis | Viremic HCV |
| Severe portal hypertension | Yes | Yes | Yes | No | Yes |
| MELD score | 19 | 9 | 22 | 6 | 18 |
| HCC | No | Yes | No | No | No |
| Comorbidities | Myelofibrosis | Giant condyloma | Common variable immunodeficiency | Silicosis | Epilepsy |
| Therapy | Hydroxyurea | – | Endovenous immunoglobulin | Steroids | – |
| Past COVID‐19 infection | No | No | No | No | Yes |
| Comirnaty doses (Pfizer‐BioNTech, New York, NY) | 2 | 2 | 2 | 2 | 1 |
| IgG anti‐SARS‐Cov‐2 (BAU/mL)* | |||||
| T1 | 12 | 16 | 13 | 5 | 32 |
| T2 | 9 | 9 | – | 5 | 18 |
Liaison SARS‐CoV‐2 TrimericS IgG assay (DiaSorin, Saluggia, Italy); cutoff value for positivity, 34 BAU/mL.
Of 89 patients, 79 received 2 vaccine doses, and their median IgG value was 1870 BAU/mL (IQR, 602‐2080 BAU/mL) at T1 and 1380 BAU/mL (IQR, 525‐2080 BAU/mL) at T2 (45 retested patients; T1 versus T2, P = 0.33; Fig. 1A).
FIG. 1.

(A) IgG titers anti‐SARS‐CoV‐2 with median and IQR in pre‐LT candidates at baseline (T0, available for 52 patients), after completion of 2 vaccine doses at T1 (available for all 79 enrolled patients) and at T2 (available for 45 patients); (T0 versus T1, P < 0.001; T1 versus T2, P = 0.33). (B) Individual variations of IgG anti‐SARS‐CoV‐2 from T0 to T1 and T2 after completion of 2 vaccine doses in 36 pre‐LT candidates (T0 versus T1, P < 0.001; T1 versus T2, P = 0.11). Liaison SARS‐CoV‐2 TrimericS IgG assay (DiaSorin, Saluggia, Italy) was used; cutoff value for positivity, 34 BAU/mL.
Of 79 pre‐LT patients, 36 underwent an IgG test before vaccination (T0) and at T1 and T2, and their median values were 10 BAU/mL (IQR, 10‐23 BAU/mL), 2080 BAU/mL (IQR, 1027‐2080 BAU/mL), and 1505 BAU/mL (IQR, 745‐2080 BAU/mL), respectively (T0 versus T1, P < 0.001; T1 versus T2, P = 0.11; Fig. 1B).
In 10 of the 89 patients who received only 1 dose because of a previous history of COVID‐19 infection, the median IgG value was 274 BAU/mL (IQR, 68‐548 BAU/mL) at T0, 2080 BAU/mL (IQR, 1165‐2080 BAU/mL) at T1, and 2030 BAU/mL (IQR, 964‐2080 BAU/mL) at T2 (T0 versus T1, P = 0.03; T1 versus T2, P = 0.99).
The median SARS‐CoV‐2 IgG values of the 64 pre‐LT patients with compensated liver disease (MELD scores <15 at the time of LT waitlist registration) were not statistically different compared with the 25 patients with decompensated liver disease (MELD scores ≥15): at T1, 2080 versus 1240 BAU/mL (P = 0.12); at T2, 1890 versus 1280 BAU/mL (P = 0.57), respectively. The MELD score did not show a correlation with IgG titer (Spearman ranked coefficient, r = −0.04, P = 0.87).
All of the participants in the control group developed IgG anti‐SARS CoV‐2, with a median value of 847 BAU/mL (IQR, 509‐1165 BAU/mL) at 4 months after vaccination (P < 0.001 versus T1 and P = 0.04 versus T2 in the study group).
Of 89 patients, 26 (29.2%) underwent an LT during the study period. No organs from executed prisoners were used. Before transplant, 84.6% received 2 doses of Comirnaty, 3.9% received 2 doses of Moderna, and 11.5% received 1 dose of Comirnaty because of previous COVID‐19 infection.
The LT patients underwent immunosuppression with steroids, tacrolimus, and mycophenolate mofetil. At the end of the study period follow‐up, 12 were retested for IgG anti‐SARS‐CoV‐2 at a median time from transplant of 37 days (IQR, 33‐58 days). The median IgG titer was 1165 BAU/mL (IQR, 425‐2080 BAU/mL) before transplant and dropped to 207 BAU/mL (IQR, 36‐732 BAU/mL) after the operation (P = 0.04). At the end of the study follow‐up, none of the patients or controls developed COVID‐19 infection.
