Abstract
Purpose
This study aimed to evaluate the contribution of vitamin A dimerization to retinal pigment epithelium (RPE) atrophic changes. Leading causes of irreversible blindness, including Stargardt disease and age-related macular degeneration (AMD), occur as a result of atrophic changes in RPE. The cause of the RPE atrophic changes is not apparent. During the vitamin A cycle, vitamin A dimerizes, leading to vitamin A cycle byproducts, such as vitamin A dimers, in the RPE.
Methods
To study the consequence of vitamin A dimerization to RPE atrophic changes, we used a rodent model with accelerated vitamin A dimerization, Abca4−/−/Rdh8−/− mice, and the vitamin A analog C20D3-vitamin A to selectively ameliorate the accelerated rate of vitamin A dimerization.
Results
We show that ameliorating the rate of vitamin A dimerization with C20D3-vitamin A mitigates pathological changes observed in the prodromal phase of the most prevalent retinal degenerative diseases, including fundus autofluorescence changes, dark adaptation delays, and signature RPE atrophic changes.
Conclusions
Data demonstrate that the dimerization of vitamin A during the vitamin A cycle is sufficient alone to cause the prerequisite RPE atrophic changes thought to be responsible for the leading causes of irreversible blindness and that correcting the dimerization rate with C20D3-vitamin A may be sufficient to prevent the RPE atrophic changes.
Translational Relevance
Preventing the dimerization of vitamin A with the vitamin A analog C20D3-vitamin A may be sufficient to alter the clinical course of the most prevalent forms of blindness, including Stargardt disease and age-related macular degeneration (AMD).
Keywords: Stargardt disease, AMD, vitamin A
Introduction
According to histopathological analysis, the most prevalent retinal diseases, including age-related macular degeneration (AMD) and Stargardt disease, occur as a result of atrophic changes in the retinal pigment epithelium (RPE).1–8 These atrophic changes include a filling of the RPE with lipofuscin, RPE attenuation, hypertrophy, hyperplasia, migration and atrophy, pigment clumping, and RPE detachment, herein termed RPE atrophic changes. The above pathological changes are followed by the formation of retinal atrophic lesions, which are responsible for loss of vision. The early development of RPE abnormalities has led to the theory that the above retinal degenerations result primarily from a disease of the RPE.9
The cause of the above RPE pathology is not apparent. Constitutive opsin signaling,10 increased retinaldehyde flux,11 oxidative stress,12 the misfolded protein response,13 complement activation,14–16 inflammation, autophagy dysfunction,17 Alu RNA accumulation,18 a thickened Bruch's membrane (BM), thinned choriocapillaris, lipofuscin, blocked choroidal vessels,19 drusen, and environmental factors such as diet, smoking, and sunlight, to name a few, have been argued to contribute to the development of Stargardt disease and/or AMD.
During the vitamin A cycle, vitamin A reacts with another molecule of vitamin A to form a dimer. These vitamin A dimers can be transformed into oxidative catabolites20–26 and react with additional vitamin A molecules to generate higher-order oligomers,27,28 together termed vitamin A cycle byproducts (VAB) (Fig. 1). Vitamin A dimerizes at an accelerated rate in Stargardt disease and presumably after the seventh decade of life, when similar concentrations of VAB are estimated in the human eye.29 We hypothesized that the dimerization of vitamin A is responsible for the RPE atrophic changes that lead to retinal atrophic lesions and vision loss in retinal diseases marked by the formation of lipofuscin, such as AMD and Stargardt disease.
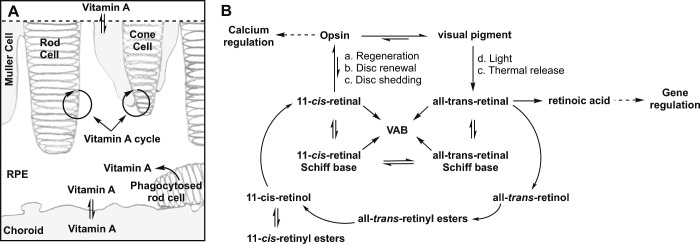
Figure 1.
