Key Points
Question
Is annual residential exposure to particulate matter 2.5 μm or less in diameter (PM2.5) associated with neuroimaging diffusion markers of white matter microstructure?
Findings
This cross-sectional study of 7602 children 9 to 10 years of age found evidence of an association between PM2.5 exposure and hemispheric differences in white matter microstructure. In hemisphere-specific models, adjusted for confounding variables, statistically significant positive associations were observed between PM2.5 and restricted isotropic diffusion, and statistically significant negative associations were observed between PM2.5 and mean diffusivity.
Meaning
Findings from this study suggest that exposure to PM2.5 may be associated with differences in white matter microarchitecture, supporting a need for further improvements in air quality to protect the developing brain.
Abstract
Importance
Outdoor particulate matter 2.5 μm or less in diameter (PM2.5) is a ubiquitous environmental neurotoxicant that may affect the developing brain. Little is known about associations between PM2.5 and white matter connectivity.
Objectives
To assess associations between annual residential PM2.5 exposure and white matter microstructure health in a US sample of children 9 to 10 years of age and to examine whether associations are specific to certain white matter pathways or vary across neuroimaging diffusion markers reflective of intracellular and extracellular microstructural processes.
Design, Setting, and Participants
This cross-sectional study, the Adolescent Brain and Cognitive Development (ABCD) Study, was composed of 21 study sites across the US and used baseline data collected from children 9 to 10 years of age from September 1, 2016, to October 15, 2018. Data analysis was performed from September 15, 2020, to June 30, 2021.
Exposures
Annual mean PM2.5 exposure estimated by ensemble-based models and assigned to the primary residential addresses at baseline.
Main Outcomes and Measures
Diffusion-weighted imaging (DWI) and tractography were used to delineate white matter tracts. The biophysical modeling technique of restriction spectrum imaging (RSI) was implemented to examine total hindered diffusion and restricted isotropic and anisotropic intracellular diffusion in each tract. Hierarchical mixed-effects models with natural splines were used to analyze the associations between PM2.5 exposure and DWI.
Results
In a study population of 7602 children (mean [SD] age, 119.1 [7.42] months; 3955 [52.0%] female; 160 [ 21.%] Asian, 1025 [13.5%] Black, 1616 [21.3%] Hispanic, 4025 [52.9%] White, and 774 [10.2%] other [identified by parents as American Indian/Native American or Alaska Native; Native Hawaiian, Guamanian, Samoan, other Pacific Islander; Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, or other Asian; or other race]), associations were seen between annual ambient PM2.5 and hemispheric differences in white matter microstructure. Hemisphere-stratified models revealed significant associations between PM2.5 exposure and restricted isotropic intracellular diffusion in the left cingulum, in the left superior longitudinal fasciculus, and bilaterally in the fornix and uncinate fasciculus. In tracts with strong positive associations, a PM2.5 increase from 8 to 12 μg/m3 was associated with increases of 2.16% (95% CI, 0.49%-3.84%) in the left cingulum, 1.95% (95% CI, 0.43%-3.47%) in the left uncinate, and 1.68% (95% CI, 0.01%-3.34%) in the right uncinate. Widespread negative associations were observed between PM2.5 and mean diffusivity.
Conclusions and Relevance
The findings of this cross-sectional study suggest that annual mean PM2.5 exposure during childhood is associated with increased restricted isotropic diffusion and decreased mean diffusivity of specific white matter tracts, potentially reflecting differences in the composition of white matter microarchitecture.
This cross-sectional study of children 9 to 10 years of age assesses associations between exposure to annual ambient particulate matter 2.5 μm or less in diameter and white matter microarchitecture.
Introduction
Ambient airborne particulate matter is composed of suspended particles with an aerodynamic diameter of 2.5 μm or less (PM2.5).1 Long-term exposure to PM2.5 is reportedly associated with adverse nervous system effects.2 Animal studies3,4,5,6 have indicated that inhaled PM2.5 leads to neuroinflammation and oxidative stress, which may induce neuronal injury and affect glial support cells. Recent magnetic resonance imaging (MRI) studies have suggested an association of PM2.5 exposure with brain structure and volume,7,8 including white matter,9,10,11,12 which is primarily made up of myelinated axons and glial support cells.13
The potential impingement on key neurodevelopmental processes by PM2.5 exposure may cause lifelong health effects.14 Myelination and improved microstructural organization of white matter pathways continue throughout childhood and into young adulthood, ultimately allowing for improved signal transduction and communication between distal brain regions among cognitive and emotional systems.15,16,17 Studies have found that air pollution is associated with smaller white matter surface area in children,11 increases in myo-inositol, a brain metabolite involved in cell membrane and myelination,18 and reduced fractional anisotropy (FA).12,19 These studies11,12,18,19 are primarily based on smaller, localized populations, and results may have limited generalizability. Furthermore, exposure levels in these populations average above the current US Environmental Protection Agency standard of 12 μg/m3.3,9,20 Further research is warranted to examine the potential effects of exposure to levels of PM2.5 at or below regulatory standards across larger, more geographically diverse populations of children and using more advanced diffusion weighted imaging (DWI) techniques.
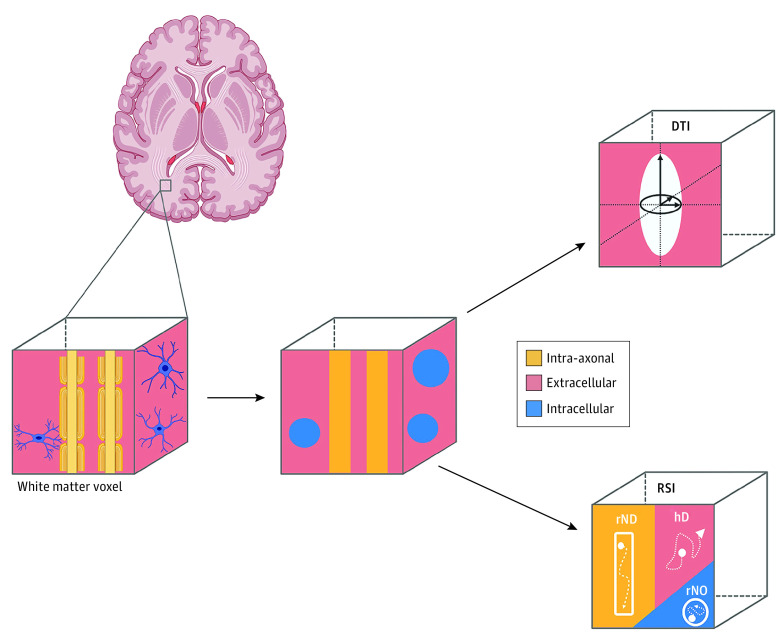
By modeling multishell high-angular resolution DWI data with a novel framework called restriction spectrum imaging (RSI), we aimed to characterize associations between ambient PM2.5 exposure and white matter microarchitecture in children 9 to 10 years of age from the Adolescent Brain Cognitive Development (ABCD) Study. Restriction spectrum imaging adopts a biophysical model that goes beyond conventional diffusion tensor imaging (DTI) techniques to distinguish different types of microstructural tissue compartments, including restricted water bounded by cell membranes (intracellular) and hindered water primarily within the extracellular space, where glial cell bodies and other neural processes increase the tortuosity of diffusion (Figure 1).22,23,24,25 Restriction spectrum imaging improves sensitivity and specificity in understanding tissue damage26 and normative changes in microstructural development.21,27 Given that particle pollution has been linked with impaired myelination and alteration of glial cells,28,29 we hypothesized that higher levels of ambient PM2.5 exposure would be associated with decreased restricted directional intracellular diffusion (rND) (eg, organized myelination) and increased restricted isotropic intracellular diffusion (rN0) (eg, glia and cell bodies). Previous work21 suggests a moderately positive correspondence between FA and rND and a large correspondence (in opposite directions) between mean diffusivity (MD) and rN0 in age-related white matter changes in early adolescence; thus, we hypothesized that PM2.5 exposure may be associated with decreased FA and MD. We also examined whether these associations varied by hemisphere and sex, given previous evidence of hemispheric and sex differences in associations between PM2.5 exposure and health outcomes.7,8,30,31,32,33,34
Figure 1. Diffusion-Weighted Imaging (DWI) Modeling Approaches.
