Highlights
-
•
Meta-ICA and dual regression demonstrated significantly reduced connectivity within the default mode network (DMN) in early-onset psychosis, compared to healthy controls.
-
•
The observed effect was strongest in the diagnostic subgroup affective psychosis (AFP).
-
•
There was no significant effect of psychotropic medication on DMN connectivity.
Keywords: Early-onset psychosis (EOP), Schizophrenia, Affective psychosis, Default mode network (DMN), Resting state fMRI (rsfMRI), Independent component analysis (ICA)
Abstract
Abnormal default mode network (DMN) connectivity has been found in schizophrenia and other psychotic disorders. However, there are limited studies on early onset psychosis (EOP), and their results show lack of agreement. Here, we investigated within-network DMN connectivity in EOP compared to healthy controls (HC), and its relationship to clinical characteristics.
A sample of 68 adolescent patients with EOP (mean age 16.53 ± 1.12 [SD] years, females 66%) and 95 HC (mean age 16.24 ± 1.50 [SD], females 60%) from two Scandinavian cohorts underwent resting state functional magnetic resonance imaging (rsfMRI). A group independent component analysis (ICA) was performed to identify the DMN across all participants. Dual regression was used to estimate spatial maps reflecting each participant’s DMN network, which were compared between EOP and HC using voxel-wise general linear models and permutation-based analyses. Subgroup analyses were performed within the patient group, to explore associations between diagnostic subcategories and current use of psychotropic medication in relation to connectivity strength.
The analysis revealed significantly reduced DMN connectivity in EOP compared to HC in the posterior cingulate cortex, precuneus, fusiform cortex, putamen, pallidum, amygdala, and insula. The subgroup analysis in the EOP group showed strongest deviations for affective psychosis, followed by other psychotic disorders and schizophrenia. There was no association between DMN connectivity strength and the current use of psychotropic medication.
In conclusion, the findings demonstrate weaker DMN connectivity in adolescent patients with EOP compared to healthy peers, and differential effects across diagnostic subcategories, which may inform our understanding of underlying disease mechanisms in EOP.
1. Introduction
Psychotic disorders in children and adolescents below the age of 18 years, are defined as early-onset psychosis (EOP) disorders (World Health Organization 1992). Relative to adult-onset psychotic disorders, EOP disorders are associated with greater negative symptom severity and increased risk of poor outcomes (Ballageer et al., 2005, Joa et al., 2009, Díaz-Caneja et al., 2015). Only 11–18% of patients with schizophrenia and other psychoses experience their first psychotic episode before the age of 18 years (Amminger et al., 2011, Fraguas et al., 2017). EOP affects youth during a transformative period of the human lifespan. The adolescent brain is characterized by protracted biological and neurodevelopmental processes, including synaptic pruning and myelination (Patel et al., 2020), supporting the acquirement of cognitive and social skills and coping strategies required for social adjustment and independence in early adulthood (Patel et al., 2020). Most brain imaging studies in patients with severe mental illness have been performed in adults, where it is often difficult to disentangle primary disease-related effects from the correlates and consequences of long-term illness, including institutionalization, alcohol or substance abuse or dependence and long-term use of psychotropic medication. These factors are likely to affect brain function and contribute to brain heterogeneity observed in severe mental illness (Alnæs et al., 2019, Wolfers et al., 2018). The current EOP sample offers an opportunity to study neurodevelopmental mechanisms which are hypothesized to play a pivotal role in the pathophysiology of psychosis (Patel et al., 2020).
Dysconnectivity in large-scale resting state networks has been implicated in the development and clinical expression of psychotic disorders (Satterthwaite and Baker, 2015, Dong et al., 2018, Pelletier-Baldelli et al., 2018). Only a limited number of studies have measured functional connectivity in EOP (Nair et al., 2020, Ioakeimidis et al., 2020). Among the studied resting state networks, the default mode network (DMN) is of particular interest, as it has been implicated in a wide range of mental disorders, including psychosis (Hu et al., 2017). The DMN is a set of functionally connected brain regions, comprising the medial prefrontal cortex (MPFC), lateral posterior cortices, and the posterior cingulate cortex (PCC)/precuneus. It shows increased spontaneous activation at rest and decreased activity during goal-directed behavior (Raichle et al., 2001), and has been linked to emotion processing, self-referential thought, and recall of prior experiences (Menon, 2011, Raichle, 2015). Group-level comparisons in DMN resting state functional connectivity (RSFC) between adult patients with schizophrenia/other psychotic disorders and healthy controls (HC) have revealed mixed effects, with reports of both DMN hyper-connectivity (Whitfield-Gabrieli et al., 2009, Zhou et al., 2007) and hypoconnectivity (Mannell et al., 2010, Skudlarski et al., 2010, Pankow et al., 2015) in patients. In youth with clinical high risk for psychosis, decreased connectivity within the DMN and between DMN and other networks has been found, compared to HC (Satterthwaite et al., 2015, Hua et al., 2019). Among EOP patients, early-onset schizophrenia (EOS) has been most studied. Several EOS studies have reported abnormal DMN RSFC, identifying patterns of both decreased and increased connectivity, see e.g. (Wang et al., 2018, Peng et al., 2020, Tang et al., 2013, Wang et al., 2017, Zhang et al., 2020). A recent study reported altered RSFC between key regions of the DMN using a seed-based approach, with lower whole brain connectivity from a seed in the MPFC, and both lower and higher whole brain connectivity from a PCC seed in EOS, compared to HC (Peng et al., 2020). Another recent study used an automated network homogeneity approach and reported significantly higher within-DMN connectivity in the left MPFC, and significantly lower connectivity in the bilateral PCC and precuneus in EOS compared to HC (Zhang et al., 2020). In adolescents with bipolar disorder (BD), a recent study showed aberrant within-network DMN connectivity in several brain regions, including anterior cingulate cortex, medial prefrontal cortex, bilateral caudate nucleus, bilateral angular gyri, and left middle temporal gyrus, in BD with psychotic symptoms, but not in non-psychotic BD or HC, suggesting a more severe impact on brain function associated with psychosis (Zhong et al., 2019). A recent meta-analysis on first episode psychosis including adolescents and young adults, reported robust hypoconnectivity within the DMN and between DMN and other networks, compared to HC (O’Neill et al., 2019). Interestingly, a review summarizing 14 DMN studies in EOP, found that six studies reported DMN hypoconnectivity and five studies reported both hyper- and hypoconnectivity in EOP (Nair et al., 2020). In sum, while abnormal DMN in youth with psychotic disorders has been observed across several studies, both the specific anatomical regions involved and the direction of the effects have been inconsistent. This can be due to a combination of factors such as small sample sizes, variability in processing and analytical pipelines, and demographic and clinical heterogeneity including age range (Hulvershorn et al., 2014, Jalbrzikowski et al., 2019), disease severity and medication status (Lottman et al., 2017, Wang et al., 2019).
