Abstract
Objective:
Negative symptoms of schizophrenia are substantially disabling and treatment resistant. Novel treatments like repetitive transcranial magnetic stimulation (TMS) need to be examined for the same using the experimental medicine approach that incorporates tests of mechanism of action in addition to clinical efficacy in trials.
Methods:
Study was a double-blind, parallel, randomized, sham-controlled trial recruiting schizophrenia with at least a moderate severity of negative symptoms. Participants were randomized to real or sham intermittent theta burst stimulation (iTBS) under MRI-guided neuro-navigation, targeting the cerebellar vermis area VII-B, at a stimulus intensity of 100% active motor threshold, two sessions/day for five days (total = 6000 pulses). Assessments were conducted at baseline (T0), day-6 (T1) and week-6 (T2) after initiation of intervention. Main outcomes were, a) Scale for the Assessment of Negative Symptoms (SANS) score (T0, T1, T2), b) fronto-cerebellar resting state functional connectivity (RSFC) (T0, T1).
Results:
Thirty participants were recruited in each arm. Negative symptoms improved in both arms (p < 0.001) but was not significantly different between the two arms (p = 0.602). RSFC significantly increased between the cerebellar vermis and the right inferior frontal gyrus (pcluster-FWER = 0.033), right pallidum (pcluster-FWER = 0.042) and right frontal pole (pcluster-FWER = 0.047) in the real arm with no change in the sham arm.
Conclusion:
Cerebellar vermal iTBS engaged a target belonging to the class of cerebello-subcortical-cortical networks, implicated in negative symptoms of schizophrenia. However, this did not translate to a superior clinical efficacy. Future trials should employ enhanced midline cerebellar TMS stimulation parameters for longer durations that can potentiate and translate biological changes into clinical effects.
Keywords: Cerebellar vermis, Schizophrenia, Transcranial magnetic stimulation, Randomized controlled trial, Negative symptoms, Resting state functional connectivity
1. Introduction
Schizophrenia is a severely disabling medical disorder that continues to stand among the top 20 causes of disability worldwide (James et al., 2018) with a greater impact in middle-income countries (Charlson et al., 2018). Compared to delusions and hallucinations, which are more likely to remit with antipsychotic treatment (Aleman et al., 2017; Carbon and Correll, 2014; Green, 2016) negative symptoms and cognitive impairments are persistent, difficult-to-treat and strongly contribute to disability (Bhagyavathi et al., 2015; Green, 2016; Strassnig et al., 2015). The treatment of negative symptoms remains a major challenge for the field, especially since a broad range of interventions have yielded mixed results with minimal clinical utility (Fusar-Poli et al., 2015; Helfer et al., 2016; Saavedra-Velez et al., 2009; Singh and Singh, 2011; Tsapakis et al., 2015). An experimental medicine approach that combines clinical efficacy trials with tests of mechanism of action is a promising initiative that can deliver reliable and reproducible clinical benefits (Insel, 2015).
Repetitive transcranial magnetic stimulation (rTMS) alters behavior on the basis of neural network modulation and when combined with functional magnetic resonance imaging (fMRI), can be a potential neuroscience-informed treatment (Fox et al., 2014; McClintock et al., 2011; Pascual-Leone et al., 2011; Shafi et al., 2012) for persistent negative symptoms. Given the critical role of the prefrontal cortex in negative symptoms (Wolkin et al., 1992), most studies have examined the effects of high-frequency rTMS targeted to the dorsolateral prefrontal cortex (DLPFC). Although this method holds some promise for the treatment of negative symptoms, the aggregate evidence for its efficacy has been modest at best (Aleman et al., 2018; He et al., 2017; Kennedy et al., 2018; Osoegawa et al., 2018) emphasizing the necessity to explore novel target areas in the brain. Cognitive dysmetria or poor mental coordination is an integrative theory of schizophrenia characterized by the fundamental cognitive deficit of an inability to effectively coordinate information processing (Andreasen et al., 1998). This function has been pinned down to the dysfunction of cortical-subcortical-cerebellar circuitry (Andreasen et al., 1998, 1996). This dysfunction has been implicated to translate into diverse symptoms of schizophrenia including negative symptoms (Andreasen et al., 1999; Honey et al., 2005). Many recent functional connectivity studies also imply this network in the brain as a central substrate underlying negative symptoms of schizophrenia (Brady et al., 2019; Gao et al., 2019). Also given the role of the cerebellum in cognition (Allen, 1997), emotion (Buckner, 2013; Schmahmann, 2019), motivation and social behaviors (Carta et al., 2019), it could be a potentially important target for improving negative symptoms through neuromodulation. A few contemporary investigations have examined midline cerebellar stimulation (Demirtas-Tatlidede et al., 2010; Garg et al., 2016). Theta burst stimulation (TBS) is a novel patterned rTMS technique, which reduces stimulation time and causes more potent and longer-lasting effects when compared to standard rTMS (Huang et al., 2005a). A recent two-phase experiment identified a cerebellar-prefrontal network to be associated with negative symptom severity in schizophrenia; engaging this network with a five-day intermittent TBS (iTBS) treatment enhanced the connectivity in this network, which was associated with improvement in negative symptoms (Brady et al., 2019). These observations lend support to past studies identifying midline cerebellar structural changes (Heath et al., 1982) and their potential impact on regulating prefrontal activity via the striatum and thalamus as a key pathophysiological mechanism in negative symptoms of schizophrenia (Andreasen et al., 1998, 1996; Rüsch et al., 2007). The fronto-cerebellar network is a potential target network that can be engaged to improve negative symptoms in more systematic controlled trials based on an experimental medicine approach.
