Abstract
Vaccination generates a neutralizing immune response against SARS‐CoV‐2. The genomic surveillance is showing the emergence of variants with mutations in spike, the main target of neutralizing antibodies. To understand the impact of these variants, we report the neutralization potency against alpha, gamma, and D614G SARS‐CoV‐2 variants in 44 individuals that received two doses of CoronaVac vaccine, an inactivated SARS‐CoV‐2 vaccine. Plasma samples collected at 60 days after the second dose of CoronaVac were analyzed by the reduction of cytopathic effect in Vero E6 cells with the three infectious variants of SARS‐CoV‐2. Plasma showed lower neutralization with alpha (geometric mean titer [GMT] = 18.5) and gamma (GMT = 10.0) variants than with D614G (GMT = 75.1) variant. Efficient neutralization against the alpha and gamma variants was not detected in 31.8% and 59.1% of plasma, respectively. These findings suggest the alpha and gamma variants could escape from neutralization by antibodies elicited by vaccination. Robust genomic and biological surveillance of viral variants could help to develop effective strategies for the control of SARS‐CoV‐2.
Keywords: CoronaVac vaccine, neutralizing antibodies, SARS‐CoV‐2 variants
1. INTRODUCTION
Several vaccines against SARS‐CoV‐2 have been introduced worldwide. 1 The vaccines mainly used in Chile are those developed by Sinovac and Pfizer‐BioNTech; which are based on inactivated virus and lipid nanoparticles that package messenger RNA (mRNA) coding for a stabilized form of spike SARS‐CoV‐2 protein, respectively. 1 To date, 23,656,008 doses of vaccines have been administered in Chile: 475,168 corresponds to people with a single dose, 12,379,781 are people with first dose, and 11,276,227 people with the complete vaccination schedule. 2
The alpha (lineage B.1.1.7) and gamma (lineage P.1) variants of concern, and the lambda (lineage C.37) variant of interest are the main circulating strains in Chile. Few and preliminary information is available on the neutralization capacity of the Pfizer‐BioNTech vaccine against the alpha and gamma variants. 3 , 4 , 5 , 6 , 7 If this vaccine generates neutralizing antibodies against the different SARS‐CoV‐2 variants has not been resolved. 3 , 4 , 8 Also, the efficacy of the CoronaVac vaccine (Sinovac Life Sciences, Beijing, China) to neutralize the infection with SARS‐CoV‐2 variants is unknown. A preliminary study about neutralizing antibodies against the gamma variant in 14 individuals vaccinated with two doses of CoronaVac vaccine was recently reported. 9 All cases were negative for neutralizing antibodies against this variant at 158 days after receipt of the second dose of vaccine. The objective of our study was to determine in CoronaVac‐vaccinated people in Chile, with a complete two‐dose schedule, the levels of neutralizing antibodies against D614G, alpha, and gamma variants.
2. MATERIALS AND METHODS
2.1. Patients and sample collection
Blood samples were obtained from healthy healthcare workers who were immunized with the CoronaVac vaccine, according to the national immunization program established by Ministry of Health. The study protocol was approved by the Scientific‐Ethical Committee of the Servicio Salud Metropolitano Oriente, Santiago, Chile. Written informed consent was obtained from each participant before enrolment. Participants were inoculated with two doses of CoronaVac at 0‐ and 28‐days post the first immunization (p.i.). Plasma samples were obtained for all volunteers before immunization to evaluate past or ongoing SARS‐CoV‐2 infection. Volunteers with previous SARS‐CoV‐2 infection or the presence of neutralizing antibodies at the time of inoculating the first dose of vaccination were excluded.
2.2. Neutralizing antibody detection
To assess the presence of neutralizing SARS‐CoV‐2 antibodies, blood samples from 44 participants obtained at 60 days after the second dose of CoronaVac were analyzed. Neutralization assays were done essentially by the reduction of cytopathic effect (CPE) in Vero E6 cells with infectious D614G (GISAID Accession Number EPI_ISL_3509539), alpha (B.1.1.7, GISAID Accession Number EPI_ISL_1167921), and gamma (P.1, GISAID Accession Number EPI_ISL_1321471) SARS‐CoV‐2 variants. 10 The viruses were previously isolated at the Public Health Institute. All neutralization assays with infectious SARS‐CoV‐2 viruses were conducted in BSL3 laboratory. Briefly, the neutralization assays were done by incubation of serial dilutions (1:10 to 1:1280) of 100 µl heat inactivated plasma samples with 100 µl (100 TCID50) of each variant. The mixtures of samples and virus (200 µl) were added to 96‐well plates with Vero E6 cells after 1 h incubation. Cytopathic effect on cells was analyzed 7 days after infection. A normal human plasma and a plasma from a COVID‐19 convalescent patient were used as a negative and positive control in each test, respectively. The negative control was plasma from a blood donor negative for SARS‐CoV‐2 IgM and IgG by the OnSite™ COVID‐19 IgG/IgM Rapid Test (CTK Biotech, Ref. R0180C). The positive control was a plasma from a recovered COVID‐19 patient, who agreed to donate blood for plasmapheresis.