In order to address some of these inconsistencies, we combined resting-state fMRI data from patients with EOP and HC from two independent cohorts. Based on the literature reviewed above, we hypothesized that EOP patients would demonstrate DMN abnormalities compared to HC from the same age range. Due to inconsistent findings in previous studies, we did not hypothesize on the direction of the potential group difference. We used independent component analysis (ICA), a hypothesis-free approach, to estimate resting-state networks, and dual regression to derive individual level DMN spatial maps to compare within-network connectivity in the DMN between EOP and HC. We performed subgroup analyses within the patient group to explore DMN differences between patients with EOS, affective psychosis (AFP) and other psychoses (OTP), as well as for association between DMN connectivity strength and psychotropic medication use.
2. Methods
2.1. Participants
This study includes data from two cohorts; the Thematically Organized Psychosis study for Youth (YTOP), part of the Norwegian Centre for Mental Disorders Research (NORMENT), University of Oslo, Norway, and the Stockholm Child and Adolescence Psychosis Study (SCAPS), Karolinska Institutet, Stockholm, Sweden. The YTOP sample consisted of 36 EOP patients (20 EOS, 14 OTP, 2 AFP) and 43 HC. Patients were recruited from the adolescent psychiatric inpatient units and outpatient clinics in the Oslo region, and assessed by trained psychiatrists or clinical psychologists. The SCAPS sample consisted of 32 EOP patients (15 OTP and 17 AFP) and 22 HC. Patients were recruited from the psychosis and bipolar disorder unit, Child and Adolescent Psychiatry Clinic, Stockholm, Sweden, and assessed by child- and adolescent psychiatry specialists working in the clinic. Inclusion criteria for the patients in both samples were: (1) early onset psychosis, EOS (schizophrenia, schizophreniform disorder, schizoaffective disorder), AFP (bipolar I disorder and major depressive disorder with psychotic features), and OTP (psychotic disorder not otherwise specified and brief psychotic disorder), (2) age between 12 and 18 years, (3) language abilities to complete interviews and self-rating tests, and (4) written informed consent. General exclusion criteria were IQ < 70 (IQ only assessed in YTOP), previous moderate/severe head injury, a diagnosis of substance-induced psychotic disorder, and organic brain disease. HC were invited through the Norwegian population registry among individuals residing in the Oslo area, ensuring that the controls were similar to the patient group with regards to age and sex distribution. HC living in the Stockholm area were invited through the Swedish population registry and ensured for similar demographic, age and sex distributions for the controls. In both samples, HC were excluded if they had been in contact with child and adolescent mental health care units, or if they currently met the criteria of a psychiatric Axis I disorder, according to the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th edition).
2.2. Diagnostic and clinical assessment
The Norwegian patients were recruited between 2013 and 2019, and diagnosed in accordance with the DSM-IV criteria using the Norwegian version of the Schedule for Affective Disorders and Schizophrenia for School Aged Children (6–18 years): Present and Lifetime Version (Kiddie-SADS) (Kaufman et al., 1997). The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) was used to assess the presence and severity of psychotic symptoms, see Table 1. The Swedish patients were recruited between 2013 and 2019. Research diagnoses, according to the DSM-IV, were established in agreement by two clinical experts (EGJ; DA) based on the patients’ medical records.
Table 1.
Means and standard deviations or percentage within each sample. Statistical tests are based on univariate analysis of variance. EOS; Early-onset schizophrenia spectrum disorder, OTP; Early-onset other psychoses, AFP; Early-onset affective psychosis, PANSS; Positive and Negative Syndrome Scale, CPZ; Chlorpromazine equivalents (mean calculated only from patients using antipsychotic drugs), FGA; First generation antipsychotics, SGA; Second generation antipsychotics. Polypharmacy; current use of > 1 of the medication classes. *IQ and PANSS were only assessed in the Norwegian sample (mean/SD based on 36 patients and 73 HC). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (WASI. Wechsler Abbreviated Scale of Intelligence. Stockholm, Sweden: Harcourt Assessment, Inc.; 2007.)
| EOP (68) | HC (95) | F | Sig | |
|---|---|---|---|---|
| Age, years (SD) | 16.53 (1.12) | 16.24 (1.50) | 1.796 | 0.182 |
| Sex, females (%) | 45 (66) | 57 (60) | 0.65 | 0.42 |
| Parental education, years (SD) | 14.38 (2.94) | 17.30 (16.82) | 1.002 | 0.319 |
| IQ (SD)* | 99.63 (13.02) | 104.07 (12.70) | 2.660 | 0.106 |
| Age of onset, years (SD) | 15.01 (1.82) | – | – | – |
| Duration of illness, years (SD) | 1.51 (1.58) | – | – | – |
| Diagnoses | ||||
| EOS (%) | 20 (29) | – | – | – |
| OTP (%) | 29 (42) | – | – | – |
| AFP (%) | 19 (27) | – | – | – |
| Symptoms | ||||
| PANSS positive* (SD) | 16.67 (4.64) | – | – | – |
| PANSS negative* (SD) | 19.14 (6.99) | – | – | – |
| PANSS total* (SD) | 74.09 (16.35) | – | – | – |
| Current medication status | ||||
| FGA (%) | 5 (7) | – | – | – |
| SGA (%) | 42 (61) | – | – | – |
| CPZ (SD) | 163.22 (210.86) | – | – | – |
| Lithium (%) | 15 (22) | – | – | – |
| Antiepileptics (%) | 10 (14) | – | – | – |
| Antidepressants (%) | 13 (19) | – | – | – |
| Polypharmacy (%) | 25 (36) | – | – | – |
| No medication (%) | 15 (22) | – | – | – |
2.3. Medication
Information regarding use of psychotropic medication in the Swedish sample was retrieved from the patients’ medical records. For the Norwegian sample, current use of medication was collected from medical records or patients and/or their legal guardians using structured interviews. Medication classes of interest were first- and second-generation antipsychotics (FGA/SGA), lithium, antidepressants and antiepileptic medication. For antipsychotics, chlorpromazine equivalents (CPZ) was calculated using previously published formulas (Woods, 2003).