Illustration of the biological components of white matter in an imaging voxel and schematic representation of the 2 diffusion tensor imaging (DTI) modeling approaches used in this study: DTI and restricted spectrum imaging (RSI). Diffusion tensor imaging measures extracellular water diffusion across a voxel. Primary DTI outcomes include fractional anisotropy (FA) and mean diffusivity (MD). Although the magnitude and direction of these outcomes allow for inferences to be made regarding axonal structure and integrity, DTI only allows for quantification of a single principal direction of diffusion and does not allow for characterization of the relative contribution of neurite orientations within a single voxel. In contrast, RSI is a biophysical model that allows for estimates of compartmentalized hindered and restricted water diffusion. Primary RSI outcomes include total hindered diffusion (hD) as well as restricted isotropic intracellular diffusion (rN0) and restricted directional intracellular diffusion (rND), which together provide greater insight into the biological properties of the microstructure of white matter tissue. The hindered compartment could encompass diffusion within intracellular spaces that allow for diffusion greater than the diffusion length scale (typically approximately 10 μm for human DTI). Given that rN0 and rND are normalized with respect to the hD compartments, changes in restricted compartments are relative to the other compartments. Previous studies using both common DTI and novel RSI metrics have shown similarities in directionality of rND and FA but opposite associations between rN0 and MD metrics in white matter during childhood.21 Created with BioRender.com.
Methods
Study Population
Data were obtained from baseline assessments (September 1, 2016, to October 15, 2018) of the ABCD Study (2020 National Institute of Mental Health Data Archive 3.0 data release), a cohort study of participants 9 to 10 years of age in the US.35,36,37 The ABCD Study implemented identical protocols for recruitment and neuroimaging of all participants at 21 study sites across the US.35,38,39,40,41,42,43 The primary inclusion criteria were age and English proficiency; exclusion criteria included severe sensory, intellectual, medical, or neurologic issues that would affect valid data collection (eMethods in the Supplement). Data analysis was performed from September 15, 2020, to June 30, 2021. Study sites obtained approval from their local institutional review boards, and centralized institutional review board approval was obtained from the University of California, San Diego. All parents or caregivers provided written informed consent; each child provided written assent. All data were deidentified before use. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.44
We further excluded participants with nonvalid addresses, low-quality or missing MRI, and incidental abnormal MRI findings (eMethods in the Supplement). Within-family nonindependence was managed by randomly including 1 sibling per family (eFigure 1 in the Supplement). The final analytic sample included 7602 participants (Table; eFigure 1 in the Supplement).
Table. Demographic Characteristics of the Final Study Data Set Compared With the Full Baseline ABCD Study Data Seta.
| Characteristic | Final data set (n = 7602) | Full data set (n = 11 884) | P valueb |
|---|---|---|---|
| Age, mean (SD) [range], mo | 119.1 (7.42) [107-133] | 119.0 (7.50) [107-133] | .30 |
| Familial relationships | |||
| Single | 5866 (77.2) | 7900 (66.5) | <.001 |
| Sibling | 810 (10.7) | 1810 (15.2) | |
| Twin | 916 (12) | 2138 (18.0) | |
| Triplet | 10 (0.1) | 30 (0.25) | |
| Sex | |||
| Male | 3955 (52.0) | 6196 (52.2) | .85 |
| Female | 3647 (48.0) | 5682 (47.8) | |
| Race and ethnicity | |||
| Asian | 160 (2.1) | 252 (2.12) | .03 |
| Black | 1025 (13.5) | 1784 (15.1) | |
| Hispanic | 1616 (21.3) | 2411 (20.3) | |
| White | 4025 (52.9) | 6182 (52.1) | |
| Otherc | 774 (10.2) | 1247 (10.5) | |
| Educational level | |||
| Less than HS diploma | 358 (4.7) | 593 (5.0) | .25 |
| HS diploma or GED | 676 (8.9) | 1132 (9.5) | |
| Some college | 1937 (25.5) | 3080 (26.0) | |
| Bachelor | 1938 (25.5) | 3015 (25.4) | |
| Postgraduate | 2685 (35.4) | 4044 (34.1) | |
| Family income, $ | |||
| <50 000 | 1976 (26.0) | 3224 (27.1) | .29 |
| ≥50 000 to <100 000 | 1987 (26.1) | 3071 (25.9) | |
| ≥100 000 | 2998 (39.4) | 4565 (38.4) | |
| Don’t know or refuse | 641 (8.4) | 1016 (8.6) | |
| Parents employment status | |||
| Working | 5315 (70.2) | 8218 (69.5) | .48 |
| Unemployed | 407 (5.4) | 674 (5.7) | |
| Other | 1847 (24.4) | 2930 (24.8) | |
| Handedness | |||
| Left | 527 (6.9) | 848 (7.1) | .37 |
| Right | 6097 (80.2) | 9429 (79.4) | |
| Ambidextrous | 978 (12.9) | 1601 (13.5) | |
| MRI manufacturer | |||
| GE Medical Systems | 1795 (24.0) | 2974 (25.7) | <.001 |
| Philips Medical Systems | 844 (11.3) | 1516 (13.1) | |
| Siemens | 4839 (64.7) | 7100 (61.3) | |
| Perceived neighborhood safety, mean (SD) [range] | 3.9 (0.97) [1.0-5.0] | 3.9 (0.98) [1.0-5.0] | .90 |
| Annual PM2.5, mean (SD) [range], μg/m3 | 7.66 (1.56) [1.72-15.90] | 7.66 (1.56) [1.72-15.90] | .83 |
| Motion [frame displacement], mean (SD) [range], mm | 1.26 (0.26) [0.55-2.00] | 1.39 (0.58) [0.55-16.14] | <.001 |
| Date range of MRI | 09/01/2016-10/15/2018 | 09/01/2016-10/15/2018 | NA |
Abbreviations: ABCD, Adolescent Brain Cognitive Development; GED, General Educational Development; HS, high school; MRI, magnetic resonance imaging; NA, not applicable.