2.3. Statistical analysis
Statistical significance was determined using the two‐tailed Mann–Whitney U test. p values <0.05 were considered significant. Statistical analyses were done using Prism, version 8.0 (GraphPad Software). For the purpose of statistical analysis, a value of 5 was assigned for neutralizing antibodies titer <10 (ND).
3. RESULTS AND DISCUSSION
We recruited 44 volunteers who received two doses of the CoronaVac vaccine. First doses of the vaccine were inoculated from February 8 to 18, 2021. Second doses were inoculated 30 days after the first dose. Plasma were drawn 2 months after the second dose of vaccination to evaluate the neutralization potency. The volunteers were grouped into three age ranges from 20 to 35 (n = 19; 43.2%), 36 to 50 (n = 14; 31.8%) and ≥51 (n = 11; 25.0%) years old; 13 (29.6%) were men and 31 (70.4%) women. Plasma samples obtained from vaccinated individuals exhibited good neutralization of D614G variant, with a geometric mean titer (GMT) of 75.1 (Figure 1). When assessing the alpha (GMT = 18.5) and gamma (GMT = 10.0) variants, exhibited neutralization that was significantly decreased to the parental D614G variant (GMT = 75.1). The mean fold decrease in neutralization relative to D614G variant was 4.1‐fold for alpha (p < 0.0001), and 7.5‐fold for gamma (p < 0.0001) (Figure 1A). Also, the mean decrease in neutralization antibody titer in relation to the Alpha variant was 1.8 times for Gamma (p = .0163) (Figure 1A). Neutralizing antibodies against alpha and gamma variants were undetectable (below the limit of detection <1/10) in 31.8% (14/44) and 59.1% (26/44) of plasma samples, respectively (Figure 1A). A significant decrease in neutralization antibody titer was detected with the alpha and gamma variants relative to D614G variant among the three age ranges (Figure 1B). Nevertheless, the neutralization ability of the gamma variant (GMT = 24.9) was only significantly reduced in the youngest group compared to the alpha variant (GMT = 12.0) (p = 0.039). Although no significant decrease was detected, the GMT of the neutralization antibody titer of D614G variant suggested a reduced neutralization ability for older people (Figure 1B).
Figure 1.
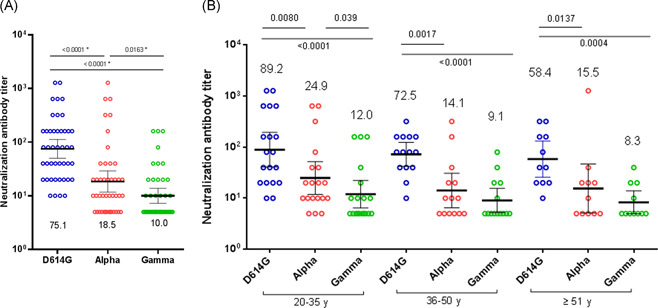
Neutralizing antibody titers of 44 CoronaVac‐vaccinated human sera against infectious SARS‐CoV‐2 variants. Neutralization assays with infectious D614G (lineage B), alpha (lineage B.1.1.7) and gamma (lineage P.1) variants were done. (A) Sera of 44 CoronaVac‐vaccinated volunteers drawn at 2 months after vaccine dose two. GMTs and 95% CIs are indicated: D614G (75.1), alpha (18.5), gamma (10.05) variant. (B) Distribution of neutralizing antibody titers of 44 human sera vaccinated with CoronaVac according to three age groups: 20–35, 36–50, and ≥51 years old. Statistical significance was determined using the two‐tailed Mann‐Whitney U test. Two‐tailed p values are reported. Non detectable results were assigned a value of 5.0. CI, confidence interval; GMT, geometric mean titer
The neutralization ability of each of the sample against the three variants showed four different patterns (Figure 2). Thirty samples showed a decrease of neutralization antibody titer with alpha and gamma variants compared to D614G variant (pattern 1). Neutralizing antibodies against alpha and gamma showed undetectable in 5 and 25 plasma samples, respectively. Furthermore, neutralizing antibodies against both variants were not simultaneously detected in five of these samples. Three samples showed a higher neutralization antibody titer against the Alpha variant than the D614G and Gamma variants (pattern 2). Nine plasma samples showed a lower potency of neutralization against the Alpha variant than D614G and Gamma variants, and with a higher neutralization antibody titer against the D614G variant than the Gamma variant (pattern 3). Finally, two samples showed a higher neutralization ability against the Gamma variant than the D614G and Alpha variants (pattern 4).
