Abstract
The pathogenesis of coronavirus disease 2019 (COVID‐19) is still not fully understood. As severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2) has a similar pathogenetic pathway to Mycobacterium tuberculosis, it has been reported that there may be a relationship between Bacille Calmette–Guérin (BCG) vaccination rate and COVID‐19 severity. This study investigated the relationship between tuberculin skin test (TST) induration diameter and the clinical course of COVID‐19. Of 1963 adult patients who underwent TST, 76 patients with SARS‐COV‐2 infection confirmed by RT‐PCR analysis of respiratory tract samples were included in the study. Relationships between COVID‐19 clinical severity and TST positivity, induration size, and other clinical parameters were analyzed. Of the 76 patients, TST results were negative for 53 patients (69.7%) and positive for 23 patients (30.3%). COVID‐19 severity was mild in 47 patients (61.8%), moderate in 22 patients (28.9%), and severe in seven patients (9.3%). All TST‐positive patients had mild disease. Patients with mild disease had a significantly higher TST positivity rate (p < 0.001) and larger induration diameter (p < 0.001). The area under the receiver operating characteristic (ROC) curve of TST induration size for the differentiation of mild with moderate and severe disease was 0.768 (p < 0.001). The maximum Youden J index value was 0.522 at an induration diameter of 6.5 mm, which had a sensitivity of 66.0% and specificity of 86.2%. COVID‐19 patients with positive TST showed a significantly higher rate of mild disease than those with negative TST. TST positivity is favorably associated with the course of COVID‐19.
Keywords: Mycobacterium tuberculosis, pathogenesis, prognosis, SARS‐COV‐2, tuberculin skin test
Highlights
TST induration diameters larger than 6.5 mm were highly specific in predicting a milder clinical presentation of COVID‐19.
Patients with positive tuberculin skin test (TST) had milder COVID‐19 symptoms.
TST induration diameter is favorably associated with the course of COVID‐19.
1. INTRODUCTION
Since December 2019, the world has been fighting coronavirus disease 2019 (COVID‐19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and scientists worldwide have been working intensively to elucidate the pathogenesis. Although descriptive studies show that the public has complied with the numerous measures recommended by authorities for disease prevention, they also reflect an increase in people's perceived stress levels since the start of the pandemic. 1 As the course of COVID‐19 varies from person to person, identifying the factors affecting the clinical course has been a primary concern. Various risk factors have been identified in relation to the complications and severity of COVID‐19, but there is still much that remains unclear about the virus. The dominant opinion remains that the host immune response affects the outcome of the disease. SARS‐CoV‐2 causes mild disease in most infected patients; however, high pro‐inflammatory cytokine levels have been associated with a more severe clinical course and systemic complications in some patients. 2
Nuclear factor‐kappa B (NF‐κB) is a ubiquitous dimeric transcription factor whose activity is regulated by various cytokines and other external stimuli. NF‐κB plays a role in the activation of immune cells by increasing the expression of many cytokines required for immune system activation. 3 NF‐κB activation is known to play a role in the pathogenesis of Mycobacterium tuberculosis infection 4 , 5 , 6 and was also shown to have a key role in the pathogenesis of SARS‐CoV infection. 7 , 8 , 9 Similar pathogenetic properties of these etiological agents gave rise to hypotheses that Bacille Calmette–Guérin (BCG) immunization may protect against COVID‐19 or reduce its severity, and various studies have been conducted to test these hypotheses. 10 , 11 , 12 In a large‐scale study evaluating the correlation between total COVID‐19 deaths and BCG vaccine coverage across 173 countries, it was observed that COVID‐19‐related mortality is lower in countries with high BCG vaccination rates. 13 BCG immunization has been shown to induce histone modifications and epigenetic reprogramming of innate immune cells and lead to a more active natural immune response to restimulation and reduced susceptibility to respiratory tract infections due to trained immunity. 14 , 15
To further investigate the possibility that BCG immunization may be protective against COVID‐19 or reduce COVID‐19‐related morbidity and mortality, this study examined the relationship between tuberculin skin test (TST) induration diameter and the clinical course of COVID‐19 in patients with a history of tuberculosis (TB) infection or BCG vaccination.