Data are expressed as number (percentage) of participants unless otherwise indicated.
P value from the Pearson χ2 test comparing the distributions of categorical variables between the full ABCD Study baseline data set and the final analytic data set or P value from the analysis of variance test comparing means of continuous variables between the full ABCD Study baseline data set and the final analytic data set.
The “other” race and ethnicity category includes participants who were identified by their parents as American Indian/Native American or Alaska Native; Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, or other Asian; Native Hawaiian, Guamanian, Samoan, or other Pacific Islander; or other race.
Estimation of PM2.5 Exposure
The methods used to estimate residential PM2.5 exposure have been previously described.7 Daily estimates of hybrid spatiotemporal PM2.5 models were used to aggregate daily PM2.5 exposure estimates across the 2016 calendar year at a resolution of 1 km2. These annual mean values were assigned to participants’ addresses at the time of their baseline visits. The mean (SD) annual PM2.5 concentration across all sites was 7.66 (1.56) μg/m3 (range, 1.72-15.90 μg/m3) (eFigure 2 in the Supplement).
MRI Acquisition and Processing
The MRI data collection was harmonized across the 21 sites using 3 T scanners (Siemens Prisma, General Electric 750, Philips).39,45 The diffusion-weighted acquisition was conducted as previously described.45 After preprocessing of DWIs (eMethods in the Supplement), RSI was used to fit fiber orientation density functions to model rND, rN0, and total hindered diffusion (hD) (eg, primarily extracellular space around neurites).45 The DTI outcomes included FA and MD. Major white matter tracts were labeled with AtlasTrack using prior probabilities and orientation of long-range projection fibers.46 Probability estimates for each white matter tract were used to calculate weighted means of the RSI and DTI measures for all white matter fibers as well as key association, commissural, and projection fiber tracts,45 including the anterior thalamic radiations (ATR), cingulum in the cingulate gyrus (CGC), cingulum adjoining the hippocampus (CGH), corpus callosum (CC), corticospinal tract (CST), fornix (FX), uncinate fasciculus (UNC), inferior frontal occipital (IFO), inferior longitudinal fasciculus (ILF), and the superior longitudinal fasciculus (SLF).
Sensitivity Analysis
We conducted sensitivity analyses adjusting models for population density and proximity to major roadways. We also evaluated the addition of random slopes by ABCD Study site to investigate geographic variability in the associations between PM2.5 exposure and DWI outcomes. Finally, we tested a possible interaction between PM2.5 exposure and assigned sex at birth.
Statistical Analysis
We used hierarchical mixed-effects models with random intercepts by study site. Natural cubic splines for PM2.5 were fit with 2 knots at 7.05 and 8.31 μg/m3 derived from tertiles of exposure. We used an interaction term of PM2.5 × hemisphere and then fit hemisphere-stratified models (eMethods in the Supplement). All models were adjusted for covariates selected based on a directed acyclic graph, including sex, child’s age, parent-declared race and ethnicity, highest educational level of any household member, household income, parental employment status, a mean score of a 3-item assessment of parent perspectives of neighborhood safety, imaging device manufacturer, handedness, and motion artifact indexed by framewise displacement (eFigure 3 and eTable 3 in the Supplement). Analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing). An α = .05 was chosen, a priori, before any models were fit or analyzed as a threshold for significance. All reported P values are 1-sided.
Results
This cross-sectional study of 7602 children (mean [SD] age, 119.1 [7.42] months; 3955 [52.0%] female; 160 [ 21.%] Asian, 1025 [13.5%] Black, 1616 [21.3%] Hispanic, 4025 [52.9%] White, and 774 [10.2%] other [identified by parents as American Indian/Native American, Alaska Native, Native Hawaiian, Guamanian, Samoan, other Pacific Islander, Asian Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, other Asian, or other race]) suggests that significant associations, moderated by hemisphere, exist between annual mean ambient PM2.5 exposure and 2 measures of white matter microarchitecture.
RSI-Derived White Matter Microstructure
Significant PM2.5 × hemisphere interactions were observed for the association of PM2.5 and all 3 RSI-derived measures for multiple tracts and the all white matter fiber summary (rN0: marginal R2 = 0.11; conditional R2 = 0.96; P < .001; rND: marginal R2 = 0.47; conditional R2 = 0.97; P < .001; hD: marginal R2 = 0.27; conditional R2 = 0.97; P < .001) (eTable 4 in the Supplement).47 Hemisphere-stratified post hoc models revealed significant, nonlinear, positive associations between PM2.5 and rN0 in the left CGH (marginal R2 = 0.16; conditional R2 = 0.17; P = .002), UNC (marginal R2 = 0.06; conditional R2 = 0.10; P = .006), and FX (marginal R2 = 0.14; conditional R2 = 0.28; P = .006) and significant linear positive associations between PM2.5 and rN0 in the right UNC (marginal R2 = 0.06; conditional R2 = 0.13; P = .02), the right FX(marginal R2 = 0.16; conditional R2 = 0.26; P = .04), and the left SLF (marginal R2 = 0.11; conditional R2 = 0.14; P = .03) (Figure 2). Hemisphere-stratified models did not reveal associations between PM2.5 and rND (marginal R2 range, 0.16-0.48; conditional R2 range, 0.21-0.51; P = .14-.98) and hD (marginal R2 range, 0.08-0.34; conditional R2 range, 0.13-0.37; P = .09-.95).
Figure 2. Associations Between Particulate Matter 2.5 μm or Less in Diameter (PM2.5) Exposure and Restricted Isotropic Diffusion.