2.4. MRI acquisition
All participants underwent protocols for rsfMRI and T1-weighted structural MRI at 3 T General Electric scanners, either in Oslo (Oslo University Hospital) or Stockholm (MR-Centre, Karolinska University Hospital, Solna). In the YTOP study, there was a scanner upgrade during the data collection period. Before the scanner upgrade (YTOP1), a Signa HDxt 3 T MRI scanner was used with an 8-channel head coil and the following parameters: a T2*-weighted 2D gradient echo planar imaging (EPI) sequence with 203 volumes [repetition time (TR) = 2638 ms; echo time (TE) = 30 ms; flip angle (FA) = 90°; field of view (FOV) 256 mm2, number of slices 45; voxel size = 4x4x3mm]. A sagittal T1-weighted FSPGR sequence was collected [TR = 7800 ms; TE = 2.956 ms; FA = 12°; FOV = 256 mm2; number of slices = 170; voxel size = 1x1x1.2]. After the scanner upgrade (YTOP2), a 750 Discovery 3 T MRI scanner was used with a 32‐channel head coil, and the following parameters: BOLD‐sensitive gradient EPI sequence with 200 volumes, [TR = 2250 ms, TE 30 = ms, flip angle = 79°, FOV 256 mm2, voxel size = 4 mm2, number of slices = 40;]. T1‐weighted images were acquired using a 3D IR‐prepared BRAVO sequence [TR = 8.16 ms; TE = 3.18 ms; flip angle = 12°; FOV = 256 mm2; voxel size = 1 mm2; number of slices = 188].
The SCAPS study collected data on a 3 T Discovery MR750. The EPI-sequence had 200 volumes [TR = 2000 ms; TE = 27 ms; FA = 90°; FOV = 240 mm2, number of slices = 40; voxel size = 1.875 mm2], and T1-weighted images were collected with a BRAVO-sequence; [TR = 7904 ms; TE = 3.06 ms; flip angle = 12°; FOV = 240 mm2, voxel size = 0.94 mm2; number of slices = 146]. At both sites, participants were instructed to lay still with their eyes open during the rsfMRI scan, and the head was fixated with foam pads to reduce motion.
2.5. rsfMRI analysis
rsfMRI analysis was performed using the FMRI Expert Analysis Tool (FEAT) from the FMRIB Software Library FSL; (Smith et al., 2004). The initial first five volumes of the rsfMRI scans were removed before analysis. The procedure included brain extraction, motion correction (MCFLIRT; (Jenkinson et al., 2002), spatial smoothing (Gaussian kernel, full-width at half-maximum = 6 mm), high pass filtering (90 s), and single-session ICA (MELODIC). FSL’s MCFLIRT was used to compute estimated mean relative in-scanner head motion (volume-to-volume displacement). To automatically classify noise components and regress them out of the data, FMRIB’S ICA-based Xnoiseifier (FIX) was applied with a threshold of 60 (Griffanti et al., 2014, Salimi-Khorshidi et al., 2014), and the cleaning step also included regression of the estimated motion parameters. FIX has shown to improve temporal signal to noise (tSNR) ratio significantly (Kaufmann et al., 2017, Skåtun et al., 2016). tSNR was computed before and after FIX (Roalf et al., 2016). T1-weighted structural images were used for registration to standard space (MNI-152) with FLIRT, normal search, 12 degrees of freedom (affine). The included participants did not have head translation movements > 2 mm or rotations > 2°.
2.6. Group ICA on rsfMRI data
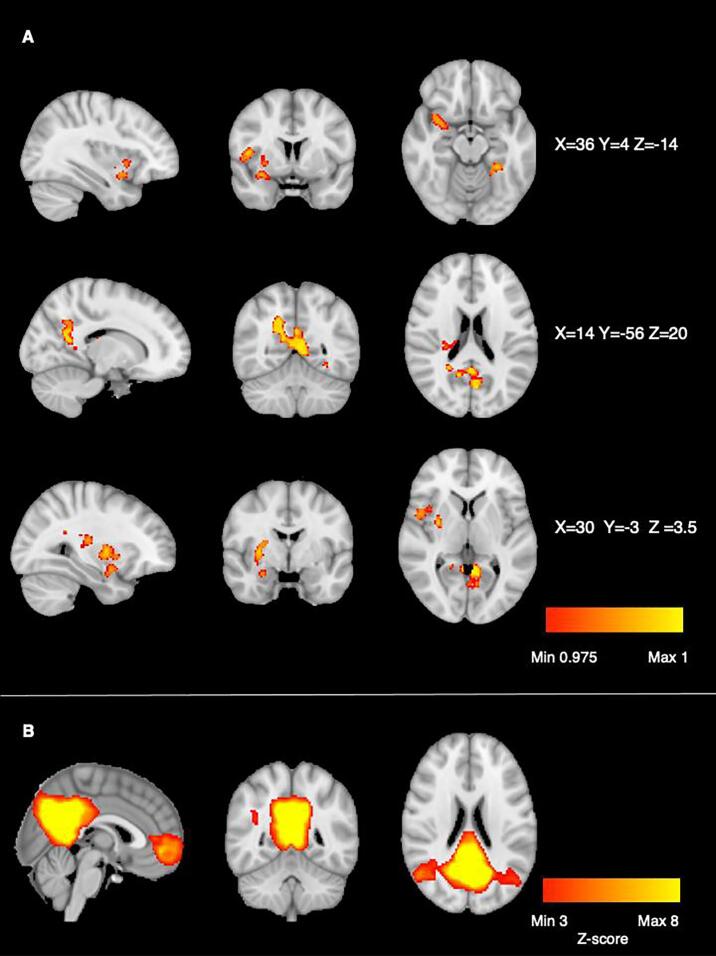
The MNI-conformed and cleaned rsfMRI datasets were submitted to temporal concatenation group independent component analysis (gICA) using FSL’s MELODIC. Based on (Ray et al., 2013), model order was set to 20. The resulting group-level components were subsequently used as spatial predictors against each participant’s rsfMRI-data to derive individual-level component time series and associated spatial maps (dual regression) (Filippini et al., 2009). To account for differences due to a scanner upgrade, the Norwegian study was treated as two samples. To integrate rsfMRI data from the Stockholm and Oslo scanners, we utilized a meta-ICA approach (see e.g. Biswal et al., 2010, Skåtun et al., 2016) using three separate sub-group ICA’s (Norwegian sample pre- and post-scanner upgrade and the Swedish sample). Each sub-group ICA was based on individual level spatial maps, and concatenated into one single meta-ICA before dual regression. The spatial maps and frequency profiles were assessed according to previous recommendations (Kelly et al., 2010). The canonical DMN was identified as a network comprising the PCC, precuneus and MPFC (see Fig. 1). Group analysis was performed to investigate differences in functional connectivity within the DMN between EOP patients and HC. FSL Randomise was run with 5000 permutations, and site, each individual’s in-scanner motion, age, sex and were modelled as covariates. The clusters from the difference maps were determined using threshold-free cluster enhancement (TFCE), corrected for multiple comparisons across voxels for the independent component of interest (Winkler et al., 2014). Between-group differences were considered statistically significant at p < 0.025. FSL’s Harvard-Oxford cortical and subcortical structural atlases to identify regions of the DMN from the meta-ICA analysis, and group difference results.
Fig. 1.
A: DMN connectivity HC > EOP group difference. Statistical map is thresholded at 1-p > 0.975 (TFCE-corrected), comparison was corrected for age, sex, scanner and motion in scanner. B: The DMN component resulting from the group ICA step carried out on the concatenated dataset across all 163 participants.