2. PATIENTS AND METHODS
2.1. Data collection
The study sample included 1963 adult patients aged 18 years or older who had a TST in the Nigde Tuberculosis Dispensary between January 1, 2017, and November 1, 2020. Of these, 76 patients had SARS‐CoV‐2 infection confirmed by reverse transcription‐polymerase reaction (RT‐PCR) analysis of respiratory tract samples. For these 76 patients, the Nigde Tuberculosis Dispensary patient records were retrospectively screened to obtain their TST induration diameter in millimeters, TST results (positive/negative), number of BCG vaccine scars, and reason for the TST. Details regarding the patients' demographic characteristics, comorbid diseases, body mass index (BMI), serum white blood cell (WBC) count, and lymphocyte count were also retrieved for analysis. COVID‐19 course and severity were recorded by evaluating thoracic computed tomography (CT) results and relevant details from the patients' medical records. Relationships between the clinical course of COVID‐19 and TST positivity, TST induration size, BCG scar count, BMI, and serum WBC and lymphocyte counts were investigated.
2.2. Definitions
TSTs were interpreted as recommended by the Centers for Disease Control and Prevention (CDC). Cases were considered TST positive if the induration was 10 mm or larger in individuals without BCG vaccination and those living or working in high‐risk collective environments, 15 mm or larger in BCG vaccinees, and 5 mm or larger in immunocompromised individuals with radiological findings suggestive of previous TB infection. 16
The clinical classification of COVID‐19 was based on the following World Health Organization (WHO) criteria in patients with PCR‐confirmed SARS‐CoV‐2: 17
-
1.
Asymptomatic: No clinical signs or symptoms
-
2.
Mild: Mild upper and lower respiratory tract symptoms without signs of hypoxia or viral pneumonia
-
3.
Moderate: Signs of pneumonia but with oxygen saturation (SpO2) ≥90 mmHg in room air
-
4.
Severe: Clinical signs of pneumonia with severe respiratory failure or SpO2 <90 mmHg in room air
-
5.
Critical: Acute respiratory distress syndrome (ARDS) accompanied clinical condition
2.3. Statistical analysis
Data were analyzed using IBM SPSS version 22.0 software (IBM Corporation). Data were evaluated using Kolmogorov–Smirnov and Shapiro–Wilk tests together with skewness and kurtosis values examination to check compliance with normal distribution. Categorical variables were expressed as frequencies (n) and percentages (%). Continuous variables that met the assumptions for parametric tests were presented as mean ± SD, and those that did not were presented as median (minimum‐maximum) values. χ 2 and Fisher's exact significance tests were used in the analysis of categorical variables. Differences between pairs of groups were evaluated using Kruskal–Wallis H with post hoc analysis using Dunn's test. Receiver operating characteristic (ROC) analysis was used to determine the value of TST diameter in the prediction of COVID‐19 severity and identify a cut‐off point. The power of the study was calculated as 87% by performing a power analysis with the OpenEpi program.
3. RESULTS
The population in Nigde province is approximately 350,000. Between January 1, 2017, and November 1, 2020, a total of 1963 individuals aged 18 years and over underwent TST testing in the Nigde Tuberculosis Dispensary. Of these, 76 patients with SARS‐CoV‐2 infection confirmed by RT‐PCR test of respiratory tract samples were included in the study. Fifty‐three (69.7%) of these patients had a negative TST result and 23 (30.3%) had a positive result.
Of the 76 patients, 33 (43.4%) were men and 43 (56.6%) were women. The mean age was 44.08 (SD: 13.597). Twenty‐two patients (28.9%) had a history of underlying comorbid disease; two of those patients had two comorbid diseases (hypertension and rheumatoid arthritis in one, rheumatoid arthritis and diabetes mellitus in one), and one patient had four comorbid diseases (hypertension, diabetes mellitus, coronary artery disease, and asthma). BMI was 25 or higher in 33 patients (43.4%). Three patients had no BCG scar. Patients were screened for TST due to risky TB contact (n = 36, 47.4%), health worker screening (n = 20, 26.3%), or pre‐immunosuppressive screening (n = 20, 26.3%). Sixteen (21.1%) of the patients with positive TST had a history of TB, but none of the patients had a latent or active TB infection. The demographic, clinical, and laboratory characteristics of the patients are shown in Table 1.
Table 1.
