Abstract
Background
Physical activity (PA) is an important determinant of cardiovascular health that may be affected the COVID‐19 pandemic. Therefore, we examined the immediate and long‐term effects of the pandemic and lockdown on PA in patients with established cardiovascular risk.
Methods
Objectively‐measured daily PA data was obtained from cardiovascular implantable electronic devices (CIEDs) from 3453 U.S patients (mean and standard deviations [SD] age, 72.65 [13.24] years; 42% women). Adjusted mixed‐effects models stratified by device type were used to compare daily PA from periods in 2020: pre‐lockdown (March 1–14), lockdown (March 15 to May 8), and the reopening phase of the pandemic (May 9 to December 31) versus 2019. Patient characteristics and events associated with inactivity during lockdown and the proportion of patients who returned to their 2019 PA‐level by the end of reopening phase (December 31, 2020) were examined.
Results
Daily PA was significantly lower during the lockdown compared to the same period in 2019 (−15%; p < .0001), especially for pacemaker patients, adults aged <65, and patients more active prior to lockdown. Non‐COVID hospitalization and ICD shock were similarly associated with low PA during lockdown (p = .0001). In the reopening phase of the pandemic, PA remained 14.4% lower in the overall sample and only 23% of patients returned to their 2019 PA level by the end of follow‐up.
Conclusions
In this large cohort of patients with CIEDs, PA was markedly lower during the lockdown and remained lower for months after restrictions were lifted. Strategies to maintain PA during a national emergency are urgently needed.
Keywords: arrhythmia, exercise, implantable cardioverter‐defibrillator, pacemaker
1. INTRODUCTION
Physical activity (PA) is an important factor for prevention and management of cardiovascular disease (CVD) 1 that has been greatly affected by the outbreak of a novel coronavirus in 2019 (SARS‐CoV‐2) and public health strategies to reduce the global burden of COVID‐19. Among the general population, preliminary data from a study of 185,000 US adults showed a 48% decrease in daily PA during a nationwide lockdown (March through April 2020). 2 Sedentary behaviors such as time spent using the computer, lying down, and watching television also increased substantially in the U.S and in other countries during mandatory lockdowns. 2 , 3 These trends are concerning, as even relatively short periods of reduced PA (1–14 days) can promote metabolic, inflammatory and hemodynamic alterations that increase risk for cardiovascular morbidity, mortality, and arrhythmogenic complications in patients with underlying cardiac dysfunction. 1 , 4 Longer durations of inactivity (≥4 weeks) have also been associated with decreased exercise tolerance, increased pulmonary congestion, and a higher risk of hospitalization in patients with heart failure. 5 , 6 Considering that millions of adults with underlying CVD may self‐isolate for a longer duration due to a heightened risk of morbidity and mortality from COVID‐19, 7 it is paramount to identify patients who may benefit from close monitoring and referral to PA interventions to prevent clinical deterioration.
Accordingly, we sought to describe and quantify the effects of the COVID‐19 pandemic and the statewide lockdown in North Carolina on daily PA in a large, well‐characterized cohort of patients with implanted cardiac devices. Since longitudinal PA is automatically collected by implanted cardiac devices and is routinely integrated into electronic health records (EHR), these devices provide a unique opportunity for analysis of PA trends before, during, and after the lockdown in a population of patients with pre‐existing CVD who are typically older with multiple comorbidities. We also examined patient characteristics and clinical events associated with inactivity during the lockdown and assessed the extent to which reductions in PA persisted after restrictions were lifted. Lastly, we identified subgroups of patients at risk for worse health outcomes due to prolonged inactivity during the COVID‐19 pandemic.
2. METHODS
2.1. Study design
To contain the spread of COVID‐19, the Governor of North Carolina issued a series of executive orders beginning on March 15, 2020 that strongly encouraged residents to stay home, and closed schools, restaurants, gyms, and other non‐essential businesses. A statewide, stay‐at‐home order was issued on March 30, 2020 until April 23, 2020, and later extended until May 8, 2020. On May 9th non‐essential businesses, public parks, and eventually restaurants and some schools re‐opened at a reduced capacity.
We examined daily PA from January 1, 2019 to December 31, 2020. Prepandemic data from 2019 were used to establish long‐term PA trends and control for seasonal variation in activity. 8 The average number of minutes of PA per day from March 1, 2020 to March 14, 2020 were used to calculate a prelockdown level of PA for each patient, allowing us to control for baseline PA in our analysis. We defined the COVID‐19 lockdown period as March 15, 2020 to May 8, 2020 and the reopening phase of the pandemic as May 9, 2020 through December 31, 2020 (end of study follow‐up). Daily PA during the COVID‐19 lockdown and reopening phase of the pandemic were compared with data from the corresponding periods in 2019. This study design and analytic approach has been used previously to assess potential adverse health impacts of the COVID‐19 pandemic. 9
The institutional review board at the University of North Carolina (UNC) at Chapel Hill School of Medicine approved the study and waiver of informed consent. The manufacturers of devices used in this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The data that support the findings of this study are available from the corresponding author upon reasonable request.
2.2. Patient cohort and data sources
The study cohort was derived from patients enrolled in the University of North Carolina Cardiovascular Device Surveillance Registry (UNC CDSR), a prospective, multicenter clinical research registry for all cardiovascular implanted electronic devices (CIEDs), including pacemakers, implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices, and implanted cardiac monitors (ICMs), implanted at the UNC Medical Center and eight affiliated hospitals from 2010 to present. Figure 1 shows the geographic distribution of participating hospitals and patients (using zip code of residence). The registry captures information from remote monitoring transmissions and routine follow‐up clinic visits for all CIED implants, upgrades, and replacements. As of January 2021, > 90% of all CIED patients implanted at participating centers were enrolled in remote monitoring (most at the time of implant), suggesting that the UNC CDSR captures a high rate of longitudinal device data from a diverse patient population in a large geographic area.
FIGURE 1.
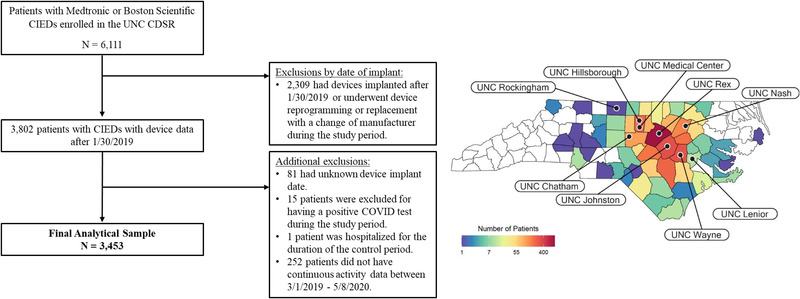
Study Cohort [Colour figure can be viewed at wileyonlinelibrary.com]
All patients in the registry who were at least 18 years old with Medtronic (Minneapolis, MN, USA) or Boston Scientific (Marlborough, MA, USA) cardiac devices (ICM, ICD or pacemaker) implanted prior to January 30, 2019 and who had continuous device data throughout the study period were eligible for inclusion. Our analyses were restricted to these two manufacturers to facilitate PA data comparisons, as they use similar methodology for PA assessment. Individuals not meeting study criteria and those who underwent device reprogramming or replacement with a change of manufacturer during follow‐up were excluded.