2.7. Subgroup analyses on DMN connectivity strength related to diagnostic subcategory and psychotropic medication
We used FSL’s fslmeants to extract the mean individual DMN connectivity strength, using the group-level clusters as mask. Analysis of covariance (ANCOVA) was used to analyze diagnostic subcategory associations with DMN connectivity. Multiple regression was used to examine the effect of medication type on DMN connectivity. Current use of psychotropic medication was modelled as a binary variable (yes/no) for each subject for the following medication classes: first generation antipsychotics (FGA), second generation antipsychotics (SGA), antiepileptics, lithium and antidepressants. A linear regression analysis was performed to investigate the possible effect of CPZ equivalents on DMN connectivity. An interaction model was performed to see whether there was an effect of sex on DMN connectivity. All subgroup analyses were done in R, version 4.0.3 and IBM SPSS Statistics, version 27, and covaried for age, sex and scanner.
3. Results
Permutation testing revealed a significant DMN connectivity group difference between patients with EOP and HC, see Fig. 1 (see Fig. S1 for mask computed by dual regression). The EOP group had significantly lower connectivity in brain areas comprising the bilateral PCC and precuneus, left lingual gyrus/fusiform cortex, right precentral gyrus, right central opercular cortex and right insula, right putamen, right pallidum and right amygdala. We tested for potential group by sex interactions for DMN connectivity, which showed no significant effect, see Table S4.
Fig. 1 Voxel-wise group comparisons.
3.1. DMN functional connectivity across diagnostic subcategories
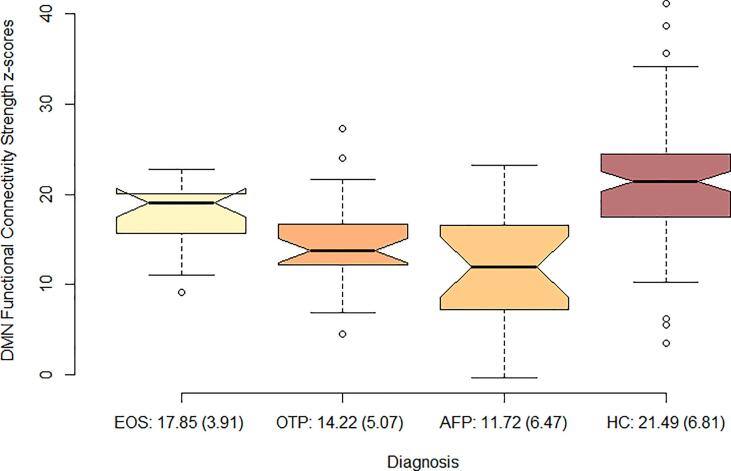
An ANCOVA with mean individual DMN connectivity as dependent variable [F 1,163 = 19.76, η2 = 0.29, p < 0.01] and age, sex and scanner as covariates, showed that the HC had the strongest connectivity 21.49 (6.81), followed by EOS; 17.85 (3.91), OTP 14.22 (5.07) and AFP 11.72 (6.47) (Fig. 2). A multiple comparisons analysis (Table S4) with diagnostic subgroup and DMN scores showed that all groups differed significantly from each other (p < 0.05), except OTP and AFP, which were not statistically different. A post-hoc within group analysis showed that effect of diagnostic subcategory was present both in the Norwegian sample [F 1,109 = 7.58, η2 = 0.17, p < 0.01] and the Swedish sample [F 1,54 = 5.25, η2 = 0.20, p < 0.01].
Fig. 2.
Distribution of connectivity strength z-scores from the DMN group difference map, in each diagnostic subcategory and in healthy controls. The values are presented in mean (standard deviation) DMN connectivity strength, covaried for age, sex and scanner. EOS; early onset schizophrenia, OTP; other psychosis, AFP; affective psychosis, HC; healthy controls.
3.2. Psychotropic medication and DMN functional connectivity
A multiple regression model with the binary variables FGA, SGA, lithium, antiepileptics and antidepressants, accounting for sex, age and scanner, revealed no significant associations between medication class and DMN connectivity strength (see Table 2). CPZ scores were not associated with DMN connectivity (See Table S3).
Table 2.
Multiple regression model with medication class, age, sex and scanner, using DMN connectivity strength as the dependent variable.
| DMN | |||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 33.02 | 14.44 to 15.61 | 0.001 |
| FGA | −0.69 | −5.95 to 4.57 | 0.794 |
| SGA | −0.39 | −3.07 to 2.28 | 0.769 |
| Lithium | −3.09 | −6.58 to 0.40 | 0.081 |
| Antiepileptics | −2.22 | −6.09 to 1.64 | 0.255 |
| Antidepressants | −2.48 | −5.77 to 0.82 | 0.138 |
| Sex | 2.22 | −0.51 to 4.96 | 0.109 |
| Age | −0.87 | −1.96 to 0.22 | 0.116 |
| Scanner | −1.73 | −3.49 to 0.03 | 0.054 |
| Observations | 68 | ||
| R2/R2 adjusted | 0.365/0.279 |
4. Discussion
The main finding of the current study was significantly lower DMN connectivity in patients with EOP, in regions encompassing the bilateral PCC and precuneus, left lingual gyrus and fusiform cortex, right precentral gyrus, right central opercular cortex and right insula, right putamen, right pallidum and right amygdala compared to HC. While both hyper- and hypoconnectivity relative to HC have been reported previously (Nair et al., 2020), the current finding is in line with studies reporting DMN hypoconnectivity in patient groups with psychotic disorders (Skudlarski et al., 2010, Pankow et al., 2015, Satterthwaite et al., 2015).
The DMN is thought to support internal mental states, such as remembering the past, thinking about the future, and envisioning scenarios in the present (Menon, 2011). In task-based fMRI studies, dynamic suppression of the DMN has been linked to better performance on attention-demanding tasks (Kelly et al., 2008, Murphy et al., 2020). On group level, cognitive impairment is a core feature in schizophrenia (Kuperberg and Heckers, 2000, Sheffield et al., 2018), and inefficient suppression of the DMN have been attributed to impairments in attention and working memory in psychosis (Fryer et al., 2013, Anticevic et al., 2012, Zhou et al., 2016). Moreover, the DMN is central for self-relevance, self-referential thought (Raichle, 2015) and mind-wandering (Godwin et al., 2017), and thus aberrant DMN connectivity has been linked to excessive self-referential and introspective processing in psychosis (Holt et al., 2011, Kühn and Gallinat, 2013, van der Meer et al., 2010).