The Dimerization of Vitamin A During the Vitamin A Cycle. (A) Vitamin A is a descriptor for retinoids that exhibit the biological activity of retinol. The retina transforms vitamin A between several congeners, all-trans-retinol, all-trans-retinyl esters, all-trans-retinaldehyde, 11-cis-retinol, 11-cis-retinyl esters, 11-cis-retinaldehyde, and retinoic acid, as it shuttles these congeners throughout its compartments. These translocations, isomerizations, oxidations, and reductions are collectively referred to as the vitamin A cycle. Vitamin A enters or exits the retina through exchanges between the RPE with choroid capillaries and the inner retina with retinal blood vessels (double arrows). Cycles between rod cells and the RPE enable rod vision, while cycles between cone and Muller cells enable cone vision. The recycling of 11-cis-retinaldehyde from phagocytosed photoreceptors and the integration of 11-cis-retinaldehyde into newly synthesized photoreceptors is also part of the vitamin A cycle. (B) The vitamin A cycle is driven by (a) the regeneration of visual pigments after bleaching, (b) photoreceptor disc renewal or synthesis (c), photoreceptor disc shedding, (d) visual pigment bleaching with light, and (e) thermal isomerization of visual pigment. As depicted, the vitamin A cycle regulates calcium homeostasis via opsin signaling and gene transcription via retinoic acid production. Vitamin A cycle byproducts (VAB) form from the reaction of a cis or trans-retinaldehyde-Schiff base with another molecule(s) of cis or trans retinaldehyde. Any primary amine in the retina can participate in Schiff base formation and catalyze the dimerization of vitamin A. Retinal: Retinaldehyde.
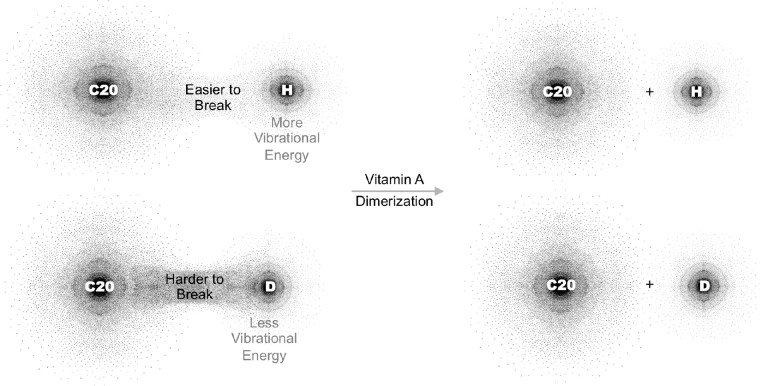
We have shown that the rate-limited step in the dimerization of vitamin A is abstraction of a proton at carbon number 20 of vitamin A.30 Selective deuterium enrichment at carbon number 20 results in a kinetic isotope effect that increases the energy required for proton abstraction and thus the dimerization of vitamin A (Fig. 2). Replacing the retina's vitamin A pool with C20D3-vitamin A thus results in a reduced rate of vitamin A dimerization and its resulting byproducts.
Figure 2.
C20D3-Vitamin A Prevents the Dimerization of Vitamin A. Shown, a C20 carbon-hydrogen bound of vitamin A at natural isotopic abundance relative to a C20 carbon-hydrogen bound of C20D3-vitamin A. To dimerize, a C20 carbon-hydrogen bond on vitamin A must be broken. Enriching the C20 carbon-hydrogen bonds (H) with deuterium (D) makes the bonds harder to break and the dimerization of vitamin A more difficult.
C20D3-vitamin A ameliorates the dimerization of vitamin without slowing or inhibiting the vitamin A cycle, altering the concentration of vitamin A congeners, or inhibiting normal retina function.30–32 Otherwise, inhibiting the delivery of vitamin A to the retina or the vitamin A cycle precipitates retinal denegation. Consequently, C20D3-vitamin A is a clinically amenable therapeutic strategy to correct the aberrant dimerization of vitamin A, which occurs in several retinal conditions.
We have administered C20D3-vitamin A to swine, mice, rats, and mouse models of Stargardt disease, Abca4−/− mice.30–33 In Abca4−/− mice, C20D3-vitamin A corrected the accelerated rate of vitamin A dimerization down to the rate observed in unaffected, wild-type mice. This rate correction correlated with a reduction in changes typically observed in the prodromal phase of human retinal degeneration, notably inflammation, the accumulation of lipofuscin in the RPE, complement activation, age-related declines in electroretinography (ERG) amplitudes, and delayed dark adaptation. However, Abca4−/− mice show limited RPE pathology. Thus, whether ameliorating the dimerization of vitamin A is sufficient alone to mitigate the signature RPE pathology has been disputed.34–38
To elucidate the contribution of inhibiting the dimerization of vitamin A to RPE pathology, we administered C20D3-vitamin A to mice with abolished activity in both the Abca4 and Rdh8 genes (Abca4−/−/Rdh8−/− double knockout mice).39 The rate of vitamin A dimerization is accelerated in these double-knockout mice relative to Abca4−/− mice. Our data suggest that the dimerization of vitamin A is sufficient alone to explain the signature RPE atrophic changes observed in retinal diseases marked by lipofuscin formation and that mitigating the rate at which vitamin A dimerizes with C20D3-vitamin A may halt the development of RPE atrophic changes.