Daily PA data were obtained from the accelerometer embedded in the CIED generator. The process of PA data collection by CIED accelerometer and a summary of validation studies are available in a recently published review. 10 Briefly, as the patient moves or accelerates, internal sensors detect changes in the frequency and amplitude of body motion, generating an electrical signal that is proportional to patient movement. 11 Patient acceleration that meets or exceeds a preprogrammed threshold is considered an active minute and the total minutes of PA per day are automatically stored in device memory. PA is recorded similarly by Medtronic and Boston Scientific devices and would be considered light‐intensity PA that is equivalent to walking at a slow pace (details provided in the Data Supplement). 12
As in previous studies, 13 PA data from the first 30 days postimplant were excluded from analysis to account for procedural recovery. In addition, data on all episodes of ICD shock were obtained from the registry because prior studies have shown a significant reduction in PA after ICD shock. 14 Electrograms for all arrhythmia episodes treated by ICD shock were adjudicated by a board‐certified electrophysiologist in a blinded manner.
Sociodemographic information, clinical history, medications, and hospitalizations were abstracted from outpatient and inpatient EHR notes using automated computer algorithms and standard methodology. 15 Sociodemographic information was collected closest to the pre‐COVID period. Comorbidities were considered present and assumed to persist if the International Classification of Diseases, Tenth Revision (ICD‐10) code for that specific condition was recorded in the EHR prior to January 1, 2020. Individuals with laboratory confirmed diagnoses of COVID‐19 during follow‐up (n = 55) were excluded from the study, as the virus may have unique behavioral effects on PA. 16 , 17 Additionally, because individuals may alter their behavior, and thus PA, in response to local disease transmissibility, data on new confirmed cases of COVID‐19 in North Carolina were obtained from publicly available datasets for the period March 1 through December 31, 2020. 18
2.3. Statistical analysis
Descriptive statistics were used to summarize the sample characteristics, and comparisons were made with Student t test or by Pearson's Chi‐Squared tests, as appropriate. Sample characteristics were also compared by quartiles of prelockdown PA. Continuous variables are expressed as means and standard deviations (SD) for continuous variables, and frequency (percentage) for categorical variables. A two‐sided p value of less than .05 was considered significant. All analyses were performed with R software, version 3.6.1 (R Core Team, 2019).
In the main analysis, PA was stratified by device type to account for known variation in activity patterns, clinical features, and potential exposures that impact PA in patients with various types of implanted cardiac devices (e.g., ICD shock). 1 We used linear mixed‐effects models with daily PA values as the unit of analysis and a random intercept for each patient to estimate trajectories of PA during the COVID‐19 lockdown and re‐opening phase of the pandemic (May 9, 2020 to December 31, 2020) relative to the corresponding periods in 2019. This analysis allows for correlations between repeated measurements on the same individuals. Effects are reported with 95% confidence intervals (CI). The base model included variables for time since implant as a piecewise linear covariate and adjusted for quartiles of prelockdown PA. All days when an ICD shock occurred and days during hospitalization were initially excluded. Subsequent analyses were performed by adding covariates to the base model. Covariates were selected a priori based on previous literature. 19 Analyses were initially adjusted for age (≥65 years), sex, race/ethnicity (white vs. non‐white/all others), and BMI. The larger numbers of patients with pacemakers and ICDs allowed us to further adjust for additional CVD risk factors in those analyses.
Since prior studies have shown a significant effect of hospitalization and ICD shock on PA in device patients, 14 separate analyses were performed to assess the additional effects of hospitalization and ICD shock on PA during the COVID‐19 lockdown period. Linear mixed‐effects models with an indicator variable were used to estimate the average difference in PA between days when hospitalization did and did not occur with ICMs as the reference group. A similar procedure was used to estimate the effects of ICD shock on PA in those with ICDs, and a separate model assessed for the effects of appropriate versus inappropriate ICD shocks. In an additional sensitivity analysis, the primary analysis was repeated in persons with an ICD after excluding patients who experienced an ICD shock at any point during the follow‐up period.
PA data from the reopening phase of the pandemic and the corresponding period in 2019 were used to identify and compare characteristics of individuals who did and did not return to their 2019 level of PA by the end of the follow‐up period (December 31, 2020) using Student t test and Pearson's Chi‐Squared tests, as appropriate. Additionally, daily PA from March 1, 2020 to December 31, 2020 were plotted in relation to new confirmed cases of COVID‐19 in North Carolina to discern whether, and to what extent, PA patterns (a proxy for a behavioral response) may have been influenced by local spread of COVID‐19.
3. RESULTS
Of the 6111 patients with Medtronic or Boston Scientific devices enrolled in the UNC CDSR, 3453 met study inclusion criteria (Figure 1). Of those included in the final cohort, 175 (5.2%) had an ICM, 1594 had a pacemaker (46.2%) and 1681 had an ICD (48.7%). Cohort demographics, comorbidities, and medications according to device type are summarized in Table 1. Patients were predominantly older (mean age of 72.65 ± 13.24 years) and white (74.7%) with multiple comorbidities. Patients with ICMs tended to be younger with fewer comorbidities relative to those with pacemakers and ICDs.
TABLE 1.