The PCC and precuneus represent core brain areas of the posterior node of the DMN (Raichle, 2015, Buckner et al., 2008). The PCC is known to integrate bottom-up implicit attention with information from memory and perception (Leech and Sharp, 2014). The precuneus is an area within the association cortices, important for episodic memory retrieval and self-referential processing (Cavanna and Trimble, 2006). Lingual gyrus and fusiform cortex are involved in higher order visual processing, and are important in the identification of faces, word recognition and reading (Kanwisher et al., 1997, Weiner and Zilles, 2016, Wandell et al., 2012, Grill-Spector and Weiner, 2014). Supramarginal gyrus and angular gyrus have been associated with a variety of functions, including language and number processing, memory and attention (Seghier, 2013). The putamen and pallidum are part of the basal ganglia, and play a key role in facilitating movement and motor learning, but are also found to be important for learning and memory in general (Packard and Knowlton, 2002). Dopaminergic abnormalities within the basal ganglia are consistently found and thought to be implicated in the pathophysiology of schizophrenia (Di Sero et al., 2019, Jørgensen et al., 2016) , and show plasticity with use of antipsychotic medication (Horga et al., 2016, McCutcheon et al., 2019). The insula and amygdala are central parts of an emotion processing circuitry, specifically involved in identifying emotional stimuli, initiating affective states, and automatic forms of emotion regulation (Phillips et al., 2003, Janak and Tye, 2015), and are key nodes of the salience network (SN), thought to have a function in filtering bodily, cognitive and emotional information (Menon and Uddin, 2010). In sum, the observed pattern of lower DMN connectivity in patients with EOP compared to HC in this study, may be associated with difficulties integrating external and internal stimuli (Scheibner et al., 2017), abnormalities in the motor system (Packard and Knowlton, 2002), impaired memory and attention function (Bortolato et al., 2015, Deng et al., 2018), and dysfunctional emotion processing (Phillips et al., 2003, Rowland et al., 2013).
The analysis of DMN functional connectivity strength across diagnostic subcategories showed that patients with AFP deviated most from the HC group, compared to EOS and OTP (Fig. 2). We know little about differences in DMN connectivity in affective psychosis compared to non-affective psychosis, in adults or adolescents. In the current sample, a majority of the AFP patients were diagnosed with BD (See Table S1). There are studies in adult samples investigating DMN dysconnectivity that have made direct comparisons between groups with schizophrenia and BD. For instance, Liu et al. (Liu et al., 2014) found lower connectivity between the amygdala and dorsolateral PFC in SCZ, likely associated with difficulties with higher order emotional and cognitive integration, while lower connectivity between amygdala and ventral PFC in BD was associated with impaired emotion regulation and inhibitory control. A recent study in adolescents with BD showed that individuals with psychotic symptoms exhibited patterns of both increased and decreased DMN connectivity, compared to both HC and non-psychotic BD (Zhong et al., 2019). A recent systematic review on rsfMRI in BD during remission (Syan et al., 2018), reported results from two studies of BD with a history of psychosis. Brady and colleagues (Brady et al., 2017) used an a-priori seed-based approach to compare manic and euthymic BD and HC. Connectivity between frontal nodes and the rest of the DMN differentiated both diagnostic group and mood state. Interestingly, the euthymic state showed hypo-connectivity, while mania demonstrated connectivity patterns more similar to the HC group. This is in accordance with the results in this study, as AFP patients in this study were not in a manic or psychotic state at scan time. The other study by Kadhka and colleagues (Khadka et al., 2013) used an ICA-based method with a sample of SCZ, BD, first degree relatives and HC. The study reported results in the posterior part of the DMN in patients relative to controls, with greatest hypoconnectivity in the left and right cingulate gyrus and left and right precuneus. This is in line with the observed group difference in PCC and precunes in the current study. These studies reporting DMN hypoconnectivity, has led to the suggestion of a potential neural phenotype of psychosis in BD (Syan et al., 2018). In recent years, there has been an increased awareness on overlapping pathology in SCZ and BD. This can be seen in common clinical features (Murray et al., 2004), neurocognitive (Reichenberg et al., 2009, Bora et al., 2010) and social cognitive deficits (Montag et al., 2010, Sparks et al., 2010), and shared genetics (Lichtenstein et al., 2009). One important difference in the clinical presentation of SCZ and BD is seen in the expression of emotions. Individuals with BD show dysregulated mood states reflected in manic or hypomanic and depressive periods (Malhi et al., 2004, Malhi et al., 2004). In SCZ spectrum disorders, emotionality is often characterized by inadequate or blunted affect, or emotional expressions that seem detached from the context (Gur et al., 2006). This known difference in emotionality between diagnostic groups, could possibly contribute to the observed differential effect in emotion processing regions including the amygdala and insula, in the current study.
When we investigated the role of psychotropic medication on DMN connectivity strength, we found no significant relationship. To date, there are no systematic investigations of putative effects of psychotropic medication on DMN connectivity in EOP. In studies of adults with affective disorders including BD, medication has shown to normalize aberrant connectivity patterns, specifically in emotional tasks (Phillips et al., 2008, Hafeman et al., 2012). In adult patients with SCZ, there is evidence that the use of antipsychotic medication can normalize dysregulated connectivity patterns within the DMN, which in turn has been associated with symptom improvement (Guo et al., 2017, Wang et al., 2017, Sambataro et al., 2010, Surguladze et al., 2011). Reports of increased DMN functional connectivity after treatment with olanzapine (Guo et al., 2017, Sambataro et al., 2010), and risperidone (Zong et al., 2019), indicate that individuals with SCZ spectrum diagnoses may show connectivity patterns more similar to HC as an effect of successful treatment with antipsychotic medication. Studies that report normalized RSFC patterns as an effect of antipsychotic medication (Guo et al., 2017, Wang et al., 2017, Sambataro et al., 2010, Surguladze et al., 2011), have suggested a possible ameliorating role of medication on DMN connectivity in SCZ spectrum disorders, an effect that would not be expected in individuals with AFP using mood stabilizing medication. However, such medication effects were not confirmed in the current study.
4.1. Strengths
The current study adds to the literature by using a well characterized clinical group of young patients with EOP, with representative subgroups. The sample size is relatively large compared to previous rsfMRI studies in similar groups (Nair et al., 2020, O’Neill et al., 2019). Most previous studies have focused on EOS, see e.g. (Wang et al., 2018, Peng et al., 2020, Tang et al., 2013, Wang et al., 2017, Zhang et al., 2020), and rsfMRI studies in adolescents with EOP, especially studies including AFP are scarce. The current study used a well-defined age range (12–18 years), which is developmentally more homogenous than studies that include children < 12 years (Watsky et al., 2018) and studies including both adolescents and young adults (Nair et al., 2020, O’Neill et al., 2019).
4.2. Limitations
A limitation to the current study was that the MRI images were obtained from two different scanners, whereas one of the scanners had an upgrade during the data collection period. Scan time, especially in the Swedish sample, was relatively short, which is suboptimal. The inclusion procedures between the Norwegian and Swedish cohorts were slightly different, and the distribution of diagnostic subcategories within each scanner/cohort were unequal, however the patient and controls groups were balanced across the two samples. The current study used adolescents with EOP which includes different diagnostic subcategories. Future studies with larger samples should focus on distinct patient groups and possible medication effects, to confirm and extend the effects observed in this study. Moreover, cognitive and clinical variables would be useful to further interpret the results in this study.