Materials and Methods
Interventions
Animal protocols were approved by Columbia University's Institutional Animal Care and Use Committee. Animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The Abca4−/−/Rdh8−/− mice were obtained from Case Western Reserve University.39 The lighting in the vivarium was ≥0.05 µmol.s−1.m−2 at the cage floor and ≈3 µmol.s−1.m−2 in the room. To modulate the accelerated dimerization of vitamin A, mice were provided standard rodent diets containing vitamin A as either retinyl acetate (Sigma Aldrich, St. Louis, MO, USA) or C20D3-retinyl acetate (Alkeus Pharmaceuticals, Somerville, MA, USA), both at 20,000 IU/kg, from weaning, for up to 18 months of age. Land O'Lakes, Inc. (Arden Hills, MN, USA) formulated the diets as reported.32 Animals were divided into the two cohorts at random. Each cohort contained approximately half male and half female. Fundus autofluorescence, quantification of qVAB, and electroretinography were performed.31,33,40,41
Histology
Eyes were removed and fixed in 10% formaldehyde. HistoWiz Inc. (Brooklyn, NY, USA) performed the histology. Eyes were sliced through the optic nerve. For each eye, eight or more cross-sections were placed on one slide for analysis. Slides were imaged at 40x magnification. For retinal thickness, we measured 1 mm from either side of the optic nerve head. For each eye, measurements from at least three slices were averaged to give the retinal layer thickness per eye. Measurements for each eye were then averaged to give the final retinal layer thickness per experimental cohort. The investigator was blinded to the group allocation when assessing the outcome.
Optical Coherence Tomography (OCT)
Images were acquired with a Spectralis OCT Angiography Module (Heidelberg Engineering GmbH, Heidelberg, Germany), during a retrospective chart review of patients with a clinical diagnosis of AMD or Stargardt disease. Columbia University's Institutional Review Board approved the retrospective analysis of anonymized data, and the research adhered to the Declaration of Helsinki.
Statistics
Values are shown as mean ± SEM or SD. A p-value of less than 0.05 was considered significant as calculated by a 2-sided, unpaired, t- or F-test, where appropriate. For comparisons of retinyl thickness, P values are from unpaired t-tests, at 12- and 18-month points, relative to baseline (3 months of age). Statistical significance was determined using the Holm-Sidak method, with α = 5%. Computations assume that all rows are samples from populations with the same scatter. For population proportions, we used two-tailed, Fisher's exact test, using 2 × 2 contingency tables and the method of summing small P values. Statistical analyses were performed with GraphPad Prism.
Results
C20D3-Vitamin A Ameliorates the Accelerated Dimerization of Vitamin A
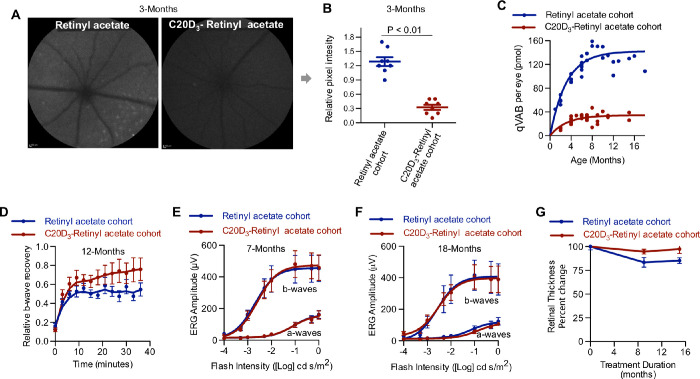
Fundus autofluorescence—observed upon 488 nm excitation and 500 to 680 nm emission—reflects the concentration of VAB and has been used as an indicator of retinal aging.42 As such, we used quantitative autofluorescence imaging to estimate the relative concentrations of VAB in the above cohorts of mice. At three months of age, the C20D3-retinyl acetate cohort's retinas were visibly less autofluorescent compared to the retinyl acetate cohort. When quantified, the C20D3-retinyl acetate cohort showed, on average, 75% less autofluorescence compared to the control cohort administered vitamin A as retinyl acetate (Figs. 3A, 3B).
Figure 3.