Demographic, clinic, and laboratory features of the patients (n = 76)
| Variables | Values |
|---|---|
| Sex, n (%) | |
| Female | 43 (56.6) |
| Male | 33 (43.4) |
| Age (years), mean ± SD | 44.08 ± 13.597 |
| Severity of disease, n (%) | |
| Mild | 47 (61.8) |
| Moderate | 22 (28.9) |
| Severe | 7 (9.3) |
| White blood cell count (103/μl), median (min‐max) | 5800.00 (1800‐18 100) |
| Serum lymphocyte count (103/μl), mean ± SD | 1688.73 ± 658.708 |
| TST induration diameter (mm), median (min‐max) | 6.00 (0–33) |
| BCG scar number, median (min‐max) | 2 (0–3) |
| Body mass index, n (%) | |
| <25 | 43 (56.6) |
| ≥25 | 33 (43.4) |
| Comorbid disease, n (%) | 24 (31.6) |
| Hypertension | 9 (11.8) |
| Asthma | 7 (9.3) |
| Diabetes mellitus | 3 (3.9) |
| Rheumatoid arthritis | 4 (5.3) |
| Others | 7 (9.2) |
Abbreviations: BCG, Bacillus Calmette–Guérin; SD, standard deviation, TST, Tuberculin skin test.
Of the 76 patients diagnosed with COVID‐19, 47 (61.8%) were classified as mild, 22 (28.9%) as moderate, and seven (9.2%) as severe cases. All patients with positive TST (n = 23) were in the mild group, moderate and severe patients were all TST‐negative patients. Patients classified as having mild COVID‐19 had significantly higher TST positivity rate (p < 0.001) and TST induration diameter (p < 0.001) than those with moderate and severe disease. The median values for TST induration diameter in mild patients were 9 (0–33) mm, 4.5 (0–11) in moderate patients, and 0 (0–5) in patients with severe disease. There is also a statistically significant difference in the ages of severe patients. Median ages were 39 (19–65) for patients with mild disease, 45 (23–70) for patients with moderate disease, and 66 (45–80) for patients with severe disease. No statistically significant differences were found when groups were compared according to BMI and WBC count. Although there are no statistically significant differences in the median number of BCG scars; there are no patients without BCG scars in mild patients but one of the patients in the moderate disease group (4.5%) and two of the patients in the severe disease group (28.6%) were not vaccinated against TB before and it was observed as being a statistically significant difference (p = 0.004). There are statistically significant differences among the groups in serum lymphocyte counts and presence of comorbidity. Serum lymphocyte counts lower than 800 (103/μl) were only present in patients with severe disease and 73.0% of mild patients (n = 27) had serum lymphocyte values higher than 1600 (103/μl). Only 19.1% of patients with mild disease (n = 9) and 31.8% of patients with moderate disease (n = 7) had a comorbid disease. The ratio for the presence of comorbidity was 85.7% in severe patients (n = 6). These results are shown in Table 2.
Table 2.
Relationship between disease severity with physical and laboratory findings of patients (n = 76)
| Severity of disease | |||||
|---|---|---|---|---|---|
| Variables | Mild (n = 47) | Moderate (n = 22) | Severe (n = 7) | Test statistics | p values |
| Age, median (min‐max) | 39 (19–65) | 45 (23–70) | 66 (45–80) a | 17.842 b | <0.001 |
| Post hoc c : Mi vs. Mo (p = 0.154), Mi vs. S ( p < 0.001), Mo vs. S ( p = 0.025) | |||||