Cohort characteristics
| Total (N = 3453) | ICM (n = 178) | Pacemaker (n = 1594) | ICD (n = 1681) | p value | |
|---|---|---|---|---|---|
| Sociodemographic characteristics | |||||
| Age (Years)a | 72.65 (13.24) | 64.92 (17.39) | 77.59 (11.45) | 68.77 (12.62) | <.001 |
| Female sex | 1431 (41.6%) | 100 (56.8%) | 800 (50.3%) | 531 (31.8%) | <.001 |
| Race/Ethnicity | <.001 | ||||
| Black or African American | 707 (20.6%) | 21 (11.9%) | 198 (12.5%) | 488 (29.2%) | |
| Hispanic or Latino | 65 (1.9%) | 4 (2.3%) | 25 (1.6%) | 36 (2.2%) | |
| White or Caucasian | 2567 (74.7%) | 144 (81.8%) | 1318 (82.9%) | 1105 (66.1%) | |
| Other | 98 (2.9%) | 7 (4.0%) | 48 (3.0%) | 43 (2.6%) | |
| Employment status | <.001 | ||||
| Full time | 360 (13.8%) | 43 (32.3%) | 125 (9.4%) | 192 (16.8%) | |
| Part time | 61 (2.3%) | 6 (4.5%) | 26 (2.0%) | 29 (2.5%) | |
| Retired | 2182 (83.8%) | 84 (63.2%) | 1179 (88.6%) | 919 (80.6%) | |
| Lifestyle factors | |||||
| Body mass index (BMI) a | 29.78 (6.57) | 29.53 (7.48) | 29.10 (6.46) | 30.46 (6.51) | <.001 |
| Alcohol abuse | 31 (0.9%) | 0 (0.0%) | 10 (0.6%) | 21 (1.3%) | .07 |
| Drug abuse | 130 (3.8%) | 5 (2.9%) | 37 (2.3%) | 88 (5.3%) | <.001 |
| Smoking status | <.001 | ||||
| Current | 250 (7.3%) | 13 (7.6%) | 75 (4.8%) | 162 (9.8%) | |
| Former | 1595 (46.9%) | 65 (37.8%) | 703 (44.6%) | 827 (50.0%) | |
| Never | 1558 (45.8%) | 94 (54.7%) | 798 (50.6%) | 666 (40.2%) | |
| Device characteristics | |||||
| Device manufacturer | <.001 | ||||
| Boston scientific | 1029 (29.8%) | 1 (0.6%) | 528 (33.1%) | 500 (29.7%) | |
| Medtronic | 2424 (70.2%) | 177 (99.4%) | 1066 (66.9%) | 1181 (70.3%) | |
| Time since implant (Years) a | 3.49 (1.91) | 1.93 (0.60) | 3.47 (1.86) | 3.67 (1.97) | <.001 |
| Clinical comorbidities | |||||
| Hypertension | 3193 (93.0%) | 143 (82.2%) | 1462 (92.1%) | 1588 (95.1%) | <.001 |
| Previous myocardial infarction | 2257 (65.8%) | 66 (37.9%) | 893 (56.2%) | 1298 (77.7%) | <.001 |
| Congestive heart failure | 2413 (70.3%) | 42 (24.1%) | 762 (48.0%) | 1609 (96.3%) | <.001 |
| Coronary artery disease | 2344 (68.3%) | 78 (44.8%) | 931 (58.6%) | 1335 (79.9%) | <.001 |
| Diabetes mellitus | 1378 (40.2%) | 47 (27.0%) | 574 (36.1%) | 757 (45.3%) | < 0.001 |
| Obstructive sleep apnea | 652 (19.0%) | 34 (19.5%) | 299 (18.8%) | 319 (19.1%) | .96 |
| Stroke/TIA | 497 (14.5%) | 30 (17.2%) | 256 (16.1%) | 211 (12.6%) | .01 |
| Lipid disorders | 2457 (71.6%) | 95 (54.6%) | 1111 (70.0%) | 1251 (74.9%) | <.001 |
| Peripheral vascular disease | 1349 (39.3%) | 60 (34.5%) | 656 (41.3%) | 633 (37.9%) | .06 |
| Valvular heart disease | 2323 (67.7%) | 86 (49.4%) | 1084 (68.3%) | 1153 (69.0%) | <.001 |
| Chronic kidney disease | 521 (15.2%) | 12 (6.9%) | 202 (12.7%) | 307 (18.4%) | <.001 |
| COPD | 1183 (34.5%) | 57 (32.8%) | 501 (31.5%) | 625 (37.4%) | .002 |
| Atrial fibrillation/atrial flutter | 2561 (74.6%) | 123 (70.7%) | 1176 (74.1%) | 1262 (75.6%) | .29 |
| Prior sudden cardiac arrest | 231 (6.7%) | 5 (2.9%) | 46 (2.9%) | 180 (10.8%) | <.001 |
| Left ventricular assist device | 51 (1.5%) | 2 (1.1%) | 4 (0.3%) | 45 (2.7%) | <.001 |
| Medications | |||||
| ACE‐inhibitor or ARB | 2591 (75.4%) | 82 (46.6%) | 1049 (66.0%) | 1460 (87.3%) | <.001 |
| Beta‐blocker | 3053 (88.8%) | 127 (72.2%) | 1287 (81.0%) | 1639 (98.0%) | <.001 |
| Statin | 2548 (74.1%) | 95 (54.0%) | 1108 (69.7%) | 1345 (80.4%) | <.001 |
| Calcium channel blockers | 1790 (52.1%) | 79 (44.9%) | 919 (57.8%) | 792 (47.4%) | .01 |
| Anti‐arrhythmic | 1439 (41.9%) | 70 (39.8%) | 658 (41.4%) | 711 (42.5%) | .69 |
| Anticoagulation | 2635 (76.7%) | 105 (59.7%) | 1242 (78.2%) | 1288 (77.0%) | <.001 |
| Antiplatelet agent/Aspirin | 2956 (86.0%) | 135 (76.7%) | 1307 (82.3%) | 1514 (90.6%) | <.001 |
| Antidepressant | 1421 (41.3%) | 74 (42.0%) | 651 (41.0%) | 696 (41.6%) | .91 |
| Psychiatric comorbidities | |||||
| Major depressive disorder | 704 (20.5%) | 40 (23.0%) | 308 (19.4%) | 356 (21.3%) | .28 |
| Prior anxiety disorder c | 159 (4.6%) | 9 (5.2%) | 76 (4.8%) | 74 (4.4%) | .84 |
Abbreviations: ACE, = angiotensin converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter defibrillator; TIA, transient ischemic attack.
Data are presented as mean ± standard deviation (SD).
Antidepressant medications include selective serotonin reuptake inhibitors/serotonin and norepinephrine reuptake inhibitors.
A composite variable was created for any prior diagnoses of anxiety disorders: generalized anxiety disorder, post‐traumatic stress disorder, acute stress disorder and panic disorder.
3.1. Patient PA prior to the outbreak of COVID‐19
In total, PA values were available for 3453 patients (mean days per patient = 657 days). During the prelockdown period, the average daily PA for the entire cohort was 133.7 ± 112 min per day (mean and SD). Table S1 of the Data Supplement presents the characteristics of the study cohort according to quartiles of prelockdown PA. Several baseline characteristics including sex, age, renal dysfunction, heart failure status, medications, and lifestyle factors were associated with PA levels prior to the lockdown period.