C20D3-Vitamin A Prevents the Prodromal Phase of Retinal Degenerations. (A) Representative quantitative fundus autofluorescence (AF) images of 3-month-old Abca4−/−/Rdh8−/− mice administered a diet containing vitamin A as either retinyl acetate or C20D3-retinyl acetate. (B) Average (with SEM) AF intensity of the retinas of mice described in panel A. Each spot corresponds to the AF intensity of a single eye. Eight eyes of eight different animals per cohort were imaged (n = 8). P-value is from a two-sided, unpaired T-test. (C) The qVAB (A2E, iso-A2E, and oxo-A2E) in mice described in panel A. Each point represents between 5 and 10 pooled eyes. (D) Average (SEM) ERG b-wave recoveries following light exposure in ∼12-month-old Abca4−/−/Rdh8−/− mice administered a diet containing retinyl acetate (n = 13 eyes, blue curve, recovered a mean and SD of 71% ± 0.7% after 30 minutes of dark-adapted maximum b-wave) or C20D3-retinyl acetate (n = 12 eyes, red curve, recovered 53 ± 0.5%, P = 0.01, two-sided F-test). (E and F) ERG dose-response curves (average with SEM) for the cohorts of dark-adapted Abca4−/−/Rdh8−/− mice described in panel A at seven months (E, retinyl acetate: n = 16 eyes; C20D3-retinyl acetate: n = 19 eyes) and 18 months of age (F, retinyl acetate: n = 12 eyes; C20D3-retinyl acetate: n = 12 eyes). (G) Percent change in retinal thickness (RPE and neuroretina) from baseline (3 months of age) in mice described in panel A, measured 1 mm from either side of the optic nerve head. Averages and SEM are shown. C20D3-retinyl acetate: n = 6 at three months, n = 14 at 12 months, and n = 4 at 18 months. Retinyl acetate: n = 5 at 3 months, n = 5 at 12 months, and n = 10 at 18 months. Each eye was from a different animal.
Because the structures of the majority of the VAB remain to be determined, to further quantify changes in VAB we quantified the amounts of three characterized VABs: N-retinylidene-N-retinylethanolamine (A2E), its geometric isomer iso-A2E, and a major oxidative metabolite of A2E, oxo-A2E, altogether denoted here as “quantifiable VAB” or qVAB. Because qVAB can be found in the neural retina, RPE,43,44 and Bruch's membrane (BM),45 we measured qVAB in whole eyes periodically from between one month and 18 months of age for animals in each cohort. We found that the C20D3-retinyl acetate cohort had, on average, 85% less qVAB compared to the retinyl acetate cohort at all ages measured (Fig. 3C), in agreement with the reduction in fundus autofluorescence.
C20D3-Vitamin A Prevents Prodromal Changes
Delayed dark adaptation has been proposed as a functional biomarker to predict the likelihood of progressing from early to late AMD.46 We thus measured the recovery of ERG b-wave amplitudes after exposing animals to light. For 12-month-old animals in the C20D3-retinyl acetate cohort, b-waves recovered up to 71% ± 0.7% of their initial values 30 minutes after exposure to light. However, the retinyl acetate cohort recovered 53% ± 0.5% of their initial values, giving a 34% improvement in the C20D3-retinyl acetate cohort (Fig. 3D). Simultaneously, we did not measure significant age-related declines in maximum, dark-adapted, ERG a- and b-wave amplitudes in mice between three months and 18 months of age (Figs. 3E, 3F). This indicated that declines in dark adaptation were not due to attenuated maximum a- and b-wave amplitudes. We observed for both cohorts, on average, ≈25% and ≈11% decreases in maximum, dark-adapted ERG a- and b-wave amplitudes, respectively, between seven and 18 months of age. But these decreases were not statistically significant (P ≥ 0.05).
C20D3-Vitamin A Prevents RPE Atrophic Changes
C20D3-vitamin A intervention prevented age-related reductions in retinyl thickness (Fig. 3G). At 12 months of age, retinal thickness was reduced by 17% in the retinyl acetate cohort and 5% in the C20D3-retinyl acetate cohort, for a difference with a standard error of 11% ± 4% (P = 0.02). At 18 months of age, retinal thickness was reduced by 15% in the retinyl acetate cohort and 3% in the C20D3-retinyl acetate cohort, for a difference with a standard error of 12% ± 5% (P = 0.03). The average thickness of outer nuclear layer (ONL), outer segment (OS), and inner nuclear layer (INL) was also thicker in the C20D3-retinyl acetate cohort relative to the control cohort, but this difference was not statically significant (Supplementary Fig. S1). The lack of significant changes in the thickness of the neuroretina was in agreement with a lack of a significant decline in dark-adapted maximum ERG a- and b-wave amplitudes.