| TST result, n (%) | |||||
| Positive | 23 (48.9) a | 0 (0) | 0 (0) | 22.623 d | <0.001 |
| Negative | 24 (51.1) | 22 (100) | 7 (100) | ||
| TST induration diameter (mm), median (min‐max) | 9 (0–33) a | 4.5 (0–11) | 0 (0–5) | 16.895 b | <0.001 |
| Post hoc c : Mi vs. Mo ( p = 0.006), Mi vs. S ( p = 0.003), Mo vs. S (p = 0.695) | |||||
| TST induration diameter, n (%) | |||||
| 0–4 mm | 14 (29.8) | 11 (50) | 6 (85.7) | ||
| 5–9 mm | 10 (21.3) | 10 (45.5) | 1 (14.3) | ||
| 10–14 mm | 12 (25.5) | 1 (4.5) | 0 (0) | 17.287 d | 0.025 |
| 15–19 mm | 6 (12.8) | 0 (0) | 0 (0) | ||
| 20–24 mm | 2 (4.3) | 0 (0) | 0 (0) | ||
| ≥25 mm | 3 (6.4) | 0 (0) | 0 (0) | ||
| Number of BCG scars, median (min‐max) | 2 (1–3) | 1 (0–3) | 1 (0–2) | 5.748 b | 0.056 |
| Number of BCG scars, n (%) | |||||
| No scar | 0 (0) | 1 (4.5) | 2 (28.6) | ||
| 1 scar | 15 (31.9) | 11 (50.0) | 3 (42.9) | 16.209 d | 0.004 |
| 2 scars | 25 (53.2) | 4 (18.2) | 2 (28.6) | ||
| 3 scars | 7 (14.9) | 6 (27.3) | 0 (0) | ||
| Body mass index, n (%) | 0.827 d | 0.689 | |||
| <25 | 28 (59.6) | 12 (54.5) | 3 (42.9) | ||
| ≥25 | 19 (40.4) | 10 (45.5) | 4 (57.1) | ||
| Body mass index, n (%) | |||||
| 16–20 | 3 (6.4) | 0 (0) | 1 (14.3) | 4.951 d | 0.524 |
| 21–25 | 25 (53.2) | 12 (54.5) | 2 (28.6) | ||
| 26–30 | 18 (38.3) | 9 (40.9) | 4 (57.1) | ||
| 31–35 | 1 (2.1) | 1 (4.5) | 0 (0) | ||
| White blood cell count (103/μl), median (min‐max) | 6300 (3200–10 400) | 5600 (3500–9800) | 4900 (1800–18 100) | 2.423 b | 0.298 |
| White blood cell count, n (%) | |||||
| <4000 | 3 (8.1) | 3 (13.6) | 1 (14.3) | ||
| 4000–11 000 | 34 (91.9) | 19 (86.4) | 5 (71.4) | 5.888 d | 0.146 |
| 11 001–15 000 | 0 (0) | 0 (0) | 0 (0) | ||
| 15 001–20 000 | 0 (0) | 0 (0) | 1 (14.3) | ||
| Serum lymphocyte count (103/μl), median (min‐max) | 1860 (990–3100) | 1490 (810–3030) | 830 (400–1680)a | 16.155 b | <0.001 |
| Post hoc c : Mi vs. Mo (p = 0.107), Mi vs. S (p < 0.001), Mo vs. S (p = 0.058) | |||||
| Serum lymphocyte count, n (%) | 8.173 d | 0.010 | |||
| <800 | 0 (0) | 0 (0) | 2 (28.6) | ||
| 800 and above | 37 (100) | 22 (100) | 5 (71.4) | ||
| Serum lymphocyte count, n (%) | |||||
| 201–400 | 0 (0) | 0 (0) | 1 (14.3) | ||
| 401–800 | 0 (0) | 0 (0) | 1 (14.3) | ||
| 801–1000 | 1 (2.7) | 4 (18.2) | 3 (42.9) | 26.126 d | 0.001 |
| 1001–1200 | 5 (13.5) | 5 (22.7) | 1 (14.3) | ||
| 1201–1400 | 1 (2.7) | 1 (4.5) | 0 (0) | ||
| 1401–1600 | 3 (8.1) | 4 (18.2) | 0 (0) | ||
| >1600 | 27 (73.0) | 8 (36.4) | 1 (14.3) | ||
| Presence of comorbidity, n (%) | |||||
| Present | 9 (19.1) | 7 (31.8) | 6 (85.7) | 11.740 d | 0.002 |
| None | 38 (80.9) | 15 (68.2) | 1 (14.3) | ||
Abbreviations: BCG, Bacillus Calmette–Guérin; Mi, mild; Mo, moderate; S, severe; SD, standard deviation; TST, tuberculin skin test.
The group from which the difference originates.
Kruskal–Wallis H.
Dunn.
Fisher's exact test.
Figure 1 shows the ROC curve obtained in the analysis examining the relationship between TST diameter and disease severity (mild/moderate) in patients diagnosed with COVID‐19. The area under the ROC curve was calculated as 0.768 (p < 0.001); values between 0.7 and 0.8 are regarded as an indicator of good diagnostic accuracy. 18
Figure 1.