Figure 2.
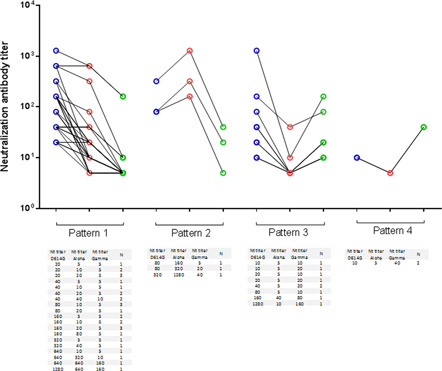
Patterns of neutralizing antibody titers of 44 CoronaVac‐vaccinated human sera against infectious D614G, alpha and gamma SARS‐CoV‐2 variants. Neutralization assays with D614G (lineage B, blue circles), alpha (lineage B.1.1.7, red circles) and gamma (lineage P.1, green circles) variants were done. Non detectable results were assigned a value of 5.0
We report that the potency of the neutralizing response against Alpha and Gamma variants was significantly lower than for D614G variant in CoronaVac‐immunized people at 2 months p.i. A notable result was that in 56.8% (25/44) of the plasma samples no neutralizing antibodies were detected against the gamma variant, which currently represents a high circulation variant in Chile. This is the first study to our knowledge that evaluates the neutralizing antibody response against the alpha, gamma, and D614G variants in vaccinated people with two doses of CoronaVac. The absence of neutralizing antibodies against the gamma variant at 150 days after the second dose was reported in all vaccinated from a very small group of subjects. 9 In our study, the neutralizing antibodies were undetectable in 56.8% of vaccinated subjects at 60 days after the second dose. Our method is more sensitive than other assays for detecting low levels of neutralizing antibodies because it uses a 1/10 initial dilution of the samples. The absence of antibodies at 6 months postvaccination using a 1/20 dilution can be misinterpreted as the absence of neutralizing antibodies and immunological escape of the SARS‐CoV‐2 variants. 9 Consequently, our results show that the neutralization potency against the alpha and gamma variants by CoronaVac is low.
Our findings suggest the potential of the variants to escape from neutralization of humoral immunity. Consequently, they warn about the need to develop broadly protective strategies against the new variants of SARS‐CoV‐2. This study is preliminary and has limitations. First, the results should be confirmed by studies with a greater number of vaccinated individuals; and second, it is necessary to follow up on vaccinated people over time. It is unknown whether the neutralizing response is maintained or varies over time in vaccinates. Increased levels of neutralizing antibodies against the wild type strain of SARS‐CoV‐2 have been reported in vaccinated people with BNT162b2 or mRNA‐1273 vaccines after several weeks of the second dose. 4 However, these vaccines showed a significant decrease of neutralization against the gamma variant. 4 Other studies suggest that this variant can evade polyclonal antibody responses, 11 , 12 and has been found in cases of SARS‐CoV‐2 reinfection. 13
Most of the neutralization studies with mRNA vaccines have been performed with pseudoviruses, which are only able to homologate the ACE2‐dependent entry step of the SARS‐CoV‐2 life cycle. Several studies have shown a correlation between the neutralization titers measured against pseudoviruses and live SARS‐CoV‐2 cultures. 14 , 15 , 16 However, the impact of additional mutations located outside of the spike on immune escape, virulence, infectivity, or pathogenesis has yet to be resolved. Furthermore, it is important to point out that many studies have been conducted under different experimental conditions (e.g., replication‐capable chimeric vesicular stomatitis virus or single entry lentiviral pseudoviruses). Thus, different spike expression plasmids and combinations of mutations have also been used. All these variations with the experimental conditions do not yet make it possible to have homologous systems to measure the neutralizing antibody titers. In the present study, the neutralization potency has been measured by incubating infectious virus variants with vaccinated sera. This procedure would better mimic the real situation that would occur in vivo viral infection.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
Jorge Fernández contributed to the study design, experimental assays, writing, critical review of the content and approved the final version of the manuscript. Nicole Bruneau contributed in the experimental assays, the analysis of results, and approved the final version of the manuscript. Héctor San Martín, and Monserrat Balanda participated in the experimental assays, and approved the final version of the manuscript. Rodrigo Fasce, Soledad Ulloa, Patricia Bustos, and Judith Mora participated in the study design, critical review of the content and approved the final version of the manuscript. Eugenio Ramírez contributed to the study design, experimental assays, writing, critical review of the content and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Lizette Muñoz, María L. Benítez, and the technical staff of the Sección Cultivos Celulares at the Instituto de Salud Pública de Chile for the excellent technical assistance with tissue cultures. We are grateful to Agencia Nacional de Investigación y Desarrollo de Chile (ANID), which supported this study (grant no. COVID0557).