Safety
No serious adverse event was reported in the control or pre‐LT group. In the pre‐LT group, 43.8% experienced pain and swelling at the injection site, 14.6% fatigue, 11.2% headache, 13.0% fever, and 3.4% muscle and joint pain. In the control group, 43.3% experienced pain and swelling at the injection site, 33.3% fatigue, 30.0% muscle and joint pain, 23.3% headache, 16.7% fever, and 3.3% nausea (global P = 0.71; Fig. 2).
FIG. 2.
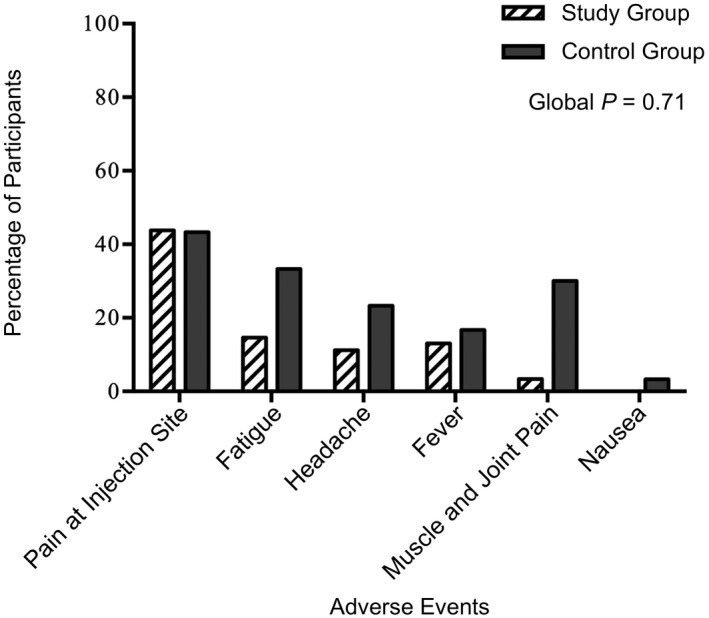
Local and systemic reactions reported by 89 pre‐LT patients and 30 healthy controls within 1 month after anti‐SARS‐CoV‐2 mRNA vaccination.
Discussion
In our multiregional, monocentric cohort of patients awaiting LT, the seropositivity rate elicited by mRNA vaccination against SARS‐CoV‐2 was surprisingly similar to the rate reported in healthy participants,( 12 , 16 ) and none of them developed COVID‐19 infection after vaccination during a median follow‐up of 110 days.
In December 2020, the US Food and Drug Administration issued emergency use authorization for mRNA vaccines developed by Pfizer‐BioNTech and Moderna, making them the first mRNA vaccines available to the public. Vaccines against SARS‐CoV‐2 showed efficacy in preventing symptomatic COVID‐19 and in reducing the length of stay in hospital and mortality rates. Despite more than 200,000 participants enrolled in phase 3 trials, there is scarcity of data in patients with CLD.( 12 , 17 ) The number of patients with liver disease enrolled was too low to conclude unequivocally regarding safety and efficacy. In the Pfizer/BioNTech vaccination study, only 3 patients (<0.1%) had moderate to severe liver disease,( 12 ) and in the Moderna trial, only 196 (0.6%) patients with liver disease were included.( 17 ) Nevertheless, in view of the higher risk of COVID‐19–related mortality in patients with CLD, the American Association for the Study of Liver( 13 ) and the European Association for the Study of the Liver( 14 ) recommended prioritization of COVID‐19 vaccination in patients with advanced liver disease. A multicenter Chinese study( 18 ) evaluated the antibody response in 381 patients with nonalcoholic fatty liver disease at least 14 days after the second dose of alum‐adjuvanted inactivated COVID‐19 vaccine. Of the patients, 95.5% elicited detectable antibody response, similar to healthy individuals, and according to Cornberg and Eberhardt,( 19 ) the young age of the cohort (median age, 39 years) with a low rate of diabetes mellitus (3.7%) might explain the high seropositivity rate. At the previous Virtual International Liver Congress in 2021, Hakimiah et al.( 20 ) showed that among 88 patients with nonalcoholic fatty liver disease, 98.9% achieved a good response, but advanced fibrosis was correlated with lower IgG titers. The authors therefore suggested a third dose vaccine booster in those at‐risk patients. According to current literature, however, we do not know the relevance of excellent versus good response to the vaccine( 20 ) and antibody thresholds for protection; monitoring the incidence of COVID‐19 infection in our pre‐LT vaccinated patients will clarify this point. In our study that enrolled 89 pre‐LT patients, with a median MELD at registration on the LT list of 12, more than half of them with severe portal hypertension and HCC in 41.6%, with a median age of 56 years, the rate of immunization was similar to the data of Wang et al.( 18 ) and healthy participants.( 12 , 17 ) Comparable results have been recently published by Thuluvath et al.,( 21 ) who enrolled 79 patients with cirrhosis (10 with decompensation) and 92 with CLD: 95.9% developed detectable antibody levels after 41 days from the final vaccine dose (91.8% mRNA vaccine). Among patients with undetectable antibodies, 1 was affected by nonalcoholic steatohepatitis (NASH) and 6 by autoimmune hepatitis (2 with compensated and 1 with decompensated cirrhosis, and 5 of 6 were on immunosuppression therapy). On multiple logistic regression analysis, immunosuppressive status was associated with a poor antibody response, whereas cirrhosis did not correlate.