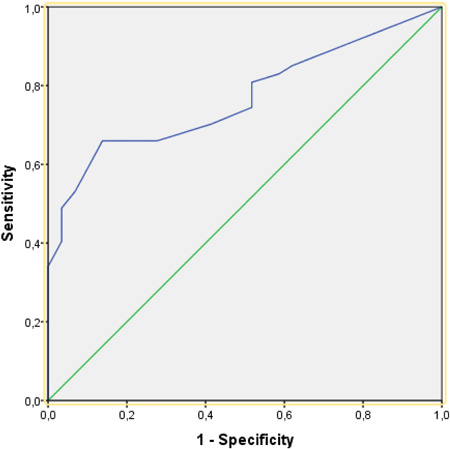
Receiver operating characteristic (ROC) curve for the relationship between TST induration size and COVID‐19 disease severity
The Youden J index was calculated to determine the TST induration diameter cut‐off point that most accurately predicts whether a patient will have mild or moderate COVID‐19 according to the WHO clinical classification. 17 , 19 The highest index value was 0.522 at a TST diameter of 6.5 mm, which had a sensitivity of 0.660 and specificity of 0.862 (Table 3).
Table 3.
Sensitivity, specificity, and Youden J index values according to TST induration size
| TST induration diameter (mm) | Sensitivity | Specificity | Youden's J index |
|---|---|---|---|
| 4.50 | 0.702 | 0.586 | 0.288 |
| 5.50 | 0.660 | 0.724 | 0.384 |
| 6.50 | 0.660 | 0.862 | 0.522 |
| 7.50 | 0.596 | 0.897 | 0.492 |
| 8.50 | 0.532 | 0.931 | 0.463 |
| 9.50 | 0.489 | 0.966 | 0.455 |
| 10.50 | 0.404 | 0.966 | 0.370 |
| 11.50 | 0.340 | 1.000 | 0.340 |
| 12.50 | 0.298 | 1.000 | 0.298 |
Abbreviation: TST, Tuberculin skin test.
4. DISCUSSION
Studies have shown that many factors influence the course of COVID‐19, such as age, sex, comorbid diseases, obesity, and genetic predisposition. 20 , 21 , 22 , 23 , 24 , 25 In addition, there is evidence that COVID‐19 incidence and COVID‐19‐related mortality rates are lower in countries with a high incidence of TB and a high BCG vaccination rate. 26 , 27 This association between TB and COVID‐19 could be attributed to cross‐immunity between Mycobacterium species and COVID‐19, previous active or latent TB infection, or BCG vaccination. TST positivity is detected as a skin induration that appears 24–72 h after intradermal administration of M. tuberculosis TST due to a CD4+ T cell‐mediated Type IV delayed hypersensitivity reaction in most people infected with M. tuberculosis. 28 The TST, which is frequently used in studies of latent tuberculosis infection (LTBI), demonstrates previous exposure to TB bacilli. In addition to BCG vaccination, LTBI or active TB is thought to affect the course of COVID‐19. Singh et al. 29 compared the frequency of LTBI and the number of COVID‐19 cases in 20 European countries and found a significant negative correlation between them. In the same study, LTBI frequency and mortality rate were also compared, but the correlation was not found to be significant, possibly because it was still early in the pandemic. Madan et al. reviewed data from 174 countries and showed that COVID‐19 incidence and fatality rates were lower in countries with a high TB incidence when compared with countries with low TB incidence and especially in low‐TB countries with higher BCG vaccine coverage when compared with low‐TB countries with lower BCG vaccine coverage. 26
There were no BCG scars in three patients, two of whom were in the severe and one in the moderate patient group. In correlation with the results of many studies on BCG vaccination in the literature, at least one BCG scar was detected in all patients in the mild disease group. These results suggest that in BCG vaccinees, the course of COVID‐19 may be milder due to the immune response to SARS‐CoV‐2, which has a pathogenetic effect on the same pathway of CD4+ cell‐mediated trained immunity activation. In a study investigating the effect of TB contact and BCG vaccination on the course of COVID‐19 in healthcare workers, it was found that the rate of BCG vaccination was higher in those with a history of COVID‐19‐related hospitalization, in contrast to many studies in the literature and our results. In the same study, higher mean TST results were found in outpatients compared to inpatients, although there was no significant difference. 30 We observed that patients with positive TST results were significantly more likely to have mild COVID‐19 disease compared to those with negative TST, too.