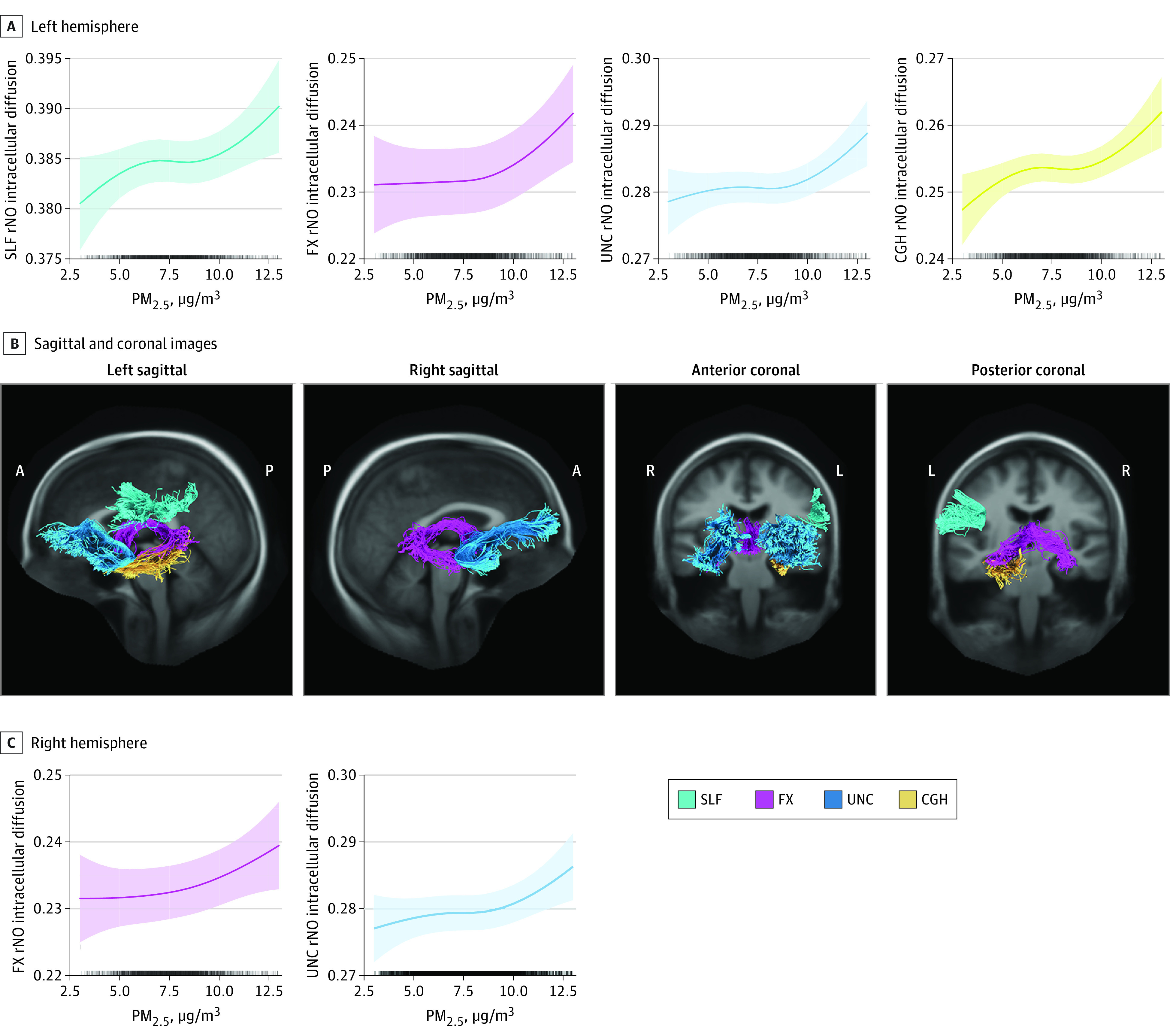
Annual mean PM2.5 exposure is associated with increases in restricted isotropic intracellular diffusion (rN0) microarchitecture in specific white matter tracts of the left or right hemisphere. Spline plots reflect model-predicted values of rN0 associated with annual mean PM2.5 exposure, with all other model covariates held constant. Sagittal and coronal images of relevant white matter tracts are provided for reference and colored to match spline plots. A indicates anterior; CGH, cingulum hippocampal portion; FX, fornix; L, left hemisphere; P, posterior; R, right hemisphere; SLF, superior longitudinal fasciculus; and UNC, uncinate fasciculus.
All models used the full exposure distribution of PM2.5 (1.72-15.90 μg/m3). For interpretability and quantification of associations, percent changes in outcomes associated with 4-μg/m3 increments of PM2.5 exposure were calculated using model-estimated predictions and SEs (eMethods in the Supplement). An increase in PM2.5 exposure from 4 to 8 μg/m3 was associated with percent increases that ranged from 0.25% (95% CI, −3.08% to 3.58%) to 1.44% (95% CI, −0.22% to 3.10%) in the 6 tracts evaluated (plus the all fiber summary), whereas an increase in PM2.5 exposure from 8 to 12 μg/m3 was associated with larger rN0 percent increases that ranged from 0.93% (95% CI, −0.10% to 1.97%) to 3.01% (95% CI, −0.39% to 6.40%) (eTable 5 in the Supplement). In tracts with strong positive associations, a PM2.5 increase from 8 to 12 μg/m3 was associated with increases of 2.16% (95% CI, 0.49%-3.84%) in the left cingulum, 1.95% (95% CI, 0.43%-3.47%) in the left uncinate, and 1.68% (95% CI, 0.01%-3.34%) in the right uncinate. Percent changes in rN0 according to household income and 6-month increases in age were estimated to contextualize the observed air pollution associations. In all models, age was included as a continuous variable with 1-month units, but for percent change calculations, 6-month increments were chosen as a reasonable time frame to capture developmental changes in white matter microstructure, given the 2-year age range of the study population. Increases in household income categories were not associated with rN0; percent changes ranged from −0.76% (95% CI, −2.4% to 0.61%) to 0.20% (95% CI, −1.17% to 1.60%); a 6-month increase in age was associated with percent changes in rN0 that ranged from 0.57% (95% CI, −2.44% to 3.58%) to 1.26% (95% CI, 0.11 to 2.41%) (eTable 5 in the Supplement).
DTI-Derived White Matter Microstructure
For mean MD and FA, significant PM2.5 × hemisphere interactions were observed for all tract estimates and the all white matter fiber summary (FA: marginal R2 = 0.64; conditional R2 = 0.98; P < .001; MD: marginal R2 = 0.52; conditional R2 = 0.98; P < .001) (eTable 6 in the Supplement). Hemisphere-stratified models did not reveal significant associations between PM2.5 and FA (marginal R2 range, 0.32-0.61; conditional R2 range, 0.37-0.68; P = .09-.82). Models revealed significant, nonlinear, negative associations between PM2.5 and MD in the left hemisphere all white matter fiber summary estimate (marginal R2 = 0.51; conditional R2 = 0.58; P = .003); the left ATR (marginal R2 = 0.52; conditional R2 = 0.61; P = .004), CGH (marginal R2 = 0.62; conditional R2 = 0.65; P < .001), FX (marginal R2 = 0.62; conditional R2 = 0.71; P < .001), SLF (marginal R2 = 0.21; conditional R2 = 0.24; P = .009), and UNC (marginal R2 = 0.36; conditional R2 = 0.41; P = .001); and the right ILF (marginal R2 = 0.30; conditional R2 = 0.33; P = .02), and UNC (marginal R2 = 0.43; conditional R2 = 0.50; P = .008). Linear negative associations were observed between PM2.5 and MD in the right hemisphere all white matter fiber summary estimate (marginal R2 = 0.51; conditional R2 = 0.57; P = .04), the left IFO (marginal R2 = 0.47; conditional R2 = 0.54; P = .02) and ILF (marginal R2 = 0.28; conditional R2 = 0.30; P = .02), and the right CGH (marginal R2 = 0.64; conditional R2 = 0.67; P = .046) and FX (marginal R2 = 0.63; conditional R2 = 0.71; P = .01) (Figure 3, eFigure 4 in the Supplement).