3.2. Changes in PA during the COVID‐19 lockdown versus non‐COVID control period in 2019
In the overall study cohort (Figure 2), we observed significantly lower PA during the lockdown compared to the same period in 2019 (15% lower overall PA; p < .0001). Patterns were generally consistent when stratified by device type (Table 2 and Figure 3). Among patients with ICMs, daily PA was 9.5% lower during the lockdown relative to the 2019 control period (−28.34 min/day, CI: −38.36–−17.91, p < .0001), and was 15% lower for patients with pacemakers (−29.32 min/day, CI: −30.67, −27.96, p < .0001) and those with ICDs (−22.77 min/day, CI: −23.93–−21.62, p < .0001). The adverse effects of the lockdown on PA remained significant in multivariate models that were stratified by device type and adjusted for pre‐COVID PA, time since implant, sociodemographic characteristics, clinical comorbidities, medications, and depression (all p's < 0.0001).
FIGURE 2.
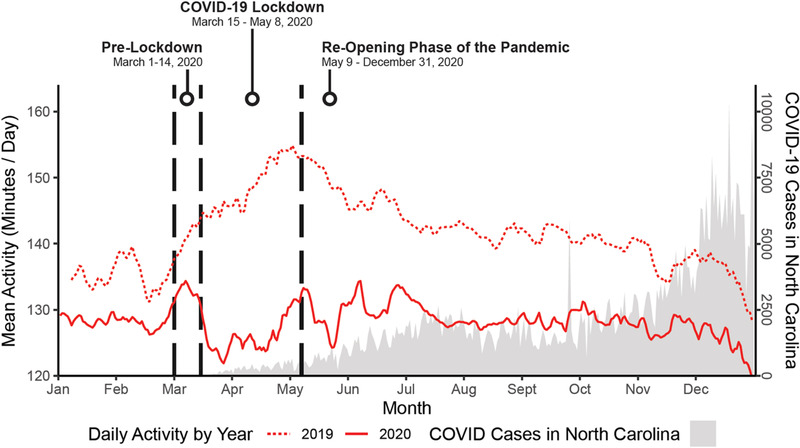
Impact of COVID‐19 lockdown on daily PA relative to new confirmed cases of COVID‐19 in North Carolina [Colour figure can be viewed at wileyonlinelibrary.com]
TABLE 2.
Changes in activity during the COVID‐19 lockdown compared to the same period in 2019 stratified by device type
| ICM | Pacemaker | ICD | ||||
|---|---|---|---|---|---|---|
| Changes in daily activity during the COVID‐19 lockdown (min/day) a | −28.76 (−38.31, −17.78) | < 0.001 | −29.02 (−30.35, −27.63) | < 0.001 | −22.80 (−23.91, −21.64) | 0.001 |
| Time since implant (years) | 0.04 (0.01, 0.07) | 0.004 | 0.009 (0.01, 0.01) | < 0.001 | 0.01 (0.01, 0.02) | 0.006 |
| Pre‐COVID PA level b | ||||||
| Low quartile of activity | 64.81 (43.87, 85.67) | < 0.001 | 52.71 (45.64, 59.76) | < 0.001 | 54.54 (48.57, 60.51) | <0.001 |
| Mid quartile of activity | 153.25 (131.91, 174.51) | <0.001 | 157.99 (150.81, 165.16) | < 0.001 | 133.8 (127.58, 140.02) | < 0.001 |
| Highest quartile of activity | 277.84 (251.53, 304.34) | <0.001 | 314.07 (304.94, 323.18) | < 0.001 | 266.38 (258.08, 274.68) | < 0.001 |
| Sociodemographic characteristics | ||||||
| Age (≥65 years) | −8.84 (−22.92, 5.07) | 0.23 | −22.58 (−30.93, −14.24) | < 0.001 | −13.98 (−18.95, −9.02) | < 0.001 |
| Female | −11.46 (−23.93, 1.01) | 0.08 | −13.37 (−18.35, −8.39) | < 0.001 | −11.85 (−16.41, −7.29) | < 0.001 |
| Non‐white race/ethnicity | −4.54 (−21.51, 12.44) | 0.61 | −3.38 (−9.86, 3.10) | 0.31 | −1.85 (−6.26, 2.55) | 0.42 |
| Lifestyle factors | ||||||
| Body mass index (BMI) | −5.77 (−11.64, 0.08) | 0.06 | −1.19 (−3.67, 1.28) | 0.35 | −3.67 (−5.82, −1.52) | < 0.001 |
| Current smoker | −6.66 (−17.96, 4.61) | 0.25 | −4.42 (−11.82, 2.99) | 0.25 | ||
| Former smoker | ‐0.54 (−5.53, 4.46) | 0.83 | 3.07 (−1.30, 7.45) | 0.17 | ||
| Clinical comorbidities and medications | ||||||
| Atrial fibrillation/atrial flutter | −3.29 (−8.34, 1.77) | 0.21 | −4.16 (−8.37, 0.06) | 0.06 | ||
| Stroke/TIA | 2.24 (−4.24, 8.73) | 0.50 | −7.26 (−13.40, −1.11) | 0.02 | ||
| Myocardial infarction | −0.94 (−14.67, 12.71) | 0.89 | −3.26 (−16.49, 9.96) | 0.63 | ||
| Congestive heart failure | −3.91 (−9.05, 1.21) | 0.14 | 10.15 (−1.33, 21.61) | 0.09 | ||
| Coronary artery disease | 3.13 (−10.63, 16.94) | 0.66 | −4.83 (−18.61, 8.98) | 0.50 | ||
| Diabetes mellitus | −2.03 (−7.19, 3.13) | 0.44 | −3.03 (−7.23, 1.17) | 0.16 | ||
| ACEs/ARBs | 2.91 (−2.24, 8.04) | 0.27 | −3.78 (−10.19, 2.63) | 0.25 | ||
| Beta blockers | 1.11 (−5.34, 7.56) | 0.73 | 4.45 (−11.02, 19.92) | 0.58 | ||
| Psychiatric comorbidities | 1.09 (−4.97, 7.14) | 0.73 | −1.49 (−6.50, 3.51) | 0.56 | ||
| Major depressive disorder | 1.09 (−4.97, 7.14) | 0.73 | −1.49 (−6.50, 3.51) | 0.56 |
Mean changes in activity (min/day).
Quartiles of pre‐COVID activity (inactive = reference group) based on the average number of minutes of activity per day recorded by their implanted cardiac device between March 1, 2020 and March 14, 2020.
FIGURE 3.