The IgG titer both at T1 and at T2 was higher in our pretransplant candidates compared with healthy participants, most likely because of the longer interval between the IgG test and vaccination in our control group. The initial decline of the IgG titer between T1 and T2 in our study group seems to support this hypothesis; however, ongoing longitudinal assessment of antibody titers will add more insight. To date, antibody kinetics data after vaccination remain fragmented; however, Doria‐Rose et al.( 22 ) showed persistence of antibodies 6 months after the second dose of mRNA‐1273 vaccine in 33 healthy adult participants included in the phase 1 follow‐up of the Moderna study.( 17 )
With other vaccines, which usually contain inactivated disease‐causing organisms or antigens that work by mimicking the infectious agent, such as against influenza and hepatitis B virus, patients with advanced CLD have been shown to elicit lower humoral immune response,( 10 ) and vaccine formulation with more stimulating adjuvants have been used to improve hypo‐responsiveness.( 23 ) Our preliminary data suggest instead that mRNA vaccination may represent a promising alternative to conventional vaccine approaches, especially in pre‐LT patients, even if IgG titers seem to significantly drop early after LT, but longer follow‐up is needed. This technology has until recently been restricted by the instability and inefficient in vivo delivery of mRNA. Recent advances have now largely overcome these issues, and multiple mRNA vaccine platforms against infectious diseases and several types of cancer have demonstrated encouraging results in both animal models and humans. RNA vaccines are not made with pathogen particles or inactivated pathogens, so they are noninfectious. RNA does not integrate itself into the host genome, and the RNA strand in the vaccine is degraded once the protein is made. On the other hand, the mRNA technology allowed to achieve seropositivity rate of only 47.5% in another study looking at immunosuppressed LT recipients and their antibody titers were significantly lower compared to healthy controls.( 24 ) For this reason, recent articles suggested the administration of a third dose of mRNA vaccine to solid organ transplant recipients to significantly improve its immunogenicity.( 15 , 16 )
Our data and the data of Thuluvath et al.( 21 ) seem to support the premise that efforts should be made to vaccinate patients on LT waiting list, considering the poor outcomes from COVID‐19 in patients with cirrhosis and the disappointing post‐LT seroconversion rate.( 5 , 14 , 24 ) Furthermore, the increasing number of vaccinated LT candidates might allow the use of precious organs from donors with active COVID‐19 infection.( 25 )
The main limitations of this study are the monocentric even if multiregional enrollment, the small number of patients, the low median MELD score, the different elapsed time between vaccination and IgG tests in the pre‐LT and control groups, and the lack of long‐term post‐LT follow‐up, but we believe that preliminary data are necessary to support our ongoing everyday policy with very fragile pretransplant candidates.
In conclusion, as we wait for the results from ongoing trials, especially in patients with severe decompensated cirrhosis, our real‐life data on 89 candidates listed for LT suggest (1) a surprisingly high rate of immunization that together with mask wearing indoors likely confers protection against SARS‐CoV‐2 infection; (2) a confirmation of the safety of the mRNA COVID‐19 vaccines, also in very fragile patients, and (3) vaccination efforts should be encouraged for patients affected by liver disease and those on the LT waiting list. Persistence of humoral response should be monitored over time, in particular after LT, and T cell immunity must be explored to better understand the global vaccine effect. The growing number of variants that may evade individual immunity is 1 of the major challenges we now have to face.( 26 )
Acknowledgments
The authors thank Giovanna Porricelli and all staff members who are working in our pre‐LT outpatient clinic for precious collaboration.