Figure 3. Associations Between Particulate Matter 2.5 μm or Less in Diameter (PM2.5) Exposure and Mean Diffusivity (MD).
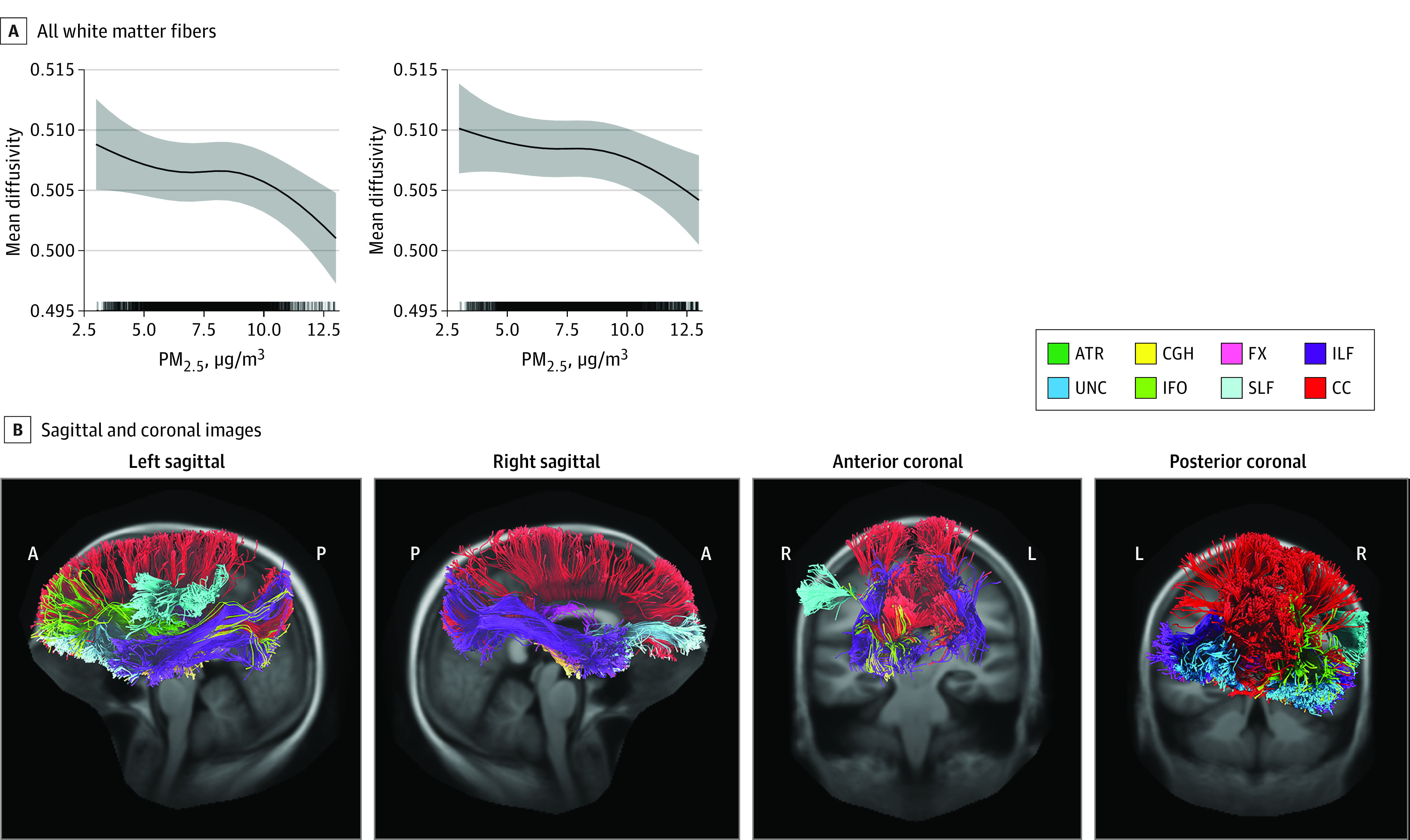
Annual mean PM2.5 exposure is associated with decreases in MD in all white matter fibers and 8 tracts of interest. Sagittal and coronal illustrations of relevant white matter tracts are provided for reference and colored to match spline plots (for details, see eFigure 4 in the Supplement). A indicates anterior; ATR, anterior thalamic radiations; CC, corpus callosum; CGH, cingulum hippocampal portion; FX, fornix; IFO, inferior fronto-occipital; ILF, inferior longitudinal fasciculus; L, left hemisphere; P, posterior; R, right hemisphere; SLF, superior longitudinal fasciculus; and UNC, uncinate.
Similar to rN0, more pronounced negative associations were observed with an exposure increase from 8 to 12 μg/m3, ranging from −1.06% (95% CI, −1.85% to −0.27%) to −0.56% (95% CI, −1.31% to 0.18%) compared with percent changes associated with lower levels exposure (ranging from −0.43% [95% CI, −1.13% to 0.27%] to −0.01% [95% CI, −0.77% to 0.74%]) (eTable 7 in the Supplement). A 6-month increase in age was associated with decreasing MD, ranging from −0.53% (95% CI, −1.02% to −0.03%) to −0.23% (95% CI, −1.31% to 0.84%). Increases in household income were not associated with MD; changes ranged from −0.13% (95% CI, −0.88% to 0.63%) to 0.30% (95% CI, −0.46% to 1.06%) (eTable 7 in the Supplement).
Sensitivity Analysis
The addition of 1 or both of our sensitivity covariates (distance to major roadways and population density) did not improve model fit or change associations. We did not see any meaningful variability in the random slopes across ABCD Study sites. In addition, no associations with sex were found for any outcomes.