5. Conclusion
The current study revealed significantly lower DMN connectivity in adolescent patients with EOP compared to HC in regions encompassing the posterior cingulate cortex, precuneus, fusiform cortex, putamen, pallidum, amygdala and insula. The effect was strongest in patients with AFP, but was also present in EOS and OTP. EOP encompass several diagnoses, and the observed differential effect of diagnostic subcategory may offer important cues to understand mechanisms in psychosis development.
CRediT authorship contribution statement
Eva Hilland: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Cecilie Johannessen: Conceptualization, Data curation, Formal analysis, Writing – review & editing. Rune Jonassen: Formal analysis, Methodology, Software, Writing – review & editing. Dag Alnæs: Formal analysis, Methodology, Software, Writing – review & editing. Kjetil N. Jørgensen: Data curation, Writing – review & editing. Claudia Barth: Data curation, Writing – review & editing. Dimitrios Andreou: Data curation, Writing – review & editing. Stener Nerland: Data curation, Writing – review & editing. Laura A. Wortinger: Data curation, Writing – review & editing. Runar E. Smelror: Data curation, Writing – review & editing. Kirsten Wedervang-Resell: Data curation, Writing – review & editing. Hannes Bohman: Funding acquisition, Project administration, Resources, Writing – review & editing. Mathias Lundberg: Funding acquisition, Project administration, Resources, Writing – review & editing. Lars T. Westlye: Funding acquisition, Project administration, Resources, Writing – review & editing. Ole A. Andreassen: Funding acquisition, Project administration, Resources, Writing – review & editing. Erik G. Jönsson: Funding acquisition, Supervision, Project administration, Resources, Writing – review & editing. Ingrid Agartz: Funding acquisition, Supervision, Project administration, Resources, Writing – review & editing.
Acknowledgments
Acknowledgements
We thank the study participants, and the clinicians as well as research assistants for participant recruitment and assessment at the Norwegian Centre for Mental Disorders (NORMENT) and the SCAPS study at the Karolinska Institutet.
This work was supported by the Research Council of Norway (grant numbers: 223273, 213700, 250358), the South-Eastern Norway Regional Health Authority (2019107, 2020086, 2019099, 2020020), the Swedish Research Council (2017-00949) and FORTE 2012.
Ethical approval
The Youth-TOP Study was approved by the The Regional Ethical Committee for Medical and Health Research for Southern Norway and the SCAPS study was approved by Stockholm Ethical Committee in Sweden. Both studies were conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from participants, parents or guardians (if the participant was under 16 years), prior to inclusion.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102881.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- World Health Organization. The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines: Geneva: World Health Organization; 1992.
- Ballageer T., Malla A., Manchanda R., Takhar J., Haricharan R. Is adolescent-onset first-episode psychosis different from adult onset? J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(8):782–789. doi: 10.1097/01.chi.0000164591.55942.ea. [DOI] [PubMed] [Google Scholar]
- Joa I., Johannessen J.O., Langeveld J., et al. Baseline profiles of adolescent vs. adult-onset first-episode psychosis in an early detection program. Acta Psychiatr. Scand. 2009;119(6):494–500. doi: 10.1111/j.1600-0447.2008.01338.x. [DOI] [PubMed] [Google Scholar]
- Díaz-Caneja C.M., Pina-Camacho L., Rodríguez-Quiroga A., Fraguas D., Parellada M., Arango C. Predictors of outcome in early-onset psychosis: a systematic review. NPJ Schizophr. 2015;1(1):1–10. doi: 10.1038/npjschz.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amminger G.P., Henry L.P., Harrigan S.M., Harris M.G., Alvarez-Jimenez M., Herrman H., Jackson H.J., McGorry P.D. Outcome in early-onset schizophrenia revisited: findings from the early psychosis prevention and intervention centre long-term follow-up study. Schizophr. Res. 2011;131(1–3):112–119. doi: 10.1016/j.schres.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Fraguas D., Díaz-Caneja C.M., Rodríguez-Quiroga A., Arango C. Oxidative stress and inflammation in early onset first episode psychosis: a systematic review and meta-analysis. Int. J. Neuropsychopharmacol. 2017;20(6):435–444. doi: 10.1093/ijnp/pyx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P.K., Leathem L.D., Currin D.L., Karlsgodt K.H. Adolescent Neurodevelopment and Vulnerability to Psychosis. Biol. Psychiatry. 2020 doi: 10.1016/j.biopsych.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnæs D., Kaufmann T., van der Meer D., et al. Brain Heterogeneity in Schizophrenia and Its Association With Polygenic RiskBrain Heterogeneity in Schizophrenia and Polygenic RiskBrain Heterogeneity in Schizophrenia and Polygenic Risk. JAMA Psychiatry. 2019 doi: 10.1001/jamapsychiatry.2019.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T., Doan N.T., Kaufmann T., et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative modelsmapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative modelsmapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75(11):1146–1155. doi: 10.1001/jamapsychiatry.2018.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Baker J.T. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr. Opin. Neurobiol. 2015;30:85–91. doi: 10.1016/j.conb.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D., Wang Y., Chang X., Luo C., Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr. Bull. 2018;44(1):168–181. doi: 10.1093/schbul/sbx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier-Baldelli A., Andrews-Hanna J.R., Mittal V.A. Resting state connectivity dynamics in individuals at risk for psychosis. J. Abnorm. Psychol. 2018;127(3):314. doi: 10.1037/abn0000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Jolliffe M., Lograsso Y.S.S., Bearden C.E. A review of default mode network connectivity and its association with social cognition in adolescents with autism spectrum disorder and early-onset psychosis. Front. Psychiatry. 2020;11:614. doi: 10.3389/fpsyt.2020.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioakeimidis V., Haenschel C., Yarrow K., Kyriakopoulos M., Dima D. A meta-analysis of structural and functional brain abnormalities in early-onset schizophrenia. Schizophrenia Bull. Open. 2020;1(1) sgaa016. [Google Scholar]