Fernández J, Bruneau N, Fasce R, et al. Neutralization of alpha, gamma, and D614G SARS‐CoV‐2 variants by CoronaVac vaccine‐induced antibodies. J Med Virol. 2021;94:399‐403. 10.1002/jmv.27310
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Craven J. COVID‐19 vaccine tracker. Accessed July 10, 2021. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- 2. Ministerio de Salud . Accessed July 10, 2021. https://www.minsal.cl/74-de-la-poblacion-objetivo-ha-completado-su-esquema-de-vacunacion-contra-sars-cov-2/
- 3. Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine‐elicited antibodies to SARS‐CoV‐2 and circulating variants. Nature. 2021;592(7855):616‐622. 10.1038/s41586-021-03324-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garcia‐Beltran WF, Lam EC, St Denis K, et al. Multiple SARS‐CoV‐2 variants escape neutralization by vaccine‐induced humoral immunity. Cell. 2021;184(9):2372‐2383. 10.1016/j.cell.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuzmina A, Khalaila Y, Voloshin O, et al. SARS‐CoV‐2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post‐vaccination sera. Cell Host Microbe. 2021;29(4):522‐528.e2. 10.1016/j.chom.2021.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muik A, Wallisch AK, Sänger B, et al. Neutralization of SARS‐CoV‐2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine‐elicited human sera. Science. 2021;371(6534):1152‐1153. 10.1126/science.abg6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xie X, Liu Y, Liu J, et al. Neutralization of SARS‐CoV‐2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine‐elicited sera. Nat Med. 2021;27(4):620‐621. 10.1038/s41591-021-01270-4 [DOI] [PubMed] [Google Scholar]
- 8. Hacisuleyman E, Hale C, Saito Y, et al. Vaccine Breakthrough Infections with SARS‐CoV‐2 Variants. N Engl J Med. 2021;384(23):2212‐2218. 10.1056/NEJMoa2105000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Souza WM, Amorim MR, Sesti‐Costa R, et al. Neutralisation of SARS‐CoV‐2 lineage P.1 by antibodies elicited through natural SARS‐CoV‐2 infection or vaccination with an inactivated SARS‐CoV‐2 vaccine: an immunological study. Lancet Microbe. 2021. Published online July 08, 2021. 10.1016/S2666-5247(21)00129-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teresa Valenzuela M, Urquidi C, Rodriguez N, Castillo L, Fernández J, Ramírez E. Development of neutralizing antibody responses against SARS‐CoV‐2 in COVID‐19 patients. J Med Virol. 2021;93(7):4334‐4341. 10.1002/jmv.26939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Greaney AJ, Starr TN, Gilchuk P, et al. Complete mapping of mutations to the SARS‐CoV‐2 spike receptor‐binding domain that escape antibody recognition. Cell Host Microbe. 2021;29(1):44‐57. 10.1016/j.chom.2020.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jangra S, Ye C, Rathnasinghe R, et al. SARS‐CoV‐2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe. 2021;2(7):e283‐e284. 10.1016/S2666-5247(21)00068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Resende PC, Bezerra JF, Teixeira Vasconcelos RH, et al. Severe acute respiratory syndrome coronavirus 2 P.2 lineage associated with reinfection case, Brazil, June‐October 2020. Emerg Infect Dis. 2021;27(7):1789‐1794. 10.3201/eid2707.210401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS‐CoV‐2 infection. Nat Commun. 2020;11(1):2251. 10.1038/s41467-020-16256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ju B, Zhang Q, Ge J, et al. Human neutralizing antibodies elicited by SARS‐CoV‐2 infection. Nature. 2020;584(7819):115‐119. 10.1038/s41586-020-2380-z [DOI] [PubMed] [Google Scholar]
- 16. Riepler L, Rössler A, Falch A, et al. Comparison of four SARS‐CoV‐2 Neutralization Assays. Vaccines. 2020;9(1):13. 10.3390/vaccines9010013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