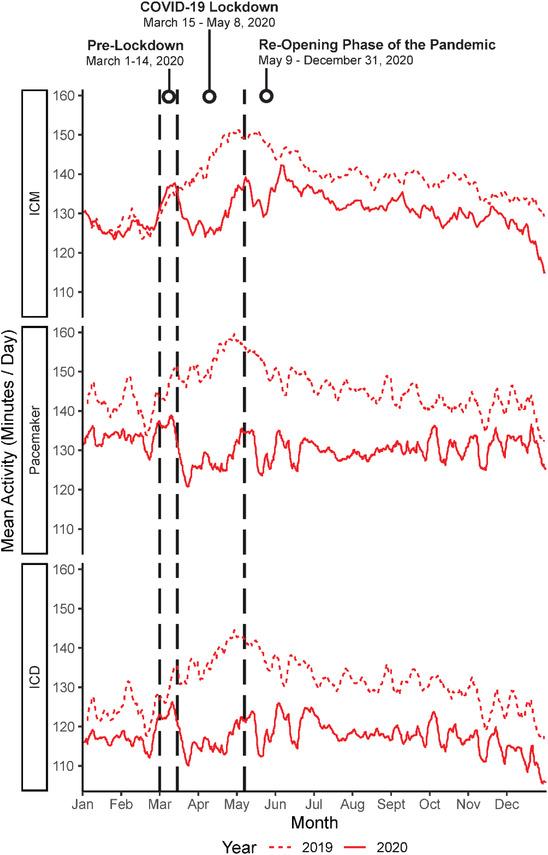
Daily PA during the COVID‐19 lockdown and the same period in 2019 stratified by device type [Colour figure can be viewed at wileyonlinelibrary.com]
For all three device groups, the single most important factor associated with lower PA during the lockdown was prelockdown PA (Table 2 and Figure 4), with data suggesting that individuals having the highest PA immediately prior to the lockdown demonstrated the largest absolute decline in daily PA during the lockdown period. Similarly, men and adults age <65 demonstrated significantly lower PA during the lockdown relative to the 2019 control period.
FIGURE 4.
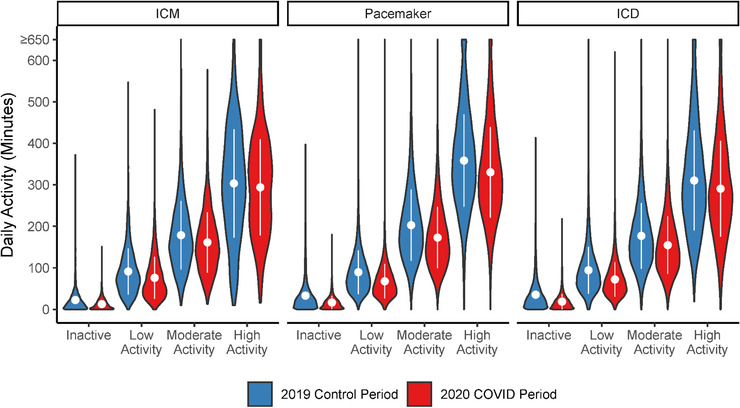
Changes in daily PA during the COVID‐19 lockdown according to pre‐lockdown levels of PA [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. Effect of hospitalization and ICD shocks on PA during the COVID‐19 lockdown
Overall, 137 (4.0%) patients were admitted to the hospital during the COVID‐19 lockdown, which was associated with significantly lower PA during lockdown, after adjusting for device type and time since implant (−54.27 min/day, CI: −55.25–−53.29, p < .0001). In analyses stratified by device groups, the data show that a hospital admission was associated with significantly lower PA in patients with pacemakers (−31.14 min/day) and ICDs (−13.75 min/day) relative to individuals with ICMs. Only one patient was admitted to the hospital for ICD shock during the lockdown.
In addition, 18 shocked episodes occurred in 17 patients (1.0% of patients with ICDs) during the lockdown. PA was significantly lower on days when patients experienced ICD shock (−11.24 min/day, CI: −17.96–−4.52, p = 0.0001) after adjusting for time since implant. There was no difference in effect between individuals who received appropriate versus inappropriate shocks (p = .47). In post hoc analyses, exclusion of persons who experienced ICD shock at any point during the follow‐up period did not significantly alter the primary findings (Table S2 of the Data Supplement).
3.4. Long‐term PA trends during the reopening Phase of the pandemic
After the lockdown orders were lifted on May 9, 2020, average daily PA remained 14.4% lower in the overall sample during the subsequent reopening phase of the pandemic (May 9, 2020 to Dec 31, 2020), compared with the corresponding period in 2019 (−13.91 min/day, CI: −14.10–−13.72, p < .0001). In analyses stratified by device groups, patients with ICMs had 3.6% lower PA during the reopening phase of the pandemic (−7.75 min/day, CI: −8.71–−6.78, p < .0001). Similarly, patients with pacemakers had 17.2% lower PA (−15.58 min/day, CI: −15.86–−15.30, p < .0001) and patients with ICDs had 12.5% lower PA (−12.99 min/day, CI: −13.26–−12.72, p < .0001).
As shown in Table 3, only 819 out of 3453 patients (23%) returned to their 2019 PA level by the end of follow‐up (December 31, 2020). Patients who returned to their previous level of PA tended to be younger (p < .0001), male (p < .0001), and were employed full‐time (p = .04). They were also less likely to have a history of diabetes (p = .04) or stroke (p = .03). In addition, Figure 2 shows the potential impact of daily case rates of COVID‐19 in North Carolina on overall PA trends. The data show an inverse association between new cases of COVID‐19 in North Carolina and daily PA, suggesting that changes in PA during the reopening phase of the pandemic were often affected by fluctuations in COVID‐19 infections.
TABLE 3.