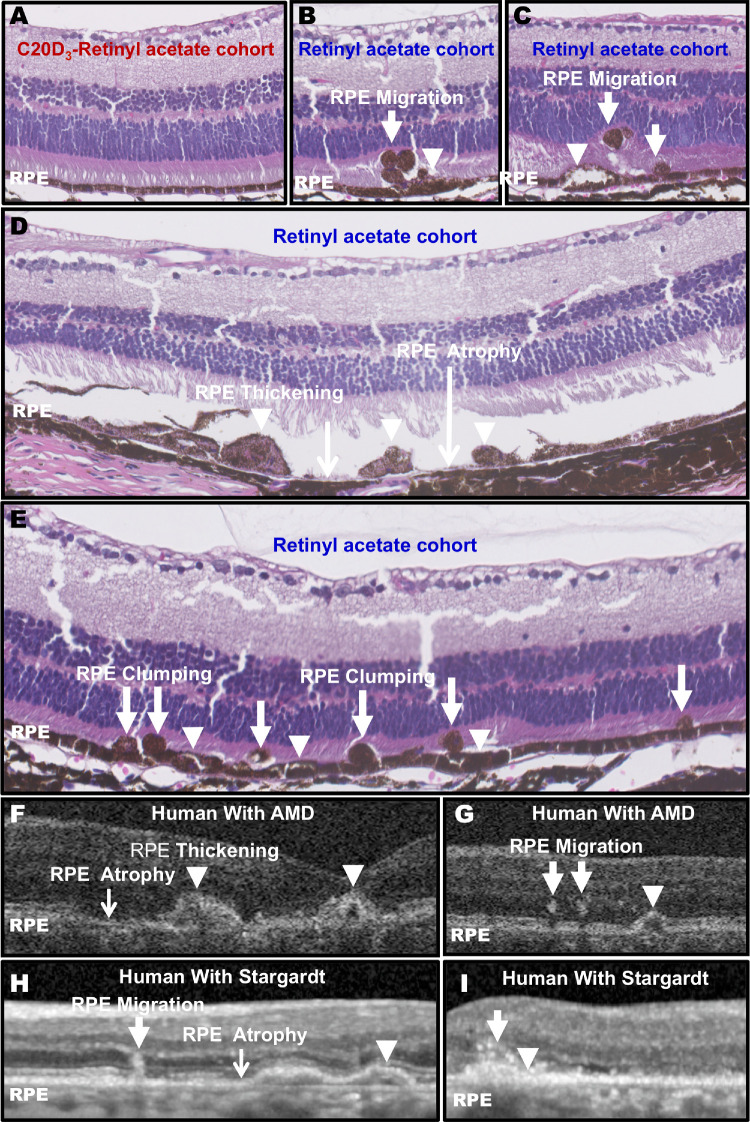
By 12 months of age, animals in the retinyl acetate cohort began to display focal RPE atrophic changes (Figs. 4B–D). After 13 months of age, all animals in the retinyl acetate cohort (n = 16 eyes from eight animals) showed focal areas of enlarged RPE cells, RPE clumping, and RPE migrating into the subretinal space and/or neural retina. Morphology in areas away from the focal RPE atrophic changes remained grossly normal (Supplementary Fig. S2). The focal RPE pathology was similar to what is observed in humans with AMD and Stargardt by OCT (Figs. 4F, 4G, Supplementary Fig. S2). No RPE pathology was observed in any 18-month-old animals in the C20D3-retinyl acetate cohort (n = 12 eyes from six animals) (Fig. 4A). The difference in histology, 16 of 16 eyes (100%) showing RPE abnormalities in the retinyl acetate cohort and 0 out of 12 eyes (0%) with RPE abnormalities in the C20D3-retinyl acetate cohort, was statistically significant (P < 0.0001).
Figure 4.
C20D3-Vitamin A Prevents the Signature RPE Atrophic Changes. (A) Representative hematoxylin and eosin (H&E) cross-section of the C20D3-retinyl acetate cohort at 18 months of age. Histology was performed on n = 12 eyes of 12 mice between 13 and 18 months of age. No RPE pathology was observed in the C20D3-retinyl acetate cohort at any age. (B–E) Representative H&E cross-sections of 18-month-old Abca4−/−/Rdh8−/− animals administered vitamin A as retinyl acetate. Each panel represents a slice from a different eye. RPE migration into the neuroretinal space (arrows), RPE swelling (arrowheads), RPE clumping, or regions of missing or atrophic RPE (black arrows) were observed. Histology was performed on n = 16 eyes of 16 mice between 13 and 18 months of age. All eyes displayed areas of RPE pathology after 13 months of age. (F–I) Representative OTC images of human AMD (F, G) and Stargardt disease (H, I) show similar RPE atrophic changes. Each image is from a different eye.