We therefore used a double-blind randomized controlled experiment to characterize changes in (a) severity of negative symptoms and cognitive impairment, following 10 sessions of real or sham midline cerebellar iTBS in schizophrenia patients with predominant negative symptoms, (b) fronto-cerebellar resting state functional connectivity (RSFC). We hypothesized a greater improvement in negative and cognitive symptoms and greater enhancement in the fronto-cerebellar RSFC in patients receiving real (as compared to sham) cerebellar stimulation.
2. Materials and methods
This study was a double-blind, randomized, parallel, sham-controlled superiority trial with an allocation ratio of 1:1. The trial was registered prior to recruitment of participants under Clinical Trials Registry – India (CTRI) with registration number CTRI/2017/09/009636 (ww.ctri.nic.in). The study was approved by the institutional ethics committee and all participants provided written informed consent.
2.1. Sample size calculation
The open label study that demonstrated benefits of iTBS to cerebellar midline using a similar protocol in schizophrenia demonstrated improvements in negative symptoms to the magnitude (effect size) of 0.69 (Demirtas-Tatlidede et al., 2010). Our sample of 30 in each group had 80% power at an alpha of 0.05 to detect a standardized mean difference of about 0.7 in negative symptoms between real and sham iTBS groups (Cohen, 1988).
2.2. Participant recruitment
Participants (aged 18–50 years) meeting the Diagnostic and Statistical Manual-5 (DSM-5) diagnosis of schizophrenia as evaluated by two clinicians independently, and confirmed using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998), were recruited for the trial from both outpatient and inpatient services at a south Indian tertiary care center, National Institute of Mental Health and Neurosciences (NIMHANS). Each participant’s medications had to be unchanged in the last three months with no Electroconvulsive Therapy (ECT) prescribed for the last six months before recruitment. Participants needed to have a minimum required severity of negative symptoms defined as a score of three (moderate) or more on each of the five global rating items of Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989; Andreasen et al., 2005). To avoid confounders likely to affect cognition or treatment response, participants with clinically determined neurological disorders (e.g., seizure disorder, traumatic brain injury), substance abuse or dependence (except nicotine) within the past three months and self-reported visual or auditory impairment interfering with study assessments were excluded. We also excluded participants with persistent suicidal or homicidal behavior, pregnant, lactating or postpartum states. To minimize transcranial magnetic stimulation/TMS-related complications, participants fulfilling any of the risk factors for TMS procedures as assessed using a standard screening tool (Rossi et al., 2011) were excluded. The randomization sequence was generated through a computer by MK. UMM ensured allocation concealment by maintaining opaque sealed envelopes containing allocation status. DI/MVT opened the opaque envelopes only after the informed consent was obtained by RB from the participant thus ensuring allocation concealment. TMS was administered by DI/MVT and the assessments were performed by RB who was unaware of the treatment status. Subjective appraisals from participants and rater (RB) were recorded regarding real versus sham status of the treatment at the end of sessions to assess the effectiveness of blinding.
