Key Points
Question
In patients at high risk for lumbar disc reherniation owing to a large annular defect following lumbar microdiscectomy, does implantation with an annular closure device reduce the risk of reherniation and reoperation?
Findings
In this secondary analysis of a randomized clinical trial of 554 patients with large annular defects following lumbar microdiscectomy for symptomatic lumbar disc herniation, the addition of an annular closure device statistically significantly reduced the risk of symptomatic reherniation (18.8% vs 31.6%) and reoperation (16.0% vs 22.6%) through 5 years follow-up with no statistical difference in the risk of serious adverse events.
Meaning
These findings suggest that the use of an annular closure device may reduce the risk of symptomatic reherniation and reoperation in patients with large annular defects following lumbar microdiscectomy.
This randomized clinical trial investigates whether a bone-anchored annular closure device is associated with lower reherniation and reoperation rates among patients who undergo lumbar microdiscectomy to treat large annular defects.
Abstract
Importance
Patients with large annular defects following lumbar microdiscectomy for disc herniation are at increased risk for symptomatic recurrence and reoperation.
Objective
To determine whether a bone-anchored annular closure device in addition to lumbar microdiscectomy resulted in lower reherniation and reoperation rates vs lumbar microdiscectomy alone.
Design, Setting, and Participants
This secondary analysis of a multicenter randomized clinical trial reports 5-year follow-up for enrolled patients between December 2010 and October 2014 at 21 clinical sites. Patients in this study had a large annular defect (6-10 mm width) following lumbar microdiscectomy for treatment of lumbar disc herniation. Statistical analysis was performed from November to December 2020.
Interventions
Lumbar microdiscectomy with additional bone-anchored annular closure device (device group) or lumbar microdiscectomy only (control group).
Main Outcomes and Measures
The incidence of symptomatic reherniation, reoperation, and adverse events as well as changes in leg pain, Oswestry Disability Index, and health-related quality of life when comparing the device and control groups over 5 years of follow-up.
Results
Among 554 randomized participants (mean [SD] age: 43 [11] years; 327 [59%] were men), 550 were included in the modified intent-to-treat efficacy population (device group: n = 272; 270 [99%] were White); control group: n = 278; 273 [98%] were White) and 550 were included in the as-treated safety population (device group: n = 267; control group: n = 283). The risk of symptomatic reherniation (18.8% [SE, 2.5%] vs 31.6% [SE, 2.9%]; P < .001) and reoperation (16.0% [SE, 2.3%] vs 22.6% [SE, 2.6%]; P = .03) was lower in the device group. There were 53 reoperations in 40 patients in the device group and 82 reoperations in 58 patients in the control group. Scores for leg pain severity, Oswestry Disability Index, and health-related quality of life significantly improved over 5 years of follow-up with no clinically relevant differences between groups. The frequency of serious adverse events was comparable between the treatment groups. Serious adverse events associated with the device or procedure were less frequent in the device group (12.0% vs 20.5%; difference, −8.5%; 95% CI, −14.6% to −2.3%; P = .008).
Conclusions and Relevance
In patients who are at high risk of recurrent herniation following lumbar microdiscectomy owing to a large defect in the annulus fibrosus, this study’s findings suggest that annular closure with a bone-anchored implant lowers the risk of symptomatic recurrence and reoperation over 5 years of follow-up.
Trial Registration
ClinicalTrials.gov Identifier: NCT01283438
Introduction
Radicular pain is characterized by radiating buttock and leg pain in a lumbar nerve root distribution, which may be accompanied by neurosensory and motor deficits. Radicular pain caused by lumbar disc herniation improves in most patients with conservative treatment only. Yet, 1 in 5 individuals will experience persistent symptoms and/or neurological deficit1,2 such that lumbar discectomy may be indicated to surgically remove the herniated disc material. Recurrent herniation occurs in 7% to 18% of patients following discectomy3,4,5 and a large defect in the annulus fibrosus after surgery is a major risk factor for reherniation.6 In a meta-analysis involving more than 1600 patients undergoing lumbar discectomy, the risk of symptom recurrence (odds ratio, 2.5; P = .004) and reoperation (odds ratio, 2.3; P < .001) was higher in patients with large vs small annular defects.6 Reoperations for lumbar disc herniation are more costly,4 less effective,7,8 and are associated with higher risk of disability9 than primary procedures. Therefore, intraoperative strategies intended to close or occlude large annular defects may lower the clinical and economic burden related to surgery for recurrent herniation. This randomized clinical trial evaluated the 5-year clinical and radiographic outcomes of patients with large annular defects after lumbar microdiscectomy treated with or without a bone-anchored annular closure device.