Discussion
Abca4 −/−/Rdh8−/− mice developed delayed dark adaptation and focal RPE pathology without a decrease in ERG a- and b-wave amplitudes and otherwise limited retinal histopathology. These observations are similar to humans in the prodromal phase of retinal degeneration secondary to AMD and Stargardt, where RPE atrophic changes can be observed by OCT and delayed dark adaptation can be measured by electrophysiology or psychologically. Progression to advanced stages of retinal degeneration, such as overt choroidal retinal atrophy or choroidal neovascularization, was not observed in these mice. However, these animals have a fraction of VAB compared to humans.33 The VAB presumably did not form in sufficient amounts to cause the advanced disease.
Replacing dietary vitamin A with C20D3-vitamin A retarded the rate of vitamin A dimerization as measured by the decreased concentrations of the resulting VAB, which prevented increased fundus autofluorescence, dark adaptation delays, and signature RPE atrophic changes. From these observations—and because the administration of C20D3-retinyl acetate did not correct the genetic defects—we can conclude that the genetic defects were not sufficient alone to cause the above changes. Instead, the above changes are best explained by the accelerated rate of vitamin A dimerization.
It has been argued that retinaldehyde is responsible for retinal pathology in this Abca4−/−/Rdh8−/− mouse model.37,39 Based on this argument, it was further argued that dimerization of vitamin A would protect the retina from retinaldehyde. C20D3-vitamin A inhibits the dimerization of vitamin A without altering retinaldehyde concentrations, yet C20D3-vitamin A prevents the development of retinal pathology. From these observations, it can be concluded that retinaldehyde does not play a role in RPE pathology relative to the dimerization of vitamin A. This conclusion is in accord with our prior in vitro work showing that isolated RPE cells can tolerate large amounts of retinaldehyde but not A2E47 and our animal work with C20D3-retinyl acetate in wild-type, Abca4−/− pigmented, and Abca4−/− albino mice.30–32
Pathological changes in the choriocapillaris and BM might precede RPE abnormalities.48 As others and we have shown, byproducts of the vitamin A cycle can damage the choriocapillaris49,50 and are located in the BM.45 Whether the vitamin A byproducts first damage the choriocapillaris or BM, which cause the RPE abnormalities, or first damage the RPE, which cause choriocapillaris or BM damage, or damage all three structures concurrently is not clear. Because the choriocapillaris, BM, and RPE are interdependent, it would be challenging to show damage to one and not the other.
In humans, sub-RPE deposits, such as drusen, are correlated with RPE abnormalities. We observed the RPE abnormalities in the absence of sub-RPE deposits, indicating that the sub-RPE deposits are not prerequisites for the RPE abnormalities. This conclusion is in accord with sub-RPE deposits originating from excreted RPE debris.51 Because sub-RPE deposits perturb the RPE, and RPE abnormalities are typically seen over sub-RPE deposits,1 the additional presence of sub-RPE deposits likely contributes to RPE abnormalities.
There are changes observed in AMD, such as drusen, linear deposits, and choroidal neovascularization that are typically not observed in the juvenile retinal dystrophies, such as Stargardt disease. Retinopathy induced by the accelerated vitamin A dimerization would mean that disease heterogeneity results from the body's reaction to the accelerated dimerization. In Stargardt disease, vitamin A dimerizes abnormally in the young retina. In AMD, vitamin A dimerizes abnormally in the aged retina. The differences observed between the two diseases presumably result from the age of the eye in which the vitamin A cycle byproducts form. The molecular derangements observed during Stargardt disease and AMD would be best explained by an individual's responses to the dimerization of vitamin A or the dimerization byproducts: as with all poisons, individuals would show a range of responses, dictated by genetic and environmental factors.
We show that the accelerated dimerization of vitamin A is sufficient to cause the RPE atrophic changes thought to be a prerequisite to atrophic lesions and vision loss in human Stargardt and AMD, presumably by chronically poisoning the RPE, BM, or choriocapillaris with dimerization byproducts. Data indicate that preventing the dimerization of vitamin A with C20D3-vitamin A may be sufficient to mitigate the development of RPE atrophic changes to alter the clinical course of these conditions.