Potential conflict of interest: Nothing to report.
References
- 1. Piano S, Brocca A, Mareso S, Angeli P. Infections complicating cirrhosis. Liver Int 2018;38(suppl 1):126‐133. [DOI] [PubMed] [Google Scholar]
- 2. Albillos A, Lario M, Álvarez‐Mon M. Cirrhosis associated immune dysfunction: distinctive features and clinical relevance. J Hepatol 2014;61:1385‐1396. [DOI] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu YI, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID‐19 in patients with liver and kidney diseases: an early systematic review and meta‐analysis. Trop Med Infect Dis 2020;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID‐19: systematic review with meta‐analysis. Eur Rev Med Pharmacol Sci 2020;24:13072‐13088. [DOI] [PubMed] [Google Scholar]
- 7. The OpenSAFELY Collaborative , Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, et al. OpenSAFELY: factors associated with COVID‐19 related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020. 10.1101/2020.05.06.20092999. [DOI] [Google Scholar]
- 8. Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID‐19: a multicentre United States experience. Liver Int 2020;40:2515‐2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marjot T, Moon AM, Cook JA, Abd‐Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2021;74:567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shouval D. Hepatitis B vaccines. J Hepatol 2003;39:S70‐S76. [DOI] [PubMed] [Google Scholar]
- 11. Marjot T, Webb GJ, Barritt AS, Ginès P, Lohse AW, Moon AM, et al. SARS‐CoV‐2 vaccination in patients with liver disease: responding to the next big question. Lancet Gastroenterol Hepatol 2021;6:156‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID‐19 vaccine. N Engl J Med 2020;383:2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, et al. AASLD COVID‐19 Vaccine Working Group . AASLD expert panel consensus statement: vaccines to prevent COVID‐19 infection in patients with liver disease. Hepatology 2021;74:1049‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cornberg M, Buti M, Eberhardt CS, Grossi PA, Shouval D. EASL position paper on the use of COVID‐19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol 2021;74:944‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of mRNA COVID‐19 vaccine in solid‐organ transplant recipients. N Eng J Med 2021;385:661‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hall VG, Ferreira VH, Ku T, Ierullo M, Majchrzak‐Kita B, Chaparro C, et al. Randomized trial of a third dose of mRNA‐1273 vaccine in transplant recipients. N Engl J Med 2021;385:1244‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. COVE Study Group . Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021;384:403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang J, Hou Z, Liu J, Gu YE, Wu Y, Chen Z, et al. Safety and immunogenicity of COVID‐19 vaccination in patients with non‐alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol 2021;75:439‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cornberg M, Eberhardt CS. Protected or not protected, that is the question. First data on COVID‐19 vaccine responses in patients with NAFLD and liver transplant recipients. J Hepatol 2021;75:265‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hakimiah D, Shfrir A, Milgrom Y, Masarwa M, Hazou W, Amer J, et al. Elderly with advanced liver fibrosis had lower response to Pfizer’s SARS‐CoV‐2 vaccine response [abstract]. J Hepatol 2021;75:S220. [Google Scholar]
- 21. Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID‐19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doria‐Rose N, Suthar MS, Makowski M, O’Connell S, McDermott AB, Flach B, et al. Antibody persistence through 6 months after the second dose of mRNA‐1273 vaccine for Covid‐19. N Engl J Med 2021;384:2259‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vesikari T, Langley JM, Segall N, Ward BJ, Cooper C, Poliquin G, et al. Immunogenicity and safety of a tri‐antigenic versus a mono‐antigenic hepatitis B vaccine in adults (PROTECT): a randomised, double‐blind, phase 3 trial. Lancet Infect Dis 2021;21:1271‐1281. [DOI] [PubMed] [Google Scholar]
- 24. Rabinowich L, Grupper A, Baruch R, Ben‐Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol 2021;75:435‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romagnoli R, Gruttadauria S, Tisone G, Maria Ettorre G, De Carlis L, Martini S, et al. Liver transplantation from active COVID‐19 donors: a lifesaving opportunity worth grasping? Am J Transplant 2021;21:3919–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keehner J, Horton LE, Binkin NJ, Laurent LC, Pride D, Longhurst CA, et al. Resurgence of SARS‐CoV‐2 infection in a highly vaccinated health system workforce. N Engl J Med 2021;385:1330‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]