Discussion
This cross-sectional analysis used data from a diverse cohort of 7602 children 9 to 10 years of age located at 21 geographically diverse locations across the US. Our objective was to characterize associations between annual ambient PM2.5 exposure and white matter microarchitecture. We found evidence of an interaction with hemisphere for nearly every white matter tract analyzed. In hemisphere-stratified models, higher PM2.5 exposure was associated with increased rN0 in 2 tracts in the left hemisphere only and bilaterally in 2 tracts. Higher exposure was associated with decreases in MD in 3 tracts in the left hemisphere only, bilaterally in 4 tracts, and in the corpus callosum. Significant hemisphere-specific associations were not observed between PM2.5 and rND, hD, or FA. These findings suggest that higher PM2.5 exposure is linked to increases in cellular barriers in white matter (reflected by decreases in MD), including increases in the isotropic compartment (reflected by increases in rN0), which may indicate changes to the cellular composition of key white matter tracts. To our knowledge, this is the first study to investigate how PM2.5 is associated with RSI-measured restricted and hindered water diffusion. The robustness of these findings is supported by strict quality control criteria for MRI inclusion, selection of covariates to control confounding, and sensitivity analyses (eMethods in the Supplement). Although the observed associations are small, it is plausible that repeated daily exposure to ambient PM2.5 across adolescence may have important implications for long-term neurophysiologic health outcomes of today’s youth.48
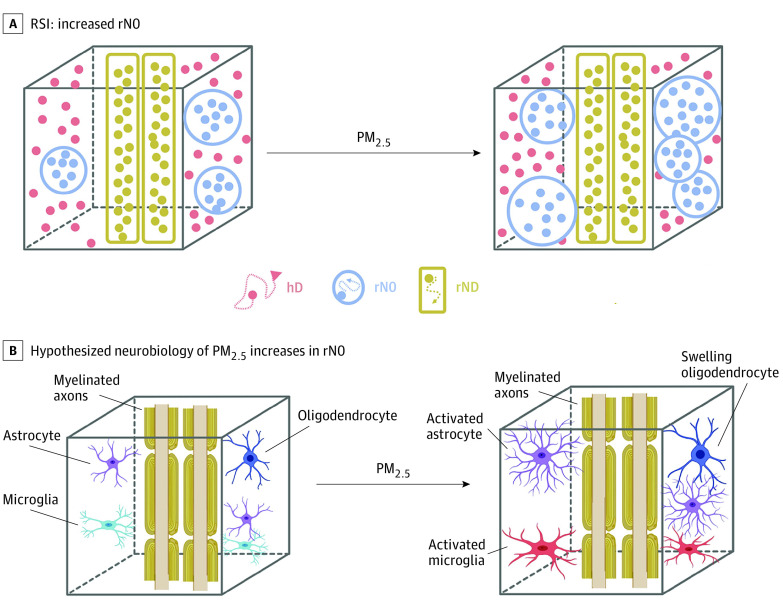
Our findings suggest that PM2.5 exposure may be associated with changes in intracellular microarchitecture of frontoparietal and limbic white matter circuitry, important for attention (SLF), emotional processing (UNC, CGH, and SLF), and memory (FX and SLF).49,50,51,52 Particle pollution may pass from the lungs into the bloodstream to infiltrate the blood brain barrier or create systematic secondary effects through inflammation.4 Microglia, the resident immune cells of the central nervous system, respond to pollutants and cause inflammatory activation.28 Activated microglia in animal and cell models exhibit increases in somal size compared with resting microglia.53 The rN0 reflects diffusion bound within cell membranes in spherical structures less than approximately 10 μm or within multiple cylindrical structures oriented such that diffusion is occurring equally in all directions. Therefore, our findings of increased rN0 may reflect differences in the number or size of glial cells (eg, oligodendrocytes, oligodendrocyte precursor cells, astrocytes, and/or microglia) in white matter tracts (Figure 4).13 Because increases in the restricted signal fraction along white matter tracts have been associated with normative development in the ABCD Study cohort, it is unclear to what extent these microstructural associations with PM2.5 reflect an acceleration of developmental processes vs an inflammatory response.21 A clinical study54 examining rN0 in patients with Parkinson disease found a 9.09% increase in rN0 in the bilateral thalamus of patients compared with healthy controls, pointing to the possibility that rN0 changes may reflect mechanisms underlying symptom origins. Further experimental animal studies using both cellular and neuroimaging techniques are warranted to better understand the implications of white matter microstructural changes.
Figure 4. Hypothesized Neurobiological Mechanisms Associated With Increased Restricted Isotropic Diffusion.
Hypothesized neurobiological underpinning of particulate matter 2.5 μm or less in diameter (PM2.5)–associated increase in restricted isotropic intracellular diffusion (rN0). Given that rN0 represents diffusion within cells, it is hypothesized that increases of rN0 in white matter at 9 to 10 years may reflect an increase in the size or number of support and glial cells in response to exposure to PM2.5. An overall increase or change in cell numbers within a given white matter region could also contribute to overall decreases in mean diffusivity as measured by diffusion tensor imaging. hD indicates total hindered diffusion; rND, restricted directional intracellular diffusion; RSI, restriction spectrum imaging.
Previous research21 has found that rND and FA tend to show similar patterns, whereas rN0 and MD are inversely associated in white matter tracts during early adolescence. Given these known patterns, the negative associations between PM2.5 and MD observed here suggest an increased barrier to water diffusion, which is congruent with potential increases in the number or size of support cells. Given that MD quantifies the magnitude of diffusion, whereas FA depends on the overall directionality of diffusion within a voxel,55 our findings suggest that PM2.5 may be increasing the number of cellular boundaries but not changing cellular processes that contribute to unidirectional water diffusion, such as axonal organization and/or myelination. Moreover, given the known associations between FA and rND metrics, a lack of association between PM2.5 and these 2 markers corroborates this conclusion.
Despite congruent findings between DTI and RSI outcomes, the notable negative associations between PM2.5 and MD in the current study contrast with a previous positive association noted in a cohort of children 9 to 10 years of age from Rotterdam, the Netherlands.9 PM2.5 composition varies by geographic location, and previous evidence2,56 suggests that certain PM2.5 components are differentially detrimental to health, which may explain these contrasting findings. Participants from the previous study9 were also exposed to much higher overall levels of PM2.5 exposure (mean, 16.5 μg/m3), which may also contribute to this discrepancy because higher levels of exposure may lead to more severe cellular or myelin disruption (reflected by increases in MD). Finally, the current study and the study by Lubczyńska et al9 used different diffusion-MRI acquisition parameters; the previous study9 used a single shell DTI sequence, whereas the current study used a multishell high-angular resolution DWI sequence with various b values, allowing for increased sensitivity and specificity.21,57
Although the exact mechanisms underlying central nervous system asymmetries remain unknown,58 structural and functional differences have been noted between the 2 cerebral hemispheres at the macroscopic, microstructural, and molecular levels.59 A previous study58 of asymmetry in neurologic disorders suggests that typical asymmetries develop between the hemispheres, which may ultimately result in greater hemispheric differences in vulnerability to brain pathologic conditions. A previous study58 suggests that brain asymmetries occur via differences in functional genetic pathways of microtubule regulation, neurogenesis, and axonogenesis, which are involved in neuronal development and organization and the manifestation of hemispheric differences in gene expression. Thus, these asymmetries in brain structure and function may contribute to the hemisphere-specific patterns observed in this study.
Limitations
This study has limitations. Because of limits in the ABCD Study air pollution data available in the 3.0 release, participants experienced varying time lags between their air pollution exposure estimation (2016) and MRI at the baseline study visit (2016-2018); an assumption was therefore made that the spatial distribution of air pollution estimates remained stable during this 2-year period. This assumption is supported by previous research that indicates that the spatial distribution of estimates of annual mean air pollution concentrations (using the current estimation methods) remained relatively stable in the US between 2008 and 2016.60 Future data releases from the ABCD Study are expected to contain full lifetime histories of air pollution exposure, which will allow for more temporal precision in cross-sectional analyses and longitudinal investigations.