2.3. Clinical, cognitive and safety assessments
Negative symptoms were assessed using the SANS (Andreasen, 1989). Positive symptoms were assessed using Scale for Assessment of Positive Symptoms (SAPS) (Andreasen, 1984). Depressive and extrapyramidal symptoms were assessed by Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al., 1990) and Simpson Angus Scale (SAS) (Simpson and Angus, 1970) respectively. General neurocognitive (Rao et al., 2004) and social cognitive assessments (Behere et al., 2008; Mehta et al., 2011) were performed to cover dimensions indicated in the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Cognitive Consensus Battery (Nuechterlein et al., 2004) (Appendix A). Pulse rate, blood pressure and motor cerebellar signs (International Co-operative Ataxia Rating Scale-ICARS) (Trouillas et al., 1997) were measured before and after each TMS session. Potential side effects of TMS (Rossi et al., 2009) were checked for each subject after each treatment session. All the assessments were performed thrice for each participant i.e., at baseline (T0), sixth day (T1) and sixth week (T2) after the initiation of iTBS (Supplemental Fig. 1).
2.4. TMS intervention
T1 structural magnetic resonance imaging (MRI) was obtained from all participants prior to initiation of TMS in which the cerebellar vermis (lobule VIIB) was identified by cerebellar anatomical landmarks within the proportional stereotaxic space of Talairach (Schmahmann et al., 1999). See Supplemental Fig. 2 for detailed localization procedure followed for each participant. rTMS was delivered as intermittent theta burst stimulation (iTBS). rTMS was delivered using MagPro X100 (MagVenture, Farum, Denmark). Dosing was at 100% of the active motor threshold, defined as the minimum intensity delivered to the left cerebral cortex, required to produce a motor evoked potential of >200 microvolts on >five out of ten trials from the contralateral (right) first dorsal interosseous, while the participant maintained voluntary contraction of about 20% of maximum (Rossini et al., 1994), measured using a hydraulic pinch gauge (Saehan Corp., Masan, South Korea). TMS was administered under MRI-guided neuronavigation using Brainsight frameless stereotaxic system (Rogue Research, Montreal, Canada) (Supplemental methods, “Neuronavigational procedure”) with the figure-of-8 Cool-B65 AP MagVenture coil, which could deliver either real or sham iTBS according to the codes fed into the TMS machine. The sides of the coil did not deliver real or sham stimulation in a fixed mode but were flexible to switch according to the codes fed into the TMS machine. The sham iTBS produced just a sound in a pattern like iTBS without any magnetic stimulation. The handle of the coil was held upwards along the sagittal plane. iTBS was administered as 20 trains of 2 s on and 8 s off cycle containing 3-pulse 50 Hz bursts at theta frequency (every 200 ms), for a total of 600 pulses delivered over 190 s (Huang et al., 2005b). The TMS protocol adhered to all safety guidelines and recommendations endorsed by the International Federation for Clinical Neurophysiology (Rossi et al., 2009). Participants underwent a total of ten TMS sessions administered as two sessions every day spaced 4 h apart and a total of 6000 pulses, for five days (Demirtas-Tatlidede et al., 2010).
2.5. Resting state functional magnetic resonance imaging (rsfMRI)
All scans were obtained with a 3 T Siemens Skyra MRI scanner using a 32-channel coil. The blood oxygenation level dependent (BOLD) echo-planar imaging (EPI) rsfMRI scan parameters were as follows: TR = 2000 ms; TE = 30 ms; flip angle = 78; slice thickness = 3 mm; slice order: Descending; slice number = 37; gap = 25%; matrix = 64 * 64 * 64 mm3, FOV = 192 * 192, voxel = 3.0 mm isotropic. The EPI sequences (10 min; 250 in number) were acquired in darkness and participants were asked to keep their eyes open during the session, lie as quietly as possible, and avoid falling asleep. A high-resolution structural T1-weighted MRI of 1-mm section with no inter-slice gap was also obtained to use for TMS-target localization, registration purposes during image processing and analyses. BOLD rsfMRI and T1-weighted MRI were obtained once at T0 and again within 2 days after completion of the 5-day TMS treatment course (T1). rsfMRI was analyzed on the imaging software FMRIB Software Library (FSL version-5.0.10). See Supplemental methods “rsfMRI_preprocessing” and “Quality check procedure of rsfMRI scans” for detailed description of the steps.