Supplementary Material
Acknowledgments
Supported by the US National Institutes of Health, National Eye Institute (grant number 1R01EY021207), Research to Prevent Blindness (RPB) Inc., New York, NY, and The BrightFocus Foundation, Clarksburg, MD.
Disclosure: D. Zhang, None; K. Robinson, None; I. Washington, IW is an inventor on patents disclosing methods to prevent retinal degeneration
References
- 1. Sarks JP, Sarks SH, Killingsworth MC.. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond). 1988; 2(Pt 5): 552–577. [DOI] [PubMed] [Google Scholar]
- 2. Ferris FL 3rd, Fine SL, Hyman L.. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102: 1640–1642. [DOI] [PubMed] [Google Scholar]
- 3. Bertolotto M, Borgia L, Iester M.. Hyperautofluorescence in outer retinal layers thinning. BioMed Res Int. 2014; 2014: 741538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bird A. Role of retinal pigment epithelium in age-related macular disease: A systematic review. Br J Ophthalmol. 2021; 105: 1469–1474. [DOI] [PubMed] [Google Scholar]
- 5. Birnbach CD, Jarvelainen M, Possin DE, Milam AH.. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994; 101: 1211–1219. [DOI] [PubMed] [Google Scholar]
- 6. Eagle RC Jr., Lucier AC, Bernardino VB Jr., Yanoff M. Retinal pigment epithelial abnormalities in fundus flavimaculatus: A light and electron microscopic study. Ophthalmology. 1980; 87: 1189–1200. [DOI] [PubMed] [Google Scholar]
- 7. Sarks SH. Ageing and degeneration in the macular region: A clinico-pathological study. Br J Ophthalmol. 1976; 60: 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972; 76: 64–80. [PubMed] [Google Scholar]
- 9. Young RW. Pathophysiology of age-related macular degeneration. Surv Ophthalmol. 1987; 31: 291–306. [DOI] [PubMed] [Google Scholar]
- 10. Lem J, Fain GL.. Constitutive opsin signaling: Night blindness or retinal degeneration? Trends Mol Med. 2004; 10: 150–157. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Okano K, Maeda T, et al.. Mechanism of all-trans-retinal toxicity with implications for Stargardt disease and age-related macular degeneration. J Biol Chem. 2012; 287: 5059–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piccardi M, Fadda A, Martelli F, et al.. Antioxidant saffron and central retinal function in ABCA4-related Stargardt macular dystrophy. Nutrients. 2019; 11: 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Q, Sabirzhanova I, Bergbower EAS, Yanda M, Guggino WG, Cebotaru L. The CFTR Corrector, VX-809 (Lumacaftor), rescues ABCA4 trafficking mutants: A potential treatment for Stargardt disease. Cell Physiol Biochem. 2019; 53: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu J, Pauer GJ, Hagstrom SA, et al.. Evidence of complement dysregulation in outer retina of Stargardt disease donor eyes. Redox Biol. 2020; 37: 101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mullins RF, Schoo DP, Sohn EH, et al.. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am J Pathol. 2014; 184: 3142–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Radu RA, Hu J, Yuan Q, et al.. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J Biol Chem. 2011; 286: 18593–18601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC.. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017; 8: e2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarallo V, Hirano Y, Gelfand BD, et al.. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012; 149: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997; 124: 677–682. [DOI] [PubMed] [Google Scholar]
- 20. Ueda K, Zhao J, Kim HJ, Sparrow JR.. Photodegradation of retinal bisretinoids in mouse models and implications for macular degeneration. Proc Natl Acad Sci USA. 2016; 113: 6904–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K.. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed Engl. 2002; 41: 814–817. [DOI] [PubMed] [Google Scholar]
- 22. Washington I, Turro NJ, Nakanishi K.. Superoxidation of retinoic acid. Photochem Photobiol. 2006; 82: 1394–1397. [DOI] [PubMed] [Google Scholar]
- 23. Washington I, Jockusch S, Itagaki Y, Turro NJ, Nakanishi K.. Superoxidation of bisretinoids. Angew Chem Int Ed Engl. 2005; 44: 7097–7100. [DOI] [PubMed] [Google Scholar]
- 24. Yoon KD, Yamamoto K, Ueda K, Zhou J, Sparrow JR.. A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PloS One. 2012; 7: e41309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR.. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc Natl Acad Sci USA. 2010; 107: 7275–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Keller LM, Dillon J, Gaillard ER.. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem Photobiol. 2006; 82: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 27. Murdaugh LS, Mandal S, Dill AE, Dillon J, Simon JD, Gaillard ER.. Compositional studies of human RPE lipofuscin: Mechanisms of molecular modifications. J Mass Spectrom. 2011; 46: 90–95. [DOI] [PubMed] [Google Scholar]