Ambient outdoor PM2.5 exposure at a primary residence does not provide a full picture of a child’s yearly air pollution exposure. Data on indoor air pollution, school air pollution, and time at the residence, although not currently available, would further clarify how PM2.5 exposure is associated with white matter connectivity. Similarly, despite efforts to account for confounding in our analyses, it is possible that unmeasured confounders and residual confounding have introduced biases in the associations reported here.
In addition, this study was limited to PM2.5 exposure, yet other types of air pollution, including nitric dioxide, may affect the morphological features and development of children’s brains and their mental health.9,19,61 In previous studies9,19 with multipollutant analyses, single pollutant associations tended to become weakened by the addition of 1 of more pollutants; thus, the possibility exists that the PM2.5 associations reported here are somewhat biased and inflated by the inclusion of only a single air pollutant in our models. When data become available for the ABCD Study population, future analyses are planned to elucidate potential associations among ambient nitric dioxide, ozone, PM2.5 components, and DWI outcomes.
Conclusions
To our knowledge, this was the first multisite US study to find associations between annual PM2.5 exposure and white matter microarchitecture. Most of the study population experienced PM2.5 exposure at or below 12 μg/m3, which is within US Environmental Protection Agency standards. These findings have important public health implications, given the ubiquity of PM2.5 exposure and its potential effects on white matter connectivity in children across the US.
eMethods. Supplemental Methods
eFigure 1. Flowchart of Final Sample
eFigure 2. Distribution of PM2.5 Annual Average Exposure by ABCD Site
eFigure 3. Directed Acyclic Graph
eFigure 4. Associations Between PM2.5 Exposure and Mean Diffusivity
eTable 1. Description of Covariates Used in Statistical Analyses
eTable 2. Comparison of Population Characteristics Across Data sets
eTable 3. Distributions of Population Characteristics at Baseline in Relation to Annual Average PM2.5
eTable 4. PM2.5-by-Hemisphere Analyses for RSI Outcomes
eTable 5. Percent Change in RSI rN0 Across Values of PM2.5 and Sociodemographic Characteristics for Significant Hemisphere-Specific Models
eTable 6. PM2.5-by-Hemisphere Analyses for DTI Outcomes
eTable 7. Percent Change in DTI MD Across Values of PM2.5 and Sociodemographic Characteristics for Significant Hemisphere-Specific Models
References
- 1.US Environmental Protection Agency. Integrated science assessment (ISA) for particulate matter: final report (December 2009). 2009. Accessed April 16, 2021. https://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=494959
- 2.US Environmental Protection Agency. Integrated science assessment (ISA) for particulate matter: final report (December 2019). 2019. Accessed April 16, 2021. https://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=539935
- 3.Babadjouni R, Patel A, Liu Q, et al. Nanoparticulate matter exposure results in neuroinflammatory changes in the corpus callosum. PLoS One. 2018;13(11):e0206934. doi: 10.1371/journal.pone.0206934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol. 2012;2012:782462. doi: 10.1155/2012/782462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Huang Y, Zhang F, et al. Macrophages treated with particulate matter PM2.5 induce selective neurotoxicity through glutaminase-mediated glutamate generation. J Neurochem. 2015;134(2):315-326. doi: 10.1111/jnc.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’Toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. 2016;119(11):1204-1214. doi: 10.1161/CIRCRESAHA.116.309279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cserbik D, Chen JC, McConnell R, et al. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int. 2020;143:105933-105933. doi: 10.1016/j.envint.2020.105933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guxens M, Lubczyńska MJ, Muetzel RL, et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol Psychiatry. 2018;84(4):295-303. doi: 10.1016/j.biopsych.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 9.Lubczyńska MJ, Muetzel RL, El Marroun H, et al. Exposure to air pollution during pregnancy and childhood, and white matter microstructure in preadolescents. Environ Health Perspect. 2020;128(2):27005. doi: 10.1289/EHP4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortamais M, Pujol J, Martínez-Vilavella G, et al. Effects of prenatal exposure to particulate matter air pollution on corpus callosum and behavioral problems in children. Environ Res. 2019;178:108734. doi: 10.1016/j.envres.2019.108734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson BS, Rauh VA, Bansal R, et al. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry. 2015;72(6):531-540. doi: 10.1001/jamapsychiatry.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujol J, Fenoll R, Macià D, et al. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 2016;6(6):e00467. doi: 10.1002/brb3.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walhovd KB, Johansen-Berg H, Káradóttir RT. Unraveling the secrets of white matter—bridging the gap between cellular, animal and human imaging studies. Neuroscience. 2014;276:2-13. doi: 10.1016/j.neuroscience.2014.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl Neurosci. 2016;7(1):24-30. doi: 10.1515/tnsci-2016-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Islam R, Kaffman A. White-matter repair as a novel therapeutic target for early adversity. Front Neurosci. 2021;15(657693):657693. doi: 10.3389/fnins.2021.657693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebel C, Treit S, Beaulieu C. A review of diffusion MRI of typical white matter development from early childhood to young adulthood. NMR Biomed. 2019;32(4):e3778. doi: 10.1002/nbm.3778 [DOI] [PubMed] [Google Scholar]
- 17.Yakovlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A. Regional Development of the Brain in Early Life. Blackwell Scientific; 1967:3-70. [Google Scholar]
- 18.Brunst KJ, Ryan PH, Altaye M, et al. Myo-inositol mediates the effects of traffic-related air pollution on generalized anxiety symptoms at age 12 years. Environ Res. 2019;175:71-78. doi: 10.1016/j.envres.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubczyńska MJ, Muetzel RL, El Marroun H, et al. Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ Res. 2021;198:110446. doi: 10.1016/j.envres.2020.110446 [DOI] [PubMed] [Google Scholar]
- 20.US Environmental Protection Agency . National primary ambient air quality standards for PM2.5. Accessed February 15, 2021. https://www.law.cornell.edu/cfr/text/40/50.18
- 21.Palmer CE, Pecheva D, Iversen J, et al. Microstructural development across white matter from 9-13 years. bioRxiv. Preprint posted online June 2021. doi: 10.1101/2021.06.04.447102 [DOI]
- 22.White NS, Leergaard TB, D’Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum Brain Mapp. 2013;34(2):327-346. doi: 10.1002/hbm.21454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White NS, McDonald C, Farid N, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014;74(17):4638-4652. doi: 10.1158/0008-5472.CAN-13-3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White NS, McDonald CR, Farid N, Kuperman JM, Kesari S, Dale AM. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol. 2013;34(5):958-964, S951. doi: 10.3174/ajnr.A3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferizi U, Schneider T, Witzel T, et al. White matter compartment models for in vivo diffusion MRI at 300mT/m. Neuroimage. 2015;118:468-483. doi: 10.1016/j.neuroimage.2015.06.027 [DOI] [PubMed] [Google Scholar]