Characteristics of patients who did and did not return to their 2019 activity levels during the reopening phase of the COVID‐19 pandemic
| Did not return to previous activity level (n = 2633) | Returned to previous activity level (n = 819) | Total (N = 3453) | p value | |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Age (Years) a | 73.698 (12.791) | 69.291 (14.068) | 72.654 (13.237) | <.001 |
| Female sex | 1140 (43.5%) | 291 (35.7%) | 1431 (41.6%) | <.001 |
| Race/Ethnicity | .67 | |||
| Black or African American | 536 (20.4%) | 171 (21.0%) | 707 (20.6%) | |
| Hispanic or Latino | 53 (2.0%) | 12 (1.5%) | 65 (1.9%) | |
| White or Caucasian | 1961 (74.8%) | 605 (74.3%) | 2566 (74.7%) | |
| Other | 72 (2.7%) | 26 (3.2%) | 98 (2.9%) | |
| Employment status | .04 | |||
| Full time | 264 (13.0%) | 96 (17.0%) | 360 (13.8%) | |
| Part time | 47 (2.3%) | 14 (2.5%) | 61 (2.3%) | |
| Retired | 1727 (84.7%) | 454 (80.5%) | 2181 (83.8%) | |
| Device characteristics | ||||
| Device type | .13 | |||
| ICM | 132 (5.0%) | 46 (5.6%) | 178 (5.2%) | |
| Pacemaker | 1261 (47.9%) | 420 (51.3%) | 1681 (48.7%) | |
| ICD | 1261 (47.9%) | 420 (51.3%) | 1681 (48.7%) | |
| Device manufacturer | .07 | |||
| Boston scientific | 805 (30.6%) | 223 (27.2%) | 1028 (29.8%) | |
| Medtronic | 1828 (69.4%) | 596 (72.8%) | 2424 (70.2%) | |
| Time since implant (Years) a | 3.492 (1.923) | 3.462 (1.866) | 3.485 (1.910) | .98 |
| Lifestyle factors | ||||
| Body mass index (BMI) b | 29.845 (6.664) | 29.572 (6.250) | 29.781 (6.569) | .47 |
| Alcohol abuse c | 22 (0.8%) | 9 (1.1%) | 31 (0.9%) | .48 |
| Drug abuse c | 93 (3.6%) | 37 (4.6%) | 130 (3.8%) | .19 |
| Smoking status | .31 | |||
| Current | 184 (7.1%) | 66 (8.2%) | 250 (7.3%) | |
| Former | 1209 (46.5%) | 386 (48.1%) | 1595 (46.9%) | |
| Never | 1206 (46.4%) | 351 (43.7%) | 1557 (45.8%) | |
| Clinical comorbidities | ||||
| Hypertension | 2445 (93.4%) | 748 (92.1%) | 3193 (93.1%) | .23 |
| Previous myocardial infarction | 1719 (65.6%) | 538 (66.3%) | 2257 (65.8%) | .75 |
| Congestive heart failure | 1843 (70.4%) | 570 (70.2%) | 2413 (70.3%) | .93 |
| Coronary artery disease | 1787 (68.2%) | 557 (68.6%) | 2344 (68.3%) | .85 |
| Diabetes mellitus | 1076 (41.1%) | 301 (37.1%) | 1377 (40.1%) | .04 |
| Obstructive sleep apnea | 502 (19.2%) | 150 (18.5%) | 652 (19.0%) | .66 |
| Stroke/TIA | 399 (15.2%) | 98 (12.1%) | 497 (14.5%) | .03 |
| Lipid disorders | 1882 (71.9%) | 574 (70.7%) | 2456 (71.6%) | .52 |
| Peripheral vascular disease | 1045 (39.9%) | 304 (37.4%) | 1349 (39.3%) | .21 |
| Valvular heart disease | 1790 (68.3%) | 533 (65.6%) | 2323 (67.7%) | .15 |
| Chronic kidney disease | 401 (15.3%) | 120 (14.8%) | 521 (15.2%) | .71 |
| COPD | 900 (34.4%) | 283 (34.9%) | 1183 (34.5%) | .79 |
| Atrial fibrillation/atrial flutter | 1961 (74.9%) | 599 (73.8%) | 2560 (74.6%) | .53 |
| Prior sudden cardiac arrest | 180 (6.9%) | 51 (6.3%) | 231 (6.7%) | .56 |
| Left ventricular assist device | 37 (1.4%) | 14 (1.7%) | 51 (1.5%) | .53 |
| Medications | ||||
| ACE‐inhibitor or ARB | 1965 (74.9%) | 625 (76.8%) | 2590 (75.4%) | .29 |
| Beta‐Blocker | 2321 (88.5%) | 731 (89.8%) | 3052 (88.8%) | .31 |
| Statin | 1956 (74.6%) | 591 (72.6%) | 2547 (74.1%) | .26 |
| Calcium channel blockers | 1381 (52.7%) | 409 (50.2%) | 1790 (52.1%) | .23 |
| Anti‐Arrhythmic | 1083 (41.3%) | 356 (43.7%) | 1439 (41.9%) | .22 |
| Anticoagulation | 2020 (77.0%) | 614 (75.4%) | 2634 (76.7%) | .34 |
| Antiplatelet agent/Aspirin | 2264 (86.3%) | 691 (84.9%) | 2955 (86.0%) | .29 |
| Antidepressant | 1079 (41.2%) | 342 (42.0%) | 1421 (41.4%) | .66 |
| Psychiatric comorbidities | ||||
| Major depressive disorder | 536 (20.5%) | 168 (20.7%) | 704 (20.5%) | .89 |
| Prior anxiety disorder d | 120 (4.6%) | 39 (4.8%) | 159 (4.6%) | .79 |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; ICD, implantable cardioverter defibrillator; TIA, transient ischemic attack.
Data are presented as mean ± standard deviation (SD).
Alcohol and drug abuse diagnoses were obtained from the EHR.
Antidepressant medications include selective serotonin reuptake inhibitors/serotonin and norepinephrine reuptake inhibitors.
A composite variable was created for any prior diagnoses of anxiety disorders: generalized anxiety disorder, post‐traumatic stress disorder, acute stress disorder and panic disorder.
4. DISCUSSION
Until now, information on the secondary health impacts of the pandemic has been limited, especially in medically vulnerable populations, such as those with underlying CVD, who are more susceptible to acute changes in health status. The principal findings from this study include the following: (1) daily PA was significantly lower in the overall cohort (15%) during the mandatory lockdown in North Carolina relative to the same period in 2019, even when accounting for demographic factors, baseline PA, comorbidities, medications, and depression; (2) the effect on PA was greatest in the pacemaker group, adults <65 years of age, men, and for those who were more active prior to the lockdown period; (3) after the lockdown orders were lifted, PA initially increased but often declined as cases of COVID‐19 increased in North Carolina; (4) our results further suggest that nearly 1 year into the COVID‐19 pandemic–7 months after the lockdown orders were lifted–77% of patients in this study had not returned to their previous level of PA from the same period in 2019. These findings suggest that targeted strategies should be developed to maintain the health and wellness of vulnerable populations during the current pandemic and may help us prepare for future public health emergencies.