- 28. Murdaugh LS, Avalle LB, Mandal S, et al.. Compositional studies of human RPE lipofuscin. J Mass Spectrom. 2010; 45: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 29. Burke TR, Duncker T, Woods RL, et al.. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2014; 55: 2841–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaufman Y, Ma L, Washington I.. Deuterium enrichment of vitamin A at the C20 position slows the formation of detrimental vitamin A dimers in wild-type rodents. J Biol Chem. 2011; 286: 7958–7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma L, Kaufman Y, Zhang J, Washington I.. C20-D3-vitamin A slows lipofuscin accumulation and electrophysiological retinal degeneration in a mouse model of Stargardt disease. J Biol Chem. 2011; 286: 7966–7974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Charbel Issa P, Barnard AR, Herrmann P, Washington I, MacLaren RE. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Natl Acad Sci USA. 2015; 112: 8415–8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang D, Robinson K, Saad L, Washington I.. Vitamin A cycle byproducts impede dark adaptation. J Biol Chem. 2021; 297: 101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ablonczy Z, Higbee D, Anderson DM, et al.. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013; 54: 5535–5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rudolf M, Vogt SD, Curcio CA, et al.. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013; 120: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wielgus AR, Chignell CF, Ceger P, Roberts JE.. Comparison of A2E cytotoxicity and phototoxicity with all-trans-retinal in human retinal pigment epithelial cells. Photochem Photobiol. 2010; 86: 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maeda A, Maeda T, Golczak M, et al.. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 2009; 284: 15173–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts JE, Kukielczak BM, Hu DN, et al.. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem Photobiol. 2002; 75: 184–190. [DOI] [PubMed] [Google Scholar]
- 39. Maeda A, Maeda T, Golczak M, Palczewski K.. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008; 283: 26684–26693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sparrow JR, Blonska A, Flynn E, et al.. Quantitative fundus autofluorescence in mice: correlation with HPLC quantitation of RPE lipofuscin and measurement of retina outer nuclear layer thickness. Invest Ophthalmol Vis Sci. 2013; 54: 2812–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Washington I, Zhou J, Jockusch S, Turro NJ, Nakanishi K, Sparrow JR.. Chlorophyll derivatives as visual pigments for super vision in the red. Photochem Photobiol Sci. 2007; 6: 775–779. [DOI] [PubMed] [Google Scholar]
- 42. Gliem M, Muller PL, Finger RP, McGuinness MB, Holz FG, Charbel Issa P. Quantitative fundus autofluorescence in early and intermediate age-related macular degeneration. JAMA Ophthalmol. 2016; 134: 817–824. [DOI] [PubMed] [Google Scholar]
- 43. Eldred GE, Lasky MR.. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993; 361: 724–726. [DOI] [PubMed] [Google Scholar]
- 44. Katz ML, Gao CL, Rice LM.. Formation of lipofuscin-like fluorophores by reaction of retinal with photoreceptor outer segments and liposomes. Mech Ageing Dev. 1996; 92: 159–174. [DOI] [PubMed] [Google Scholar]
- 45. Murdaugh LS, Wang Z, Del Priore LV, Dillon J, Gaillard ER.. Age-related accumulation of 3-nitrotyrosine and nitro-A2E in human Bruch's membrane. Exp Eye Res. 2010; 90: 564–571. [DOI] [PubMed] [Google Scholar]
- 46. Owsley C, McGwin G Jr., Clark ME, et al.. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016; 123: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mihai DM, Washington I.. Vitamin A dimers trigger the protracted death of retinal pigment epithelium cells. Cell Death Dis. 2014; 5: e1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U.. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014; 35: 2562–2573. [DOI] [PubMed] [Google Scholar]
- 49. Penn J, Mihai DM, Washington I.. Morphological and physiological retinal degeneration induced by intravenous delivery of vitamin A dimers in rabbits. Dis Model Mech. 2015; 8: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iriyama A, Inoue Y, Takahashi H, Tamaki Y, Jang WD, Yanagi Y.. A2E, a component of lipofuscin, is pro-angiogenic in vivo. J Cell Physiol. 2009; 220: 469–475. [DOI] [PubMed] [Google Scholar]
- 51. Meyer A. Zur Entstehung der geschichteten Drusen der Lamina vitrea chorioideae. Graefes Arch Ophthalmol. 1877; 23: 159–171. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.