- 26.McDonald CR, White NS, Farid N, et al. Recovery of white matter tracts in regions of peritumoral FLAIR hyperintensity with use of restriction spectrum imaging. AJNR Am J Neuroradiol. 2013;34(6):1157-1163. doi: 10.3174/ajnr.A3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer CE, Pecheva D, Iversen J, et al. Spatially heterogeneous microstructural development within subcortical regions from 9-13 years. bioRxiv. Preprint posted online June 2021. doi: 10.1101/2021.06.04.446984 [DOI]
- 28.Gómez-Budia M, Konttinen H, Saveleva L, et al. Glial smog: Interplay between air pollution and astrocyte-microglia interactions. Neurochem Int. 2020;136:104715. doi: 10.1016/j.neuint.2020.104715 [DOI] [PubMed] [Google Scholar]
- 29.Klocke C, Allen JL, Sobolewski M, Blum JL, Zelikoff JT, Cory-Slechta DA. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology. 2018;65:196-206. doi: 10.1016/j.neuro.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38-46. doi: 10.1016/j.brainres.2010.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonekamp D, Nagae LM, Degaonkar M, et al. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733-742. doi: 10.1016/j.neuroimage.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Büchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14(9):945-951. doi: 10.1093/cercor/bhh055 [DOI] [PubMed] [Google Scholar]
- 33.Butter ME. Are women more vulnerable to environmental pollution? J Hum Ecol. 2006;3:221-226. doi: 10.1080/09709274.2006.11905931 [DOI] [Google Scholar]
- 34.Peterson D, Mahajan R, Crocetti D, Mejia A, Mostofsky S. Left-hemispheric microstructural abnormalities in children with high-functioning autism spectrum disorder. Autism Res. 2015;8(1):61-72. doi: 10.1002/aur.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garavan H, Bartsch H, Conway K, et al. Recruiting the ABCD sample: design considerations and procedures. Dev Cogn Neurosci. 2018;32:16-22. doi: 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jernigan TL, Brown SA, Dowling GJ. The Adolescent Brain Cognitive Development Study. J Res Adolesc. 2018;28(1):154-156. doi: 10.1111/jora.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Koob GF, Croyle RT, et al. The conception of the ABCD study: from substance use to a broad NIH collaboration. Dev Cogn Neurosci. 2018;32:4-7. doi: 10.1016/j.dcn.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bagot KS, Matthews SA, Mason M, et al. Current, future and potential use of mobile and wearable technologies and social media data in the ABCD study to increase understanding of contributors to child health. Dev Cogn Neurosci. 2018;32:121-129. doi: 10.1016/j.dcn.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casey BJ, Cannonier T, Conley MI, et al. ; ABCD Imaging Acquisition Workgroup . The Adolescent Brain Cognitive Development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018;32:43-54. doi: 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feldstein Ewing SW, Chang L, Cottler LB, Tapert SF, Dowling GJ, Brown SA. Approaching retention within the ABCD Study. Dev Cogn Neurosci. 2018;32:130-137. doi: 10.1016/j.dcn.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagler DJ, Hatton SN, Makowski C, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luciana M, Bjork JM, Nagel BJ, et al. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018;32:67-79. doi: 10.1016/j.dcn.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uban KA, Horton MK, Jacobus J, et al. ; Adolescent Brain Cognitive Development Study . Biospecimens and the ABCD study: rationale, methods of collection, measurement and early data. Dev Cogn Neurosci. 2018;32:97-106. doi: 10.1016/j.dcn.2018.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):e296. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagler DJ Jr, Hatton S, Cornejo MD, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019;202:116091. doi: 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagler DJ Jr, Ahmadi ME, Kuperman J, et al. Automated white-matter tractography using a probabilistic diffusion tensor atlas: application to temporal lobe epilepsy. Hum Brain Mapp. 2009;30(5):1535-1547. doi: 10.1002/hbm.20619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 48.Funder DC, Ozer, DJ. Evaluating effect size in psychological research: sense and nonsense. Adv Methods Pract Psychol Sci. 2019;2:156-168. doi: 10.1177/2515245919847202 [DOI] [Google Scholar]
- 49.Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev. 2018;92:104-127. doi: 10.1016/j.neubiorev.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakajima R, Kinoshita M, Shinohara H, Nakada M. The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav. 2020;14(6):2817-2830. doi: 10.1007/s11682-019-00187-4 [DOI] [PubMed] [Google Scholar]
- 51.Senova S, Fomenko A, Gondard E, Lozano AM. Anatomy and function of the fornix in the context of its potential as a therapeutic target. J Neurol Neurosurg Psychiatry. 2020;91(5):547-559. doi: 10.1136/jnnp-2019-322375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(Pt 6):1692-1707. doi: 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019;94:112-120. doi: 10.1016/j.semcdb.2019.05.004 [DOI] [PubMed] [Google Scholar]
- 54.Hope TR, Selnes P, Rektorová I, et al. Diffusion tensor and restriction spectrum imaging reflect different aspects of neurodegeneration in Parkinson’s disease. PLoS One. 2019;14(5):e0217922-e0217922. doi: 10.1371/journal.pone.0217922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316-329. doi: 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect. 2012;120(12):1699-1704. doi: 10.1289/ehp.1205201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mori S, Tournier JD. Moving beyond DTI: high angular resolution diffusion imaging (HARDI). In: Introduction to Diffusion Tensor Imaging and Higher Order Models. 2nd ed. Academic Press; 2014:65-78. [Google Scholar]
- 58.Lubben N, Ensink E, Coetzee GA, Labrie V. The enigma and implications of brain hemispheric asymmetry in neurodegenerative diseases. Brain Commun. 2021;3(3):b211. doi: 10.1093/braincomms/fcab211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ocklenburg S. Laterality. In: Della Sala S, ed. Encyclopedia of Behavioral Neuroscience. 2nd ed.. Elsevier; 2022:350-356. doi: 10.1016/B978-0-12-819641-0.00043-8 [DOI] [Google Scholar]
- 60.Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environ Int. 2019;130:104909. doi: 10.1016/j.envint.2019.104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sram RJ, Veleminsky M Jr, Veleminsky M Sr, Stejskalová J. The impact of air pollution to central nervous system in children and adults. Neuro Endocrinol Lett. 2017;38(6):389-396. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Flowchart of Final Sample
eFigure 2. Distribution of PM2.5 Annual Average Exposure by ABCD Site
eFigure 3. Directed Acyclic Graph
eFigure 4. Associations Between PM2.5 Exposure and Mean Diffusivity
eTable 1. Description of Covariates Used in Statistical Analyses
eTable 2. Comparison of Population Characteristics Across Data sets
eTable 3. Distributions of Population Characteristics at Baseline in Relation to Annual Average PM2.5
eTable 4. PM2.5-by-Hemisphere Analyses for RSI Outcomes
eTable 5. Percent Change in RSI rN0 Across Values of PM2.5 and Sociodemographic Characteristics for Significant Hemisphere-Specific Models
eTable 6. PM2.5-by-Hemisphere Analyses for DTI Outcomes
eTable 7. Percent Change in DTI MD Across Values of PM2.5 and Sociodemographic Characteristics for Significant Hemisphere-Specific Models