2.6. Seed based analysis of rsfMRI
The cerebellar vermis area VIIB was chosen as the primary seed as that was the target of stimulation and we intended to study the change in resting state connectivity of this midline cerebellum structure with the rest of the voxels in the brain following the stimulation. Vermis VIIB was chosen as the seed as per “Cerebellar Atlas with MNI152 space after normalization with FLIRT” (Diedrichsen et al., 2009). The seed was binarized to and inverse transformed from standard subject space (cleaned functional MRIs) using the matrix file available from the initial registration step. Average time series of rsfMRI BOLD-signal from individual voxels within this seed were extracted for each subject, and used as the model predictor in a general linear model (GLM) analysis to determine brain regions temporally correlated with it using fMRI Expert Analysis Tool (FSL-FEAT) (Woolrich et al., 2001). The resultant seed connectivity maps were registered back to standard MNI-152 space. Next, to determine significant within-and between-group differences, and relationship between functional brain connectivity changes and change in negative symptom severity, FSL’s randomize permutation tool was employed (Winkler et al., 2014). Clusters were determined by using threshold-free cluster enhancement (TFCE) (Smith and Nichols, 2009) and a family-wise error rate (FWER) corrected cluster significance threshold of p < 0.05 with 5000 permutations.
2.7. Statistical methodology
2.7.1. Clinical data
Baseline clinical and sociodemographic parameters were compared between the two groups using independent t-test or chi-square test. We adopted the standard intention-to-treat (ITT) analysis. Change in negative symptom severity, cognitive scores, and physical parameters over time because of intervention were examined through Mixed Models Repeated Measures (MMRM) analysis. Group and time-point of measurement were modeled as fixed effects. Clinical ratings were performed thrice on every participant contributing to a correlation between these scores within each participant. This correlation of repeated measures on the same participant was accounted for by modeling individual participants as a random effect and by specifying a covariance structure to the random effect, in this case the autoregressive first order (AR1). Maximum Likelihood (ML) method was used for estimating parameters in the model. The residuals obtained from the model for each outcome variable were tested for normality of distribution. Group * Time interaction effect in the MMRM models, which indicated differential improvement of symptoms in the groups over time, was considered statistically significant at p ≤ 0.05. All statistical analyses were performed on R Studio version 3.6.1.
2.7.2. Neuroimaging data