- Hu M.-L., Zong X.-F., Mann J.J., et al. A review of the functional and anatomical default mode network in schizophrenia. Neuroscience Bull. 2017;33(1):73–84. doi: 10.1007/s12264-016-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cognit. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Raichle M.E. The brain's default mode network. Annu. Rev. Neurosci. 2015;38:433–447. doi: 10.1146/annurev-neuro-071013-014030. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Thermenos H.W., Milanovic S., et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Liang M., Tian L., Wang K., Hao Y., Liu H., Liu Z., Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr. Res. 2007;97(1–3):194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Mannell M.V., Franco A.R., Calhoun V.D., Cañive J.M., Thoma R.J., Mayer A.R. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Hum. Brain Mapp. 2010;31(3):424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P., Jagannathan K., Anderson K., Stevens M.C., Calhoun V.D., Skudlarska B.A., Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol. Psychiatry. 2010;68(1):61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow A., Deserno L., Walter M., Fydrich T., Bermpohl F., Schlagenhauf F., Heinz A. Reduced default mode network connectivity in schizophrenia patients. Schizophr. Res. 2015;165(1):90–93. doi: 10.1016/j.schres.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Vandekar S.N., Wolf D.H., et al. Connectome-wide network analysis of youth with psychosis-spectrum symptoms. Mol. Psychiatry. 2015;20(12):1508–1515. doi: 10.1038/mp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J.P., Karcher N.R., Merrill A.M., O’Brien K.J., Straub K.T., Trull T.J., Kerns J.G. Psychosis risk is associated with decreased resting-state functional connectivity between the striatum and the default mode network. Cognit. Affect. Behav. Neurosci. 2019;19(4):998–1011. doi: 10.3758/s13415-019-00698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhan Y., Zhang Y., et al. Abnormal long-and short-range functional connectivity in adolescent-onset schizophrenia patients: a resting-state fMRI study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;81:445–451. doi: 10.1016/j.pnpbp.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Peng Y., Zhang S., Zhou Y., et al. Abnormal functional connectivity based on nodes of the default mode network in first-episode drug-naive early-onset schizophrenia. Psychiatry Res. 2020;113578 doi: 10.1016/j.psychres.2020.113578. [DOI] [PubMed] [Google Scholar]
- Tang J., Liao Y., Song M., et al. Aberrant default mode functional connectivity in early onset schizophrenia. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang Y., Long Z., et al. Frequency-specific alteration of functional connectivity density in antipsychotic-naive adolescents with early-onset schizophrenia. J. Psychiatr. Res. 2017;95:68–75. doi: 10.1016/j.jpsychires.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Zhang S., Yang G., Ou Y., et al. Abnormal default-mode network homogeneity and its correlations with neurocognitive deficits in drug-naive first-episode adolescent-onset schizophrenia. Schizophr. Res. 2020;215:140–147. doi: 10.1016/j.schres.2019.10.056. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Wang C., Gao W., Xiao Q., Lu D., Jiao Q., Su L., Lu G. Aberrant resting-state functional connectivity in the default mode network in pediatric bipolar disorder patients with and without psychotic symptoms. Neurosci. Bull. 2019;35(4):581–590. doi: 10.1007/s12264-018-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill A., Mechelli A., Bhattacharyya S. Dysconnectivity of large-scale functional networks in early psychosis: a meta-analysis. Schizophr. Bull. 2019;45(3):579–590. doi: 10.1093/schbul/sby094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn L.A., Cullen K.R., Francis M.M., Westlund M.K. Developmental resting state functional connectivity for clinicians. Curr. Behav. Neurosci. Rep. 2014;1(3):161–169. doi: 10.1007/s40473-014-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M., Murty V.P., Tervo-Clemmens B., Foran W., Luna B. Age-associated deviations of amygdala functional connectivity in youths with psychosis spectrum disorders: relevance to psychotic symptoms. Am. J. Psychiatry. 2019;176(3):196–207. doi: 10.1176/appi.ajp.2018.18040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottman K.K., Kraguljac N.V., White D.M., Morgan C.J., Calhoun V.D., Butt A., Lahti A.C. Risperidone effects on brain dynamic connectivity–a prospective resting state fMRI study in schizophrenia. Front. Psychiatry. 2017;8:14. doi: 10.3389/fpsyt.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-X., Guo F., Zhu Y.-Q., et al. Effect of second-generation antipsychotics on brain network topology in first-episode schizophrenia: a longitudinal rs-fMRI study. Schizophr. Res. 2019;208:160–166. doi: 10.1016/j.schres.2019.03.015. [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., Williamson D., Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003 doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Griffanti L., Salimi-Khorshidi G., Beckmann C.F., et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T., Alnæs D., Doan N.T., Brandt C.L., Andreassen O.A., Westlye L.T. Delayed stabilization and individualization in connectome development are related to psychiatric disorders. Nat. Neurosci. 2017;20(4):513. doi: 10.1038/nn.4511. [DOI] [PubMed] [Google Scholar]
- Skåtun K.C., Kaufmann T., Doan N.T., Alnæs D., Córdova-Palomera A., Jönsson E.G., Fatouros-Bergman H., Flyckt L., KaSP, Melle I. Consistent functional connectivity alterations in schizophrenia spectrum disorder: a multisite study. Schizophr. Bull. 2016;43(4):914–924. doi: 10.1093/schbul/sbw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf D.R., Quarmley M., Elliott M.A., et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray K.L., McKay D.R., Fox P.M., et al. ICA model order selection of task co-activation networks. Front. Neurosci. 2013;7:237. doi: 10.3389/fnins.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G., et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B., Mennes M., Zuo X.-N., Gohel S., Kelly C., Smith S.M., Beckmann C.F., Adelstein J.S., Buckner R.L., Colcombe S. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R.E., Jr, Alexopoulos G.S., Wang Z., et al. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods. 2010;189(2):233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.C., Uddin L.Q., Biswal B.B., Castellanos F.X., Milham M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Murphy A.C., Bertolero M.A., Papadopoulos L., Lydon-Staley D.M., Bassett D.S. Multimodal network dynamics underpinning working memory. Nat. Commun. 2020;11(1):1–13. doi: 10.1038/s41467-020-15541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg G., Heckers S. Schizophrenia and cognitive function. Curr. Opin. Neurobiol. 2000;10(2):205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Sheffield J.M., Karcher N.R., Barch D.M. Cognitive deficits in psychotic disorders: a lifespan perspective. Neuropsychol. Rev. 2018;28(4):509–533. doi: 10.1007/s11065-018-9388-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer S.L., Woods S.W., Kiehl K.A., et al. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front. Psychiatry. 2013;4:92. doi: 10.3389/fpsyt.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Cole M.W., Murray J.D., Corlett P.R., Wang X.-J., Krystal J.H. The role of default network deactivation in cognition and disease. Trends Cognit. Sci. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Pu W., Wang J., et al. Inefficient DMN suppression in schizophrenia patients with impaired cognitive function but not patients with preserved cognitive function. Sci. Rep. 2016;6(1):1–10. doi: 10.1038/srep21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin C.A., Hunter M.A., Bezdek M.A., et al. Functional connectivity within and between intrinsic brain networks correlates with trait mind wandering. Neuropsychologia. 2017;103:140–153. doi: 10.1016/j.neuropsychologia.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Holt D.J., Cassidy B.S., Andrews-Hanna J.R., Lee S.M., Coombs G., Goff D.C., Gabrieli J.D., Moran J.M. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol. Psychiatry. 2011;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr. Bull. 2013;39(2):358–365. doi: 10.1093/schbul/sbr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34(6):935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. 