The results of ROC analysis to examine the relationship between TST diameter and COVID‐19 disease severity according to WHO clinical classification indicated good diagnostic accuracy, with an area under the curve of 0.768 (Figure 1). Additional analysis of the predictive value of TST induration size using the Youden J index showed that the optimal cut‐off value was 6.5 mm, which had sensitivity and specificity values of 66.0% and 86.2%, respectively. The next highest Youden J index value was 0.492 at a TST diameter of 7.5 mm, which had 59.6% sensitivity and 89.7% specificity. The third highest Youden J index value was 0.463 for a TST diameter of 8.5 mm, with a sensitivity of 53.2% and specificity of 93.1%. As with the other values in Table 3, the purified protein derivative (PPD) values of 6.5, 7.5, and 8.5 mm represent the average of two consecutive PPD test results.
Since the concepts of sensitivity and specificity entered the literature in 1947, they have been used in the field of medicine to statistically assess the performance of binary classification (yes/no) tests for the diagnosis of diseases. 31 Sensitivity refers to the ratio of positives correctly identified by a diagnostic test, and specificity refers to the ratio of negatives correctly identified by the test. A negative result in a test with high specificity helps rule out the disease, while a positive result in a test with high sensitivity is useful in determining that the disease exists in the patient being tested. 32 Accordingly the high specificity values observed for TST induration sizes greater than 9.5 mm were also consistent with the finding discussed above, that patients with TST induration size of 10 mm and larger had significantly milder disease than patients with indurations smaller than 10 mm.
As a result of the comparison of the lymphocyte counts of the patients in terms of the severity of COVID‐19, it was revealed that there was a statistically significant difference between the mild and severe patient groups. These results support the literature data.
5. LIMITATIONS OF STUDY
The present study has some limitations. As the data were collected retrospectively and the study sample was small, we could not use methods of controlling for confounding factors. In our study, patients in the COVID‐19 patient groups show a different distribution in terms of age and comorbidity. Although there are some studies in the literature on BCG vaccination and COVID‐19 prognosis, there are no studies investigating the relationship between TST positivity and the clinical course of COVID‐19. This adds value to our study, but prospective, randomized controlled studies are needed to examine this further.
6. CONCLUSIONS
This study provides a valuable contribution to the literature on the relationship between COVID 19 and TB, which is a popular research topic. Studies that reveal the relationship between SARS‐COV‐2 and TB, which have similar pathogenetic features, are based on BCG vaccination in the literature. Looking from a different perspective, we investigated the effect of TST positivity on the COVID 19 prognosis. To summarize our findings, COVID‐19 patients with positive TST showed a significantly higher rate of mild disease than those with negative TST. It is possible to say that TST positivity is favorably associated with the course of COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
The study protocol was accepted by the Ethical Committee of Nigde Omer Halisdemir University Noninterventional Clinical Research Ethics Committee. Protocol serial number: 2020/83.
Arslan Gulen T, Bayraktar M, Yaksi N, Kayabas U. Is the course of COVID‐19 associated with tuberculin skin test diameter? A retrospective study. J Med Virol. 2022;94:1020‐1026. 10.1002/jmv.27414
REFERENCES
- 1. Ersin F, Kartal M. The determination of the perceived stress levels and health‐protective behaviors of nursing students during the COVID‐19 pandemic. Perspect Psychiatr Care. 2020:1‐7. 10.1111/ppc.12636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conti P, Ronconi G, Caraffa A, et al. Induction of pro‐inflammatory cytokines (IL‐1 and IL‐6) and lung inflammation by Coronavirus‐19 (COVI‐19 or SARS‐CoV‐2): anti‐inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327‐331. [DOI] [PubMed] [Google Scholar]
- 3. Yamada H, Mizuno S, Reza‐Gholizadeh M, Sugawara I. Relative importance of NF‐kappaB p50 in mycobacterial infection. Infect Immun. 2001;69:7100‐7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fallahi‐Sichani M, Kirschner DE, Linderman JJ. NF‐κB signaling dynamics play a key role in infection control in tuberculosis. Front Physiol. 2012;3:170. 10.3389/fphys.2012.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bai X, Feldman NE, Chmura K, et al. Inhibition of nuclear factor‐kappa b activation decreases survival of Mycobacterium tuberculosis in human macrophages. PLoS One. 2013;8:e61925. 10.1371/journal.pone.0061925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Méndez‐Samperio P. Expression and regulation of chemokines in mycobacterial infection. J Infect. 2008;57:374‐384. [DOI] [PubMed] [Google Scholar]