Baseline resting state functional connectivity maps of the cerebellar seed to the rest of the brain (seed-to-voxel) were compared between the two groups using an unpaired higher-level GLM test design within FSL. Change in functional connectivity within the two groups was separately examined using paired higher-level GLM test designs. Difference maps were created using the subtraction function in FSL by subtracting pre-from post-TMS functional connectivity maps. An unpaired higher-level test was then used to compare the difference maps between real iTBS and sham iTBS groups (with and without clozapine status as a covariate). GLM model consisting of the difference maps and the change in SANS scores was used to examine the relationship between change in whole brain functional connectivity of the cerebellar seed and change in SANS severity score following TMS.
3. Results
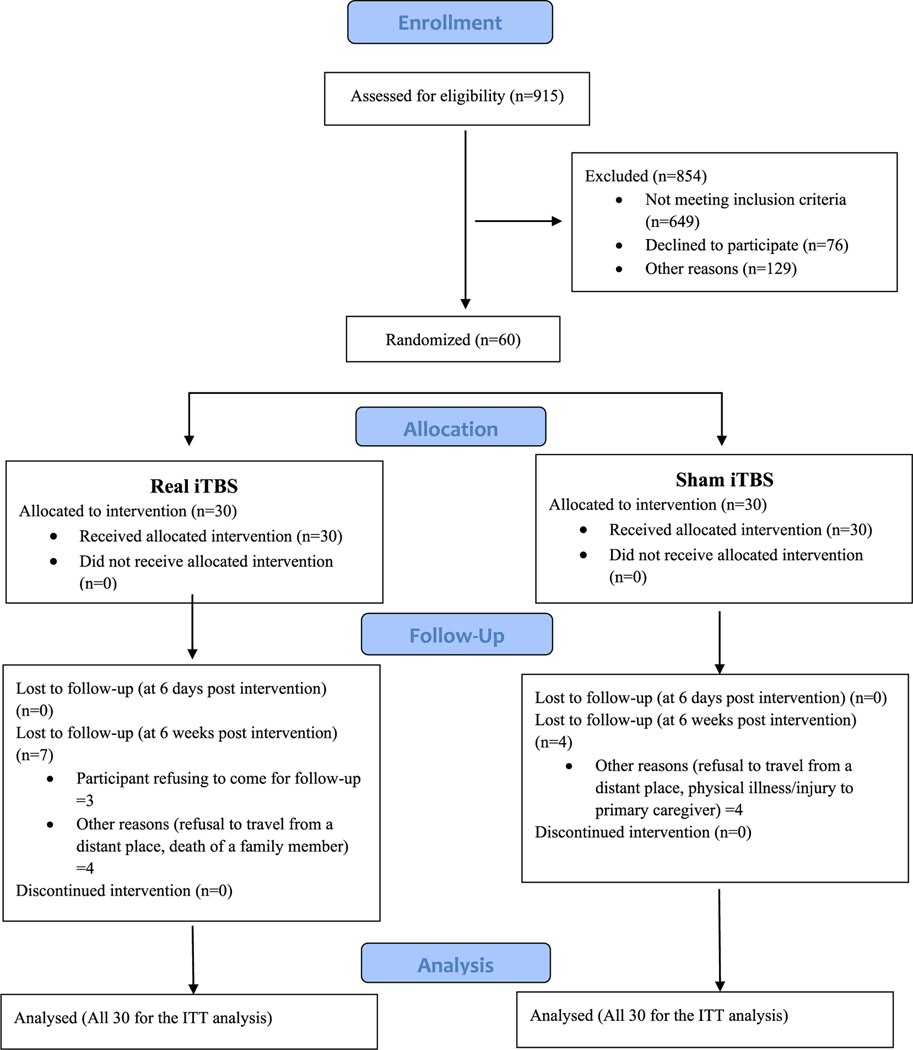
Thirty participants were recruited in each group from August 2016 to July 2018. The Consolidated Standards for Reporting Trials (CONSORT) diagram is depicted in Fig. 1.
Fig. 1.
CONSORT flow diagram.
Legend: Consolidated Standards for Reporting Trials (CONSORT) flow diagram depicting the recruitment of participants into the trial from screening to inclusion of data in the final statistical analysis. ITT = Intention to treat; iTBS = intermittent theta burst stimulation.
3.1. Baseline clinical and sociodemographic variables
The 2 groups were comparable on baseline, clinical and cognitive parameters except the long-term percent retention score of verbal memory which was higher in sham iTBS arm. However, a higher proportion of participants in the real iTBS arm were on clozapine (30%) compared to sham iTBS (6.67%) (p = 0.02) (Table 1, Supplemental Table 1).
Table 1.
Baseline symptom severity and cognitive scores.
| Real iTBS (N = 30) | Sham iTBS (N = 30) | t/X2 | p | |
|---|---|---|---|---|
| Scale for the Assessment of Positive Symptoms (SAPS) | 4.63 (6.14) | 6.10 (9.33) | −0.72 | 0.48 |
| Scale for the Assessment of Negative Symptoms (SANS) | 85.23 (11.75) | 82.47 (13.82) | 0.84 | 0.41 |
| Calgary Depression Scale in Schizophrenia (CDSS) | 1.27(2.59) | 1.7 (2.38) | −0.68 | 0.50 |
| Simpson Angus Scale (SAS) | 1.2 (1.83) | 0.83 (1.32) | 0.89 | 0.38 |
| Learning Score | 31.11 (9.11) | 32.33 (9.27) | −0.51 | 0.61 |
| Memory Score | 22.96 (7.55) | 25.9 (7.44) | −1.48 | 0.15 |
| Long Term Percent Retention Score | 63.16 (34.05) | 86.31 (38.89) | −2.41 | 0.019 |
| Complex Figure Test (CFT)-copying | 31.66 (5.24) | 29 (29.21) | 1.42 | 0.16 |
| CFT-Immediate Recall | 12.61 (8.75) | 10.91 (8.22) | 0.75 | 0.45 |
| CFT-Delayed Recall | 11.93 (8.34) | 9.67 (7.53) | 1.07 | 0.29 |
| DSST (seconds) (Digit Symbol Substitution Test) | 372.04 (206.9) | 475.17 (288.56) | −1.53 | 0.13 |
| DVT (seconds) (Digit Vigilance Test) | 772.78 (373.64) | 780.28 (349.25) | −0.08 | 0.94 |
| Colour Trails-A (seconds) | 110.48 (61.73) | 162.28 (127.01) | −1.92 | 0.06 |
| Colour Trails-B (seconds) | 255.81 (148.14) | 349.24 (221.52) | −1.82 | 0.075 |
| Verbal1back Hits | 6.54 (2.8) | 7.03 (2.25) | −0.75 | 0.46 |