Methods
Trial Design and Oversight
Members of the Annular Closure RCT Study Group representing 21 sites in Europe (eTable 1 in Supplement 3) designed the trial in collaboration with the study sponsor (Intrinsic Therapeutics [Woburn, Massachusetts]). Data were analyzed by an independent statistician and radiographic assessments were performed by an independent core laboratory blinded to patient outcomes. An independent data safety monitoring board adjudicated adverse events by seriousness and by relation to the procedure or implant. All authors had full access to the data and the data analysis. The study protocol and statistical analysis plan are available in Supplement 1 and Supplement 2, respectively. The clinical trial was approved by local ethics committees prior to commencing patient enrollment. All participants provided written informed consent prior to study entry. This secondary analysis followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Population
Details of the study design have been published previously.10 Among patients undergoing discectomy for lumbar disc herniation, key inclusion criteria were (1) age between 21 and 75 years, (2) single-level disc herniation between the first lumbar vertebra (L1) and the first sacral vertebra (S1), (3) posterior disc height at least 5 mm between end plates on sagittal magnetic resonance imaging (MRI), (4) attempted nonsurgical treatment for at least 6 weeks prior to surgery, (5) leg pain severity of at least 40 of 100 on a visual analogue scale, (6) Oswestry Disability Index (ODI) score of at least 40 of 100, and (7) a large annular defect (4 to 6 mm tall and 6 to 10 mm wide) following the discectomy procedure. Patients were excluded from enrollment if they had spondylolisthesis (grade II or higher), osteoporosis, or had previous surgery at the index level. Race data were self-reported. The complete list of inclusion and exclusion criteria for this trial are provided in eTable 2 in Supplement 3.
Study Procedures
Radiographic diagnosis of lumbar disc herniation was confirmed prior to surgery by MRI with T1- and T2-weighted axial and sagittal images, and preoperative imaging also included low-dose multiplanar computed tomography (CT) and flexion/extension x-rays. Surgeons performed a microdiscectomy and were instructed to avoid enlarging the annular defect for purposes of study inclusion. After completion of the microdiscectomy procedure but prior to surgery completion, surgeons used a sizing tool to measure the height and width of the defect in the annulus fibrosus. Patients with a large annular defect were intraoperatively randomized to the device group or the control group. Simple randomization was performed with a web-based system using a 1:1 ratio with a block size of 4. Neither surgeons nor patients were blinded to treatment group except for patients in the Netherlands (comprising 15% of the study population) who were blinded due to regional allowances. In the device group, a bone-anchored annular closure device (Barricaid, Intrinsic Therapeutics) was implanted under fluoroscopic guidance. The annular closure device is composed of a flexible polymer to physically occlude the annular defect and a titanium anchor to secure the polymer to an adjacent vertebral body. In the control group, no additional procedures were performed. Patients returned for clinical and radiographic follow-up at 6 weeks, 3 months, 6 months, and annually for 5 years (eTable 3 in Supplement 3).
Study Outcomes
The coprimary study end points were the incidence of recurrent disc herniation and a composite outcome comprised of clinical and radiographic variables, both of which were assessed after 2 years of follow-up and statistically favored the Device group.11 Here, we report final results of the study among all measured outcomes after 5 years of patient follow-up. Reherniation was confirmed during reoperation or by core laboratory identification of protrusion, extrusion, or sequestration at any location of the index-level disc.12 A reherniation was considered symptomatic if it was verified during a reoperation, identified by the imaging core laboratory where the patient reported at least moderate (40 of 100) disability, radicular symptoms, or neurologic deterioration, or if the reherniation was reported as an adverse event. Reoperations included any repeat procedure at the index level of herniation.
Patient-reported outcomes included ODI (measured on a 0-100 scale with higher scores indicating more severe disability),13 leg pain severity (measured on a 0-100 scale with higher scores indicating more severe pain),14 and health-related quality of life with the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores from the Medical Outcomes Study 36-Item Short-Form General Health Survey (SF-36) scale (each measured on a 0-100 scale with higher scores indicating better health-related quality of life).15 The minimal important differences (MID) for these outcomes were a minimum 15-point decrease from baseline for ODI,16 a 20-point decrease from baseline for leg pain,17 a 5.7-point increase from baseline for PCS,18 and a 6.3-point increase from baseline for MCS.18
Key radiographic assessments included disc height, device status, and vertebral end plate changes. Neurologic status and adverse events were assessed at each follow-up visit. The occurrence of adverse events was ascertained at each study contact and routinely monitored for accuracy. Investigators classified adverse events by seriousness and relation to the device or procedure.