2008. [DOI] [PubMed]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner K.S., Zilles K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia. 2016;83:48–62. doi: 10.1016/j.neuropsychologia.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell B.A., Rauschecker A.M., Yeatman J.D. Learning to see words. Annu. Rev. Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K., Weiner K.S. The functional architecture of the ventral temporal cortex and its role in categorization. Nat. Rev. Neurosci. 2014;15(8):536–548. doi: 10.1038/nrn3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M.G., Knowlton B.J. Learning and memory functions of the basal ganglia. Annu. Rev. Neurosci. 2002;25(1):563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Di Sero A., Jørgensen K.N., Nerland S., Melle I., Andreassen O.A., Jovicich J., Agartz I. Antipsychotic treatment and basal ganglia volumes: exploring the role of receptor occupancy, dosage and remission status. Schizophr. Res. 2019;208:114–123. doi: 10.1016/j.schres.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Jørgensen K.N., Nesvåg R., Gunleiksrud S., Raballo A., Jönsson E.G., Agartz I. First-and second-generation antipsychotic drug treatment and subcortical brain morphology in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2016;266(5):451–460. doi: 10.1007/s00406-015-0650-9. [DOI] [PubMed] [Google Scholar]
- Horga G., Cassidy C.M., Xu X., Moore H., Slifstein M., Van Snellenberg J.X., Abi-Dargham A. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry. 2016;73(8):862–870. doi: 10.1001/jamapsychiatry.2016.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon R.A., Abi-Dargham A., Howes O.D. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42(3):205–220. doi: 10.1016/j.tins.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibner H.J., Bogler C., Gleich T., Haynes J.-D., Bermpohl F. Internal and external attention and the default mode network. Neuroimage. 2017;148:381–389. doi: 10.1016/j.neuroimage.2017.01.044. [DOI] [PubMed] [Google Scholar]
- Bortolato B., Miskowiak K.W., Köhler C.A., Vieta E., Carvalho A.F. Cognitive dysfunction in bipolar disorder and schizophrenia: a systematic review of meta-analyses. Neuropsychiatr. Dis. Treat. 2015;11:3111. doi: 10.2147/NDT.S76700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M., Pan Y., Zhou L., et al. Resilience and cognitive function in patients with schizophrenia and bipolar disorder, and healthy controls. Front. Psychiatry. 2018;9:279. doi: 10.3389/fpsyt.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland J.E., Hamilton M.K., Vella N.C., Lino B.J., Mitchell P.B., Green M.J. Adaptive associations between social cognition and emotion regulation are absent in schizophrenia and bipolar disorder. Front. Psychol. 2013;3:607. doi: 10.3389/fpsyg.2012.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Tang Y., Womer F., et al. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophr. Bull. 2014;40(2):469–477. doi: 10.1093/schbul/sbt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syan S.K., Smith M., Frey B.N., Remtulla R., Kapczinski F., Hall G.B., Minuzzi L. Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. J. Psychiatry Neurosci. JPN. 2018;43(5):298. doi: 10.1503/jpn.170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R.O., Jr, Tandon N., Masters G.A., Margolis A., Cohen B.M., Keshavan M., Öngür D. Differential brain network activity across mood states in bipolar disorder. J. Affect. Disord. 2017;207:367–376. doi: 10.1016/j.jad.2016.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadka S., Meda S.A., Stevens M.C., et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol. Psychiatry. 2013;74(6):458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R.M., Sham P., Van Os J., Zanelli J., Cannon M., McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr. Res. 2004;71(2–3):405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Reichenberg A., Harvey P.D., Bowie C.R., Mojtabai R., Rabinowitz J., Heaton R.K., Bromet E. Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophr. Bull. 2009;35(5):1022–1029. doi: 10.1093/schbul/sbn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E., Yücel M., Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: implications for DSM-V criteria and beyond. Schizophr. Bull. 2010;36(1):36–42. doi: 10.1093/schbul/sbp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C., Ehrlich A., Neuhaus K., Dziobek I., Heekeren H.R., Heinz A., Gallinat J. Theory of mind impairments in euthymic bipolar patients. J. Affect. Disord. 2010;123(1–3):264–269. doi: 10.1016/j.jad.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Sparks A., McDonald S., Lino B., O'Donnell M., Green M.J. Social cognition, empathy and functional outcome in schizophrenia. Schizophr. Res. 2010;122(1–3):172–178. doi: 10.1016/j.schres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P., Yip B.H., Björk C., Pawitan Y., Cannon T.D., Sullivan P.F., Hultman C.M. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi G.S., Lagopoulos J., Sachdev P., Mitchell P.B., Ivanovski B., Parker G.B. Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord. 2004;6(4):271–285. doi: 10.1111/j.1399-5618.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Lagopoulos J., Ward P.B., Kumari V., Mitchell P.B., Parker G.B., Ivanovski B., Sachdev P. Cognitive generation of affect in bipolar depression: an fMRI study. Eur. J. Neurosci. 2004;19(3):741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- Gur R.E., Kohler C.G., Ragland J.D., Siegel S.J., Lesko K., Bilker W.B., Gur R.C. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophr. Bull. 2006;32(2):279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Travis M.J., Fagiolini A., Kupfer D.J. Medication effects in neuroimaging studies of bipolar disorder. Am. J. Psychiatry. 2008;165(3):313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafeman D.M., Chang K.D., Garrett A.S., Sanders E.M., Phillips M.L. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14(4):375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- Guo W., Liu F., Chen J., Wu R., Li L., Zhang Z., Zhao J. Olanzapine modulation of long-and short-range functional connectivity in the resting brain in a sample of patients with schizophrenia. Eur. Neuropsychopharmacol. 2017;27(1):48–58. doi: 10.1016/j.euroneuro.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Wang Y., Tang W., Fan X., et al. Resting-state functional connectivity changes within the default mode network and the salience network after antipsychotic treatment in early-phase schizophrenia. Neuropsychiatr. Dis. Treat. 2017;13:397. doi: 10.2147/NDT.S123598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F., Blasi G., Fazio L., et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35(4):904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S.A., Chu E.M., Marshall N., et al. Emotion processing in schizophrenia: fMRI study of patients treated with risperidone long-acting injections or conventional depot medication. J. Psychopharmacol. 2011;25(6):722–733. doi: 10.1177/0269881110363316. [DOI] [PubMed] [Google Scholar]
- Zong X., Hu M., Pantazatos S.P., et al. A dissociation in effects of risperidone monotherapy on functional and anatomical connectivity within the default mode network. Schizophr. Bull. 2019;45(6):1309–1318. doi: 10.1093/schbul/sby175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watsky R.E., Gotts S.J., Berman R.A., et al. Attenuated resting-state functional connectivity in patients with childhood-and adult-onset schizophrenia. Schizophr. Res. 2018;197:219–225. doi: 10.1016/j.schres.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.