- 7. Liao QJ, Ye LB, Timani KA, et al. Activation of NF‐kappaB by the full‐length nucleocapsid protein of the SARS coronavirus. Acta Biochim Biophys Sin (Shanghai). 2005;37:607‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The role and therapeutic potential of NF‐Kappa‐B pathway in severe COVID‐19 patients. Inflammopharmacology. 2020;29:1‐10. 10.1007/s10787-020-00773-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fang X, Gao J, Zheng H, et al. The membrane protein of SARS‐CoV suppresses NF‐kappaB activation. J Med Virol. 2007;79:1431‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID‐19. Lancet. 2020;395:1545‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escobar LE, Molina‐Cruz A, Barillas‐Mury C. BCG vaccine protection from severe coronavirus disease 2019 (COVID‐19). Proc Natl Acad Sci USA. 2020;117:17720‐17726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miller A, Reandelar MJ, Fasciglione K, Roumenova V, Li Y, Otazu GH. Correlation between universal BCG vaccination policy and reduced mortality for COVID‐19. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID‐19: an epidemiological study. medRxiv. 2020. 10.1101/2020.03.24.20042937 [DOI] [Google Scholar]
- 13. Urashima M, Otani K, Hasegawa Y, Akutsu T. BCG vaccination and mortality of COVID‐19 across 173 countries: an ecological study. Int J Environ Res Public Health. 2020;17(15):5589. 10.3390/ijerph17155589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arts RJW, Moorlag S, Novakovic B, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89‐100. e5. 10.1016/j.chom.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 15. Kerboua KE. The perplexing question of trained immunity versus adaptive memory in COVID‐19. J Med Virol. 2020;92:1858‐1863. [DOI] [PubMed] [Google Scholar]
- 16. CDC . Tuberculin Skin Testing. Document number CS 320275‐A. 2020. https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed September 15, 2021.
- 17. World Health Organization . Clinical management of COVID‐19 interim guidance; 27 May 2020. https://apps.who.int/iris/handle/10665/332196. Accessed September 15, 2021.
- 18. Šimundić AM. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203‐211. [PMC free article] [PubMed] [Google Scholar]
- 19. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32‐35. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID‐19. Nature. 2020;583:437‐440. [DOI] [PubMed] [Google Scholar]
- 21. Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID‐19 and individual genetic susceptibility/receptivity: Role of ACE1/ACE2 genes, immunity, inflammation and coagulation. Might the double X‐chromosome in females be protective against SARS‐CoV‐2 compared to the single X‐chromosome in males? Int J Mol Sci. 2020;21:3474. 10.3390/ijms21103474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chanana N, Palmo T, Sharma K, Kumar R, Graham BB, Pasha Q. Sex‐derived attributes contributing to SARS‐CoV‐2 mortality. Am J Physiol Endocrinol Metab. 2020;31:E562‐E567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hou W, Zhang W, Jin R, Liang L, Xu B, Hu Z. Risk factors for disease progression in hospitalized patients with COVID‐19: a retrospective cohort study. Infect Dis (Lond). 2020;52:498‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madan M, Pahuja S, Mohan A, et al. TB infection and BCG vaccination: are we protected from COVID‐19? Public Health. 2020;185:91‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wickramasinghe D, Wickramasinghe N, Kamburugamuwa SA, Arambepola C, Samarasekera DN. Correlation between immunity from BCG and the morbidity and mortality of COVID‐19. Trop Dis Travel Med Vaccines. 2020;6:17. 10.1186/s40794-020-00117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang H, Kruh‐Garcia NA, Dobos KM. Purifiedprotein derivatives of tuberculin–past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273‐280. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3491170/pdf/nihms391872.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh S, Maurya RP, Singh RK. “Trained immunity” from Mycobacterium spp. exposure or BCG vaccination and COVID‐19 outcomes. PLoS Pathog. 2020;16:e1008969(3):273–280. 10.1371/journal.ppat.1008969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torun S, Ozkaya S, Şen N, et al. The Relationship between COVID‐19 Severity and Bacillus Calmette‐Guérin (BCG) Mycobacterium tuberculosis exposure history in healthcare workers: a multi‐center study. Pathog Glob Health. 2021:May 20:1‐7. 10.1080/20477724.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yerushalmy J. Statistical problems in assessing methods of medical diagnosis, with special reference to x‐ray techniques. Public Health Rep. 1947;62:1432‐1449. [PubMed] [Google Scholar]
- 32. Altman DG, Bland JM. Statistics notes: diagnostic tests 1: sensitivity and specificity. BMJ. 1994;308:1552. 10.1136/bmj.308.6943.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]