Early studies of ICD patients in Italy suggested that PA modestly declined during a 40‐day national lockdown. 20 , 21 Similar trends have been reported in a sample of U.S adults with pacemakers and ICDs, and in pediatric device patients. 22 , 23 However, these studies were generally limited to small samples of predominantly male patients with devices from a single manufacturer and did not adequately control for risk factors known to affect PA in persons with implanted cardiac devices (e.g., comorbidities, hospitalization and ICD shocks). 14 Consequently, their data do not identify sub‐groups of patients who may benefit from targeted strategies to maintain PA during a public health emergency. Our findings extend this work by illustrating longitudinal PA trends over a 2‐year period in a heterogenous population of patients with CIEDs with continuous PA monitoring and included a wide range of implanted cardiac devices from multiple manufacturers. Moreover, the large size of our dataset and the integration of multiple sources of high‐dimensional health data allowed us to comprehensively assess the immediate and long‐term impact of the lockdown on daily PA with greater precision.
Results from the current study demonstrate concerning trends in PA among a high‐risk group of cardiac patients, especially those with pacemakers and ICDs. Similarly, individuals who were more active immediately prior to the COVID‐19 pandemic, adults < 65 years of age, and men experienced significantly lower PA during the lockdown and may benefit from targeted interventions to maintain health‐promoting behaviors during the pandemic. We also showed that adverse clinical events, such as hospitalization or ICD shock, had an immediate and detrimental effect on PA during lockdown. This finding may reflect that patients perform very little or no PA during hospitalization. 24 Alternatively, patients may experience anxiety and engage in behavioral avoidance after ICD shock. 14 More importantly, reductions in PA did not significantly differ between those who received appropriate versus inappropriate shocks. This finding is consistent with data from a prior study, 14 and suggests that the experience of ICD shock–not the underlying arrhythmia–triggered the behavioral response (i.e., immediate reduction in PA). These results suggest that there may be opportunities to leverage PA data obtained from CIEDs to proactively identify patients at risk for adverse outcomes due to prolonged inactivity and link them to interventions that can facilitate patients’ recovery, and return to activity following critical events, such as a global pandemic, lockdown, ICD shock, or even a prolonged period of time spent at home.
In addition, this study advances our understanding of the long‐term effects of the pandemic and lockdown on an important modifiable risk factor for CVD. Although there was an initial increase in PA after the statewide lockdown orders were eased in May 2020, we saw evidence of persistently lower PA for months after restrictions were lifted compared to PA trends from 1 year earlier. This pattern of prolonged inactivity was observed in patients representing a broad clinical spectrum of CVD – from younger patients with ICMs, who presumably have less cardiovascular risk compared to those with ICDs and pacemakers, to those with CRT‐D devices and advanced heart failure. Given the robust associations between inactivity and the risk of CVD morbidity andmmortality, 1 , 4 and the likely increase in other cardiovascular risk factors during the pandemic, 25 ongoing surveillance of these issues should be considered to prevent potential increases in primary and secondary care presentations for CVD in the months and years ahead.
4.1. Implications of findings
PA is an effective preventive measure that can reduce CVD progression and mortality. 1 Thus, early and ongoing education about the health benefits of PA and the risks of prolonged inactivity is essential to optimize CVD self‐management. 13 , 26 Patients may also benefit from specific guidance from their healthcare providers regarding strategies for exercising safely during the pandemic. 27 , 28 Moreover, it is clear that cost‐effective, widely‐scalable, technology‐based tools and telemedicine interventions need to be developed to supplement traditional in‐person care. Preliminary data from a remotely‐delivered intervention for patients with atrial fibrillation that was rapidly developed and deployed during the COVID‐19 lockdown demonstrated the feasibility and acceptability of this type of intervention, as well as improvements in mood and disease self‐management. 29 Whether a similar approach can be used to improve health and health outcomes in other patient populations during routine care or in future public health emergencies requires further investigation.
4.2. Limitations
This study has some limitations. First, although the UNC CDSR captures data from multiple centers and hospitals located throughout North Carolina, this is a study of patients from a single healthcare system. Second, information on some clinical parameters were not available for this study including NYHA (New York Heart Association) heart failure classification, ejection fraction, and data on lifestyle factors (e.g., adherence to medical therapies, sleep and diet). 19 This is a common limitation of registry‐based studies that utilize administrative data. 9 Information on device indication was also not available and PA patterns may differ according to underlying cardiovascular etiology. 1 However, in prior studies, PA measured by CIEDs has not been found to differ among primary and secondary prevention patients after correcting for device type. 14 Thus, we stratified our analyses by device type. Third, CIED sensors are highly sensitive to the onset of PA but they are less sensitive to short bursts of movement or vibrations, 30 and they are not able to determine the type or intensity of PA performed (i.e., light, moderate vigorous), 10 and the data cannot be used to compute metabolic equivalent tasks (METs). 31 Thus, data from CIEDs may not generalize to data obtained from other wearable PA devices. Developing CIED‐specific PA thresholds will enhance the utility of these data. Finally, we did not have sufficient statistical power to investigate whether reductions in PA during the pandemic were associated with subsequent increases in hospitalizations or deaths. Larger studies are required to ascertain the association between reduced PA during the pandemic and health outcomes.
5. CONCLUSIONS
In this longitudinal study of North Carolina adults with implanted cardiac devices, PA was significantly lower during the COVID‐19 lockdown than during a comparable period 1 year earlier. During the subsequent reopening phase of the pandemic, PA did not return to prepandemic levels for a great majority of patients. Whether technology‐based tools can proactively identify patients at risk of low PA and link them to interventions to prevent clinical deterioration during a national emergency or improve secondary prevention of CVD in routine care is an important avenue of future research.
AUTHOR CONTRIBUTIONS
Lindsey Rosman, PhD receives consulting fees from Pfizer. Anil Gehi, MD: Dr. Gehi receives research support from Bristol Myers Squib Foundation, consulting fees from Biosense‐Webster, speaker's honoraria from Abbott, Biotronik, Zoll Medical. The manufacturers of devices used in this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors take responsibility for the integrity of the data and analyses.
Supporting information
Table S1. Cohort characteristics according to pre‐COVID levels of daily physical activity
Table S2. Changes in activity during the COVID‐19 lockdown compared to the same period in 2019 after excluding ICD patients who experienced an ICD shock during follow‐up
ACKNOWLEDGEMENTS
This study was supported by a grant from the National Heart, Lung, And Blood Institute of the National Institutes of Health to Dr. Rosman (K23HL141644). Dr. Salmoirago‐Blotcher is funded by grants R01HL149672 and R21HL140492. Support for the data linkage and database management were provided by the Translational and Clinical Sciences Institute (UL1TR002489 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences, National Institutes of Health).