Statistical Analysis
The trial was designed to enroll between 400 and 800 patients using a bayesian approach to sample size selection19 where interim analyses were performed after enrollment of 400 patients and repeated at increments of 50 patients thereafter until the predictive probability of trial success on each primary end point exceeded 90% or the maximum sample size was reached. Primary end point outcomes reported through 2 years of follow-up were analyzed with bayesian approaches; all clinical outcomes beyond 2 years of follow-up were analyzed with frequentist approaches. Efficacy analyses were performed on a modified intent-to-treat population comprised of all randomized patients in whom the intended procedure was attempted. Missing data were not imputed. Adverse events were reported on a safety population where patients were analyzed as treated. Outcomes between the groups were assessed with the t test for continuous data or Fisher exact test for categorical data. Longitudinal analysis of patient -reported outcomes was performed using a linear mixed-effects model. The incidences of symptomatic reherniation and reoperation were analyzed using Kaplan-Meier methods with log-rank tests for group comparisons. The cumulative incidence of reoperations accounting for multiple procedures in individual patients was analyzed using the Nelson-Aalen cumulative hazard function. Significance testing was performed using 2-sided tests (α = .05). All analyses were performed using STATA version 16.1 (StataCorp) from November to December 2020. Additional details regarding sample size estimation and analytic methods are described in the study protocol (Supplement 1) and the statistical analysis plan (Supplement 2).
Results
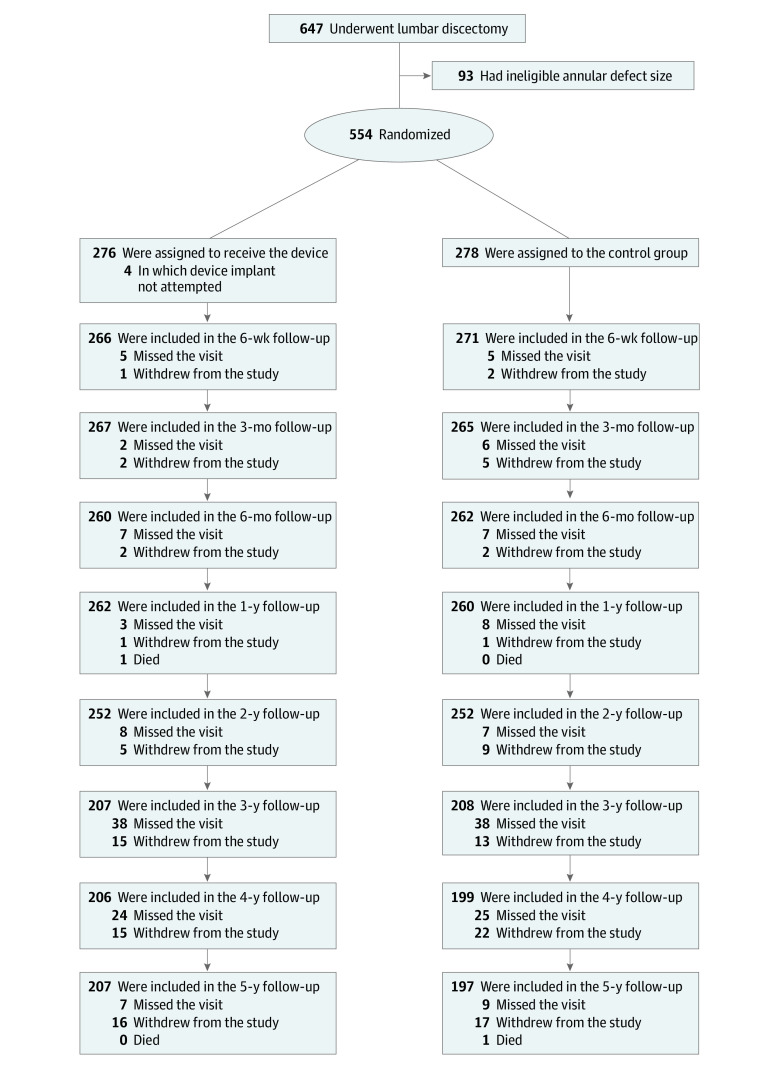
A total of 554 patients were enrolled at 21 sites in Austria, Belgium, France, Germany, Switzerland, and the Netherlands between December 2010 and October 2014; 327 [59%] were men; 276 were in the device group; 278 were in the control group; the mean [SD] age was 43 [11] years (Table). In 4 patients allocated to the device group, implantation was not attempted owing to proximity of the nerve root to the planned implant location. Therefore, the modified intention-to-treat population included 550 patients (272 in the device group [270 (99%) were White], and 278 in the control group [273 (98%) were White]). Implantation of the annular closure device was unsuccessful in 5 patients, including 4 patients in whom the polymer occlusion component did not fully enter the disc and 1 patient with nerve root injury during attempted implantation; thus, the as-treated population included 267 patients in the device group and 283 in the control group. The disposition of participants during the study is summarized in Figure 1. Baseline characteristics of patients were similar in the 2 treatment groups (Table). Median (IQR) fluoroscopy use was 18 (9-32) seconds in the device group and 5 (2-9) seconds in the control group. Median (IQR) operative time was 67 (46-90) minutes in the device group and 47 (30-70) minutes in the control group.
Table. Baseline Characteristics of the Patients.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| Device (n = 272) | Control (n = 278) | |
| Age, mean (SD), y | 43 (11) | 44 (10) |
| Sex | ||
| Female | 116 (43) | 107 (38) |
| Male | 156 (57) | 171 (62) |
| BMI, mean (SD) | 26 (4) | 26 (4) |
| Smoking history | 173 (64) | 175 (63) |
| Medical historya | ||
| Musculoskeletal | 95 (35)b | 91 (33)c |
| Head and neck | 62 (23)b | 54 (20)c |
| Gastrointestinal | 53 (20)d | 59 (21)b |
| Cardiovascular | 49 (18)b | 48 (17)b |
| Genitourinary | 39 (14)b | 35 (13)b |
| Skin | 29 (11)b | 30 (11)b |
| Respiratory | 28 (10)b | 44 (16)b |
| Visual analogue scale for leg paine | 81 (15) | 81 (15) |
| Oswestry Disability Index scoref | 59 (12) | 58 (14) |
| SF-36 component summary score, mean (SD)g | ||
| Physical | 29 (6) | 29 (6) |
| Mental | 40 (13) | 41 (13) |
| Index level | ||
| L2-L3 | 2 (1) | 1 (<1) |
| L3-L4 | 8 (3) | 5 (2) |
| L4-L5 | 123 (45) | 101 (36) |
| L5-S1 | 139 (51) | 171 (62) |
| Disc height, mean (SD), mm | 8.9 (2.1) | 8.9 (2.2) |
| Spondylolisthesis (grade I) | 6 (2) | 8 (3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); SF-36, 36-Item Short-Form General Health Survey.
Medical history variables reported with frequency of 10% or more in either group.
Data from 2 patients not reported.
Data from 1 patient not reported.
Data from 3 patients not reported.
Scores on the visual analogue scale range from 0 to 100, with higher scores indicating more severe pain.
Scores on the Oswestry Disability Index range from 0 to 100, with higher scores indicating more severe disability.
Physical Component Summary and Mental Component Summary scores from the SF-36 scale range from 0 to 100, with higher scores indicating better health-related quality of life.
Figure 1. Enrollment and Randomization of Patients.
A total of 276 patients were assigned to the device group and 278 patients assigned to the control group. Modified intention-to-treat population consisted of 272 patients with attempted device implant and 278 control patients. As-treated safety population consisted of 267 patients receiving the device and 283 receiving the control. In the as-treated safety population, failed device implantation in 5 patients from the modified intent-to-treat population resulted in assignment to the control group for analysis purposes.
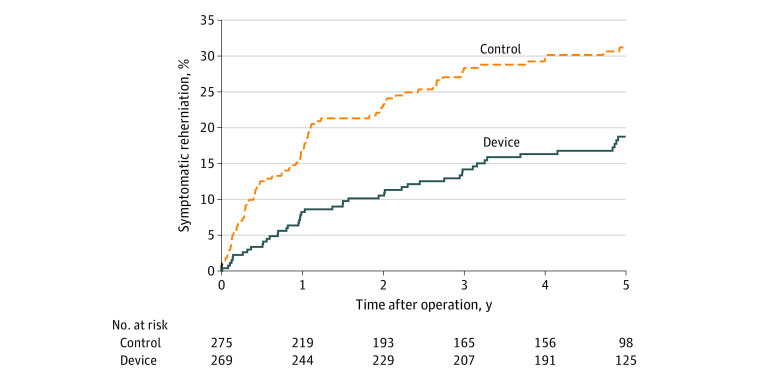
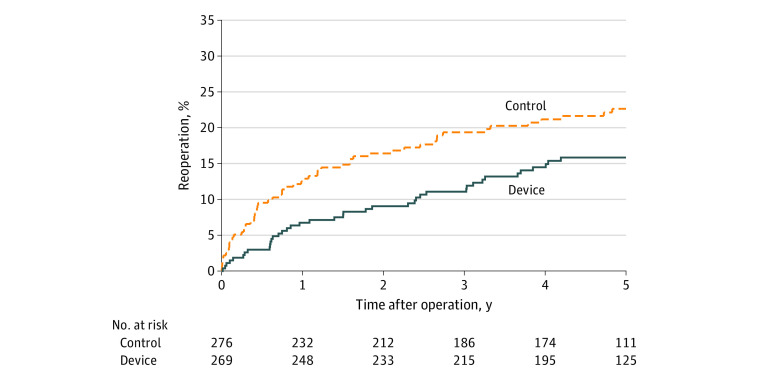
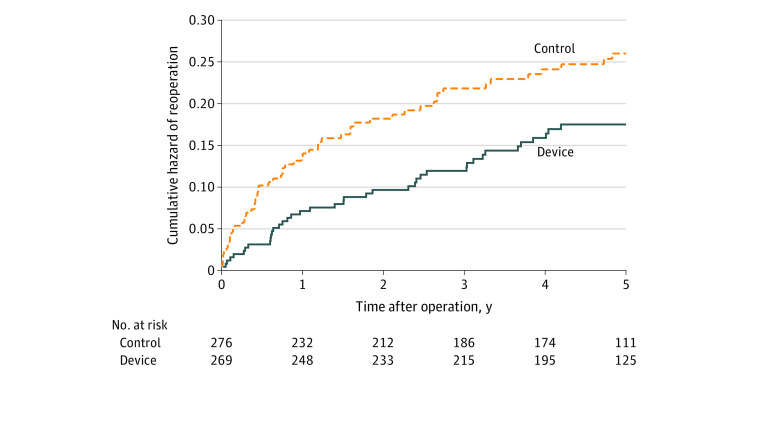
The risk of symptomatic reherniation was lower in the device group compared with the control group (18.8% [SE, 2.5%] vs 31.6% [SE, 2.9%], P < .001) over 5 years of follow-up (Figure 2). A total of 40 patients underwent 53 reoperations in the device group, and 58 patients underwent 82 reoperations in the control group (eTable 4 in Supplement 3). The risk of reoperation (16.0% [SE, 2.3%] vs 22.6% [SE, 2.6%], P = .03) (Figure 3) and the cumulative risk of reoperation accounting for multiple surgeries in individual patients (17.2% [SE, 2.7%] vs 25.5% [SE, 3.4%], P = .03) (Figure 4) were lower in the device group compared with the control group over 5 years of follow-up. Reoperation specifically for device failure was performed in 5.2% (14 of 267) of patients where 12 patients underwent device removal and 2 underwent device removal lumbar fusion. Scores for leg pain severity (eFigure 1 in Supplement 3), Oswestry Disability Index (eFigure 2 in Supplement 3), and health-related quality of life on the PCS and MCS subscales (eFigure 3 and eFigure 4 in Supplement 3) significantly improved over the 5-year follow-up period with no clinically important differences observed between groups. Comparing the device group with the control group, the percentage of patients whose scores exceeded the MID at 5 years and remained free from a reoperation was 78.0% vs 71.2% (difference, 6.8%; 95% CI, −1.4% to 14.8%; P = .12) for ODI, 77.1% vs 68.9% (difference, 8.1%; 95% CI, −0.2% to 16.3%; P = .07) for leg pain, 74.8% vs 65.3% (difference, 9.5%; 95% CI, 0.9% to 17.9%; P = .04) for PCS, and 50.9% vs 44.7% (difference, 6.2%; 95% CI, −3.2% to 15.4%; P = .21) for MCS.
Figure 2. Index Level Symptomatic Reherniation Through 5 Years.
Kaplan-Meier estimates in the modified intent-to-treat population through 5 years were 18.8% (SE, 2.5%) in the device group and 31.6% (SE, 3.0%) in the control group (log-rank P < .001).
Figure 3. Index Level Reoperation Through 5 Years.
Kaplan-Meier estimates in the modified intent-to-treat population through 5 years were 16.0% (SE, 2.3%) in the device group and 22.6% (SE, 2.7%) in the control group (log-rank P = .03).
Figure 4. Cumulative Risk of Reoperation Through 5 Years.
The Nelson-Aalen cumulative hazard for reoperation over 5 years was 0.172 (SE, 0.027) in the device group and 0.255 (SE, 0.034) in the control group (log-rank P = .03).
The frequency of serious adverse events was comparable between the treatment groups (eTable 5 in Supplement 3). Serious adverse events related to the device or procedure were less frequent in the Device group (12.0% vs 20.5%; difference −8.5%; 95% CI, −14.6% to −2.3%; P = .008; eTable 6 in Supplement 3), which was mainly attributable to the lower risk of lumbar disc reherniation. The incidence of any adverse event (85.4% vs 82.0%; difference 3.4%; 95% CI, −2.8% to 9.6%; P = .30) and any device- or procedure-related adverse event (45.3% vs 37.1%; difference, 8.2%; 95% CI, 0.0% to 16.3%; P = .06) was comparable between groups (eTable 7 and eTable 8 in Supplement 3). Vertebral end plate changes were more commonly reported in the device group (20.2% vs 1.4%; difference 18.8%; 95% CI, 13.9% to 24.1%; P < .001). No association was observed between vertebral end plate changes and clinical outcomes including leg pain severity, Oswestry Disability Index, and health-related quality of life. Disc height loss was observed in the entire sample over 5 years with no statistical differences between groups (−1.9 mm vs −1.7 mm; difference, −0.3 mm; 95% CI, −0.7 to 0.2 mm; P = .31).
Discussion
In this secondary analysis of a randomized clinical trial, implantation with an annular closure device in patients at high risk for lumbar disc reherniation owing to a large annular defect following lumbar microdiscectomy significantly reduced the risk of recurrent herniation and reoperation over 5 years of follow-up. There were no important group differences in other outcomes including leg pain, ODI, health-related quality of life, or safety. These findings support the use of an annular closure device during lumbar discectomy procedures when the intraoperatively identified defect in the annulus is greater than 6 mm width, which places the patient at elevated risk for a future lumbar disc reherniation.
The 31.6% rate of symptomatic reherniation among patients treated with lumbar discectomy only in the current trial was considerably higher than the 7% to 18% rates commonly cited following lumbar disc surgery.3,4,5 This observation is attributable to the eligibility criteria that required a large annular defect prior to randomization, a characteristic that more than doubles the risk of recurrence.6 The addition of an annular closure device during surgery lowered the risk of recurrence and reoperation in this high-risk population and the safety profile was comparable to those treated with surgery only. Importantly, annular closure is not indicated to close small defects since the risk of recurrence in this patient subgroup is acceptably low.6 However, 30% to 44% of lumbar discectomy patients have an annular defect exceeding 6 mm width6,20 and this cohort would derive the greatest benefit from annular closure. This large defect cohort is further characterized by a sufficient disc height of at least 5 mm, which has also been shown to increase reherniation risk.21
Previous analyses from this study showed a lower incidence of symptomatic herniation throughout follow-up comparing the device group to the control group, including at 3 months (1.5% vs 6.5%),22 1 year (8.4% vs 17.3%),23 2 years (12.4% vs 25.3%),11 and 3 years (14.8% vs 29.5%).24 Additionally, the main findings of this randomized trial with 5-year follow-up are comparable to those from other studies with the annular closure device. In addition to the current study, 3 controlled studies (1 randomized) have been conducted with the annular closure device.25,26,27 In a randomized trial of 60 patients, Cho and colleagues26 reported lower rates of symptomatic reherniation and reoperation (3.3% vs 20.0%) in patients who additionally received annular closure device after lumbar discectomy. In an 85-patient cohort by Barth et al,25 the rates were 2.2% vs 12.5% for symptomatic reherniation and 8.9% vs 12.5% for reoperation; and in a 102-patient study by Vukas et al,27 the rates were 0.0% vs 6.9% for both outcomes. The results of the current trial corroborate these previous reports yet provide unique information in 2 important ways. First, each of the prior controlled studies reported outcomes with the annular closure device through 2 years of follow-up compared with the 5-year follow-up results in the current report. Second, the sample sizes in previous studies were relatively small compared with 554 patients who were enrolled in the current trial. Ultimately, these findings suggest that the promising mid-term results observed in previous studies with annular closure are maintained over long-term follow-up.
Several considerations related to patient selection and long-term device integrity should be noted. In patients with small annular defects, there is no indication for annular closure. Moreover, in patients with low disc height owing to surgical access challenges or lumbar osteoporosis owing to possible device subsidence may also be poor candidates. Although the risk of osteoporosis is higher in older patients, older age is not a contraindication for treatment because older patients derive the same clinical benefit as younger patients with annular closure.28 It should also be noted that the presence of the device does not interfere with standard reoperation strategies should one be indicated in the future.29 Patients who successfully received the annular closure device and underwent reoperation were treated with similar reoperation techniques as patients in the control group. Additionally, there were no reported cases where the device complicated a reoperation procedure or altered the planned reoperative approach. Finally, radiographic changes of the vertebral end plate identified on CT were more commonly reported in patients treated with the annular closure device. The etiology of these changes remains speculative and may possibly be mechanical device-related effects, yet they appear to be clinically benign because the presence of end plate changes is not associated with inferior clinical outcomes or increased complication rates.30
Limitations
This study has several limitations. First, the results are generalizable only to patients with large defects in the annulus fibrosus following lumbar discectomy. Second, most patients and all investigators were aware of treatment assignment; therefore, it is possible that reoperation rates may have been influenced by performance bias. Third, patients in the trial were treated with limited lumbar discectomy with little to no removal of disc material within the intervertebral space.31 It is possible that lower reherniation rates could be achieved with aggressive disc resection, although intervertebral instability and spondylosis progression are potential risks with this surgical technique.32 Fourth, although end plate changes in the device group were associated with a benign clinical course through 5 years of follow-up, their natural history over longer term follow-up is currently unclear. Finally, although the 5-year follow-up visit rate of 73% is typical of long-term clinical trials of spinal devices, the potential for bias owing to missing data must be acknowledged.
Conclusions
In patients at high risk of herniation recurrence following lumbar microdiscectomy owing to a large defect in the annulus fibrosus, this study found that annular closure with a bone-anchored implant lowered the risk of symptomatic recurrence and reoperation over 5 years of follow-up. These finding suggest that implantation with an annular closure device represents a safe and durable preventative strategy in patients at high risk for lumbar disc reherniation following microdiscectomy.
Trial Protocol
Statistical Analysis Plan
eTable 1. List of Investigators and Participating Centers in the Annular Closure RCT Study Group
eTable 2. Inclusion and Exclusion Criteria
eTable 3. Study Assessments at Each Follow-up Interval
eTable 4. Reoperations by Type and Timeframe Through 5 Years
eTable 5. Serious Adverse Events Through 5 Years
eTable 6. Serious Device- and Procedure-related Adverse Events Through 5 Years
eTable 7. Any Adverse Events Through 5 Years
eTable 8. Any Device- and Procedure-related Adverse Events Through 5 Years
eFigure 1. Visual-Analogue Scale for Leg Pain Severity Over 5 Years Follow-up
eFigure 2. Oswestry Disability Index Score Over 5 Years Follow-up
eFigure 3. SF-36 Physical Component Summary Score Over 5 Years Follow-up
eFigure 4. SF-36 Mental Component Summary Score over 5 Years Follow-up
Data Sharing Statement
References
- 1.Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52(475):119-123. [PMC free article] [PubMed] [Google Scholar]
- 2.Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Lack of effectiveness of bed rest for sciatica. N Engl J Med. 1999;340(6):418-423. doi: 10.1056/NEJM199902113400602 [DOI] [PubMed] [Google Scholar]
- 3.Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S. A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976). 2006;31(6):653-657. doi: 10.1097/01.brs.0000203714.76250.68 [DOI] [PubMed] [Google Scholar]
- 4.Ambrossi GL, McGirt MJ, Sciubba DM, et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 2009;65(3):574-578. doi: 10.1227/01.NEU.0000350224.36213.F9 [DOI] [PubMed] [Google Scholar]
- 5.McGirt MJ, Eustacchio S, Varga P, et al. A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976). 2009;34(19):2044-2051. doi: 10.1097/BRS.0b013e3181b34a9a [DOI] [PubMed] [Google Scholar]
- 6.Miller LE, McGirt MJ, Garfin SR, Bono CM. Association of annular defect width after lumbar discectomy with risk of symptom recurrence and reoperation: systematic review and meta-analysis of comparative studies. Spine (Phila Pa 1976). 2018;43(5):E308-E315. doi: 10.1097/BRS.0000000000002501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdu RW, Abdu WA, Pearson AM, Zhao W, Lurie JD, Weinstein JN. Reoperation for recurrent intervertebral disc herniation in the spine patient outcomes research trial: analysis of rate, risk factors, and outcome. Spine (Phila Pa 1976). 2017;42(14):1106-1114. doi: 10.1097/BRS.0000000000002088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritzell P, Knutsson B, Sanden B, Strömqvist B, Hägg O. Recurrent versus primary lumbar disc herniation surgery: patient-reported outcomes in the Swedish Spine Register Swespine. Clin Orthop Relat Res. 2015;473(6):1978-1984. doi: 10.1007/s11999-014-3596-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klassen PD, Hsu WK, Martens F, et al. Post-lumbar discectomy reoperations that are associated with poor clinical and socioeconomic outcomes can be reduced through use of a novel annular closure device: results from a 2-year randomized controlled trial. Clinicoecon Outcomes Res. 2018;10:349-357. doi: 10.2147/CEOR.S164129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klassen PD, Hes R, Bouma GJ, et al. A multicenter, prospective, randomized study protocol to demonstrate the superiority of a bone-anchored prosthesis for anular closure used in conjunction with limited discectomy to limited discectomy alone for primary lumbar disc herniation. Int J Clin Trials. 2016;3:120-131. doi: 10.18203/2349-3259.ijct20162794 [DOI] [Google Scholar]
- 11.Thomé C, Klassen PD, Bouma GJ, et al. ; Annular Closure RCT Study Group . Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J. 2018;18(12):2278-2287. doi: 10.1016/j.spinee.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 12.Jensen MC, Kelly AP, Brant-Zawadzki MN. MRI of degenerative disease of the lumbar spine. Magn Reson Q. 1994;10(3):173-190. [PubMed] [Google Scholar]
- 13.Strömqvist B, Fritzell P, Hägg O, Jönsson B; Swedish Society of Spinal Surgeons . The Swedish Spine Register: development, design and utility. Eur Spine J. 2009;18(suppl 3):294-304. doi: 10.1007/s00586-009-1043-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72(1-2):95-97. doi: 10.1016/S0304-3959(97)00005-5 [DOI] [PubMed] [Google Scholar]
- 15.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160-164. doi: 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940-2952. doi: 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 17.Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19(4):593-607. doi: 10.1016/j.berh.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000;25(24):3130-3139. doi: 10.1097/00007632-200012150-00008 [DOI] [PubMed] [Google Scholar]
- 19.Berry DA. Interim analyses in clinical trials: classical vs. bayesian approaches. Stat Med. 1985;4(4):521-526. doi: 10.1002/sim.4780040412 [DOI] [PubMed] [Google Scholar]
- 20.Ammerman J, Watters WC, Inzana JA, Carragee G, Groff MW. Closing the treatment gap for lumbar disc herniation patients with large annular defects: a systematic review of techniques and outcomes in this high-risk population. Cureus. 2019;11(5):e4613. doi: 10.7759/cureus.4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KT, Park SW, Kim YB. Disc height and segmental motion as risk factors for recurrent lumbar disc herniation. Spine (Phila Pa 1976). 2009;34(24):2674-2678. doi: 10.1097/BRS.0b013e3181b4aaac [DOI] [PubMed] [Google Scholar]
- 22.Kienzler JC, Heidecke V, Assaker R, Fandino J, Barth M. Intraoperative findings, complications, and short-term results after lumbar microdiscectomy with or without implantation of annular closure device. Acta Neurochir (Wien). 2021;163(2):545-559. doi: 10.1007/s00701-020-04612-2 [DOI] [PubMed] [Google Scholar]
- 23.van den Brink W, Flüh C, Miller LE, Klassen PD, Bostelmann R. Lumbar disc reherniation prevention with a bone-anchored annular closure device: 1-year results of a randomized trial. Medicine (Baltimore). 2019;98(44):e17760. doi: 10.1097/MD.0000000000017760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kienzler JC, Klassen PD, Miller LE, et al. ; Annular Closure RCT Study Group . Three-year results from a randomized trial of lumbar discectomy with annulus fibrosus occlusion in patients at high risk for reherniation. Acta Neurochir (Wien). 2019;161(7):1389-1396. doi: 10.1007/s00701-019-03948-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barth M, Fontana J, Thomé C, Bouma GJ, Schmieder K. Occurrence of discal and non-discal changes after sequestrectomy alone versus sequestrectomy and implantation of an anulus closure device. J Clin Neurosci. 2016;34:288-293. doi: 10.1016/j.jocn.2016.09.013 [DOI] [PubMed] [Google Scholar]
- 26.Cho PG, Shin DA, Park SH, Ji GY. Efficacy of a novel annular closure device after lumbar discectomy in Korean patients: a 24-month follow-up of a randomized controlled trial. J Korean Neurosurg Soc. 2019;62(6):691-699. doi: 10.3340/jkns.2019.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vukas D, Ledić D, Grahovac G, Kolić Z, Rotim K, Vilendecić M. Clinical outcomes in patients after lumbar disk surgery with annular reinforcement device: two-year follow up. Acta Clin Croat. 2013;52(1):87-91. [PubMed] [Google Scholar]
- 28.Bouma GJ, Ardeshiri A, Miller LE, et al. Clinical performance of a bone-anchored annular closure device in older adults. Clin Interv Aging. 2019;14:1085-1094. doi: 10.2147/CIA.S208098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klassen PD, Lesage G, Miller LE, et al. Reoperation after primary lumbar discectomy with or without implantation of a bone-anchored annular closure device: surgical strategies and clinical outcomes. World Neurosurg. 2019;130:e926-e932. doi: 10.1016/j.wneu.2019.07.038 [DOI] [PubMed] [Google Scholar]
- 30.Kuršumović A, Bouma GJ, Miller LE, et al. Clinical implications of vertebral endplate disruptions after lumbar discectomy: 3-year results from a randomized trial of a bone-anchored annular closure device. J Pain Res. 2020;13:669-675. doi: 10.2147/JPR.S226480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spengler DM. Lumbar discectomy. results with limited disc excision and selective foraminotomy. Spine (Phila Pa 1976). 1982;7(6):604-607. doi: 10.1097/00007632-198211000-00015 [DOI] [PubMed] [Google Scholar]
- 32.Mochida J, Nishimura K, Nomura T, Toh E, Chiba M. The importance of preserving disc structure in surgical approaches to lumbar disc herniation. Spine (Phila Pa 1976). 1996;21(13):1556-1563. doi: 10.1097/00007632-199607010-00014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable 1. List of Investigators and Participating Centers in the Annular Closure RCT Study Group
eTable 2. Inclusion and Exclusion Criteria
eTable 3. Study Assessments at Each Follow-up Interval
eTable 4. Reoperations by Type and Timeframe Through 5 Years
eTable 5. Serious Adverse Events Through 5 Years
eTable 6. Serious Device- and Procedure-related Adverse Events Through 5 Years
eTable 7. Any Adverse Events Through 5 Years
eTable 8. Any Device- and Procedure-related Adverse Events Through 5 Years
eFigure 1. Visual-Analogue Scale for Leg Pain Severity Over 5 Years Follow-up
eFigure 2. Oswestry Disability Index Score Over 5 Years Follow-up
eFigure 3. SF-36 Physical Component Summary Score Over 5 Years Follow-up
eFigure 4. SF-36 Mental Component Summary Score over 5 Years Follow-up
Data Sharing Statement