Rosman L, Mazzella AJ, Gehi A, et al. Immediate and long‐term effects of the COVID‐19 pandemic and lockdown on physical activity in patients with implanted cardiac devices. Pacing Clin Electrophysiol. 2022;45:111–123. 10.1111/pace.14409
REFERENCES
- 1. Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: jACC health promotion series. J Am Coll Cardiol. 2018;72(14):1622–1639. (In eng). [DOI] [PubMed] [Google Scholar]
- 2. Evidation Health I. COVID‐19 pulse: delivering regular insights on the pandemic from a 150,000+ person connected cohort. April 15, 2020. ( https://evidation.com/news/covid‐19‐pulse‐first‐data‐evidation/).
- 3. Tison GH, Avram R, Kuhar P, et al. Worldwide effect of COVID‐19 on physical activity: a descriptive study. Ann Intern Med. 2020;173(9):767–770. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nosova EV, Yen P, Chong KC, et al. Short‐term physical inactivity impairs vascular function. J Surg Res. 2014;190(2):672–682. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003;107(8):1210–1225. (In eng). [DOI] [PubMed] [Google Scholar]
- 6. Kelly Jacob P, Ballew Nicholas G, Lin L, et al. Association of implantable device measured physical activity with hospitalization for heart failure. JACC: Heart Failure. 2020;8(4):280–288. [DOI] [PubMed] [Google Scholar]
- 7. Kiss P, Carcel C, Hockham C, Peters SAE. The impact of the COVID‐19 pandemic on the care and management of patients with acute cardiovascular disease: a systematic review. Eur. Heart j. 2020.7(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klompstra L, Jaarsma T, Strömberg A, van der Wal MHL. Seasonal variation in physical activity in patients with heart failure. Heart Lung. 2019;48(5):381–385. [DOI] [PubMed] [Google Scholar]
- 9. Andersson C, Gerds T, Fosbøl E, et al. Incidence of new‐onset and worsening heart failure before and after the COVID‐19 epidemic lockdown in Denmark: a Nationwide Cohort Study. Circ Heart Fail. 2020;13(6):e007274. (In eng). [DOI] [PubMed] [Google Scholar]
- 10. Rosman L, Lampert R, Sears SF, Burg MM. Measuring physical activity with implanted cardiac devices: a systematic review. J Am Heart Assoc. 2018;7(11):e008663. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaszala K, Ellenbogen KA. Device sensing: sensors and algorithms for pacemakers and implantable cardioverter defibrillators. Circulation. 2010;122(13):1328–1340. (In eng). [DOI] [PubMed] [Google Scholar]
- 12. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer DB, Mitchell SL, Monteiro J, et al. Patient activity and survival following implantable cardioverter‐defibrillator implantation: the ALTITUDE activity study. J Am Heart Assoc. 2015;4(5):e001775. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sears SF, Rosman L, Sasaki S, et al. Defibrillator shocks and their impact on objective and subjective patient outcomes: results from the pain free SST clinical trial. Heart Rhythm. 2017. [DOI] [PubMed] [Google Scholar]
- 15. Dusetzina SB, Tyree S, Meyer AM, Meyer A, Green L, Carpenter WR. Linking Data for Health Services Research: A Framework and Instructional Guide. United States: Agency for Healthcare Research and Quality; 2014.</bib> [PubMed] [Google Scholar]
- 16. Briguglio M, Giorgino R, Dell'Osso B, et al. Consequences for the elderly after COVID‐19 isolation: fEaR (Frail Elderly amid Restrictions). Frontiers Psychol. 2020;11(2433). (Opinion) (In English). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phelps M, Christensen DM, Gerds T, et al. Cardiovascular comorbidities as predictors for severe COVID‐19 infection or death. Eur Heart J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infectious Dis. 2020;20:533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi J, Lee M, Lee J‐k, Kang D, Choi J‐Y. Correlates associated with participation in physical activity among adults: a systematic review of reviews and update. BMC Public Health. 2017;17(1):356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sassone B, Mandini S, Grazzi G, Mazzoni G, Myers J, Pasanisi G. Impact of COVID‐19 pandemic on physical activity in patients with implantable cardioverter‐defibrillators. J Cardiopulm Rehabil Prev. 2020;40(5):285–286. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Malanchini G, Malacrida M, Ferrari P, et al. Impact of the coronavirus disease‐19 outbreak on physical activity of patients with implantable cardioverter defibrillators. J. Card. Fail. 2020;26(10):898–899. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu Y, Murugiah K, Jones PW, et al. Physical activity patterns among patients with intracardiac remote monitoring devices before, during, and after COVID‐19‐related public health restrictions. medRxiv. 2021. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzella AJ, Gehi AK, Lampert R, Buck S, Rosman L. Effects of COVID‐19 pandemic on physical activity in children and young adults with implanted devices. Heart Rhythm. 2021. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tasheva P, Vollenweider P, Kraege V, et al. Association between physical qctivity levels in the hospital setting and hospital‐acquired functional decline in elderly patients. JAMA Network Open. 2020;3:e1920185–e1920185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varga TV, Bu F, Dissing AS, et al. Loneliness, worries, anxiety, and precautionary behaviours in response to the COVID‐19 pandemic: a longitudinal analysis of 200,000 Western and Northern Europeans. Lancet Regional Health ‐ Europe. 2021;2:100020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chelu MG, Gunderson BD, Koehler J, Ziegler PD, Sears SF. Patient activity decreases and mortality increases after the onset of persistent atrial fibrillation in patients with implantable cardioverter‐defibrillators. JACC: Clin Electrophysiol. 2016;2(4):518–523. [DOI] [PubMed] [Google Scholar]
- 27. Rosman L, Gehi A, Sears SF. How to stay healthy and manage stress if you have a heart rhythm disorder: a guide for patients and their families during the COVID‐19 outbreak. Circ Arrhythm Electrophysiol. 2020;13(12):e009064–e009064. (In eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Medicine ACoS . Staying physically active during the COVID‐19 pandemic.
- 29. Rosman L, Armbruster T, Kyazimzade S, et al. Effect of a virtual self‐management intervention for atrial fibrillation during the outbreak of COVID‐19. Pacing Clin Electrophysiol. 2021;44(3):451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shoemaker MJ, Cartwright K, Hanson K, Serba D, Dickinson MG, Kowalk A. Concurrent validity of daily activity data from medtronic ICD/CRT devices and the actigraph GT3X triaxial accelerometer: a pilot study. Cardiopulm. Phys. Ther. J. 2017;28(1). [Google Scholar]
- 31. Piercy KL, Troiano RP. Physical activity guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11:e005263. (In eng). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cohort characteristics according to pre‐COVID levels of daily physical activity
Table S2. Changes in activity during the COVID‐19 lockdown compared to the same period in 2019 after excluding ICD patients who experienced an ICD shock during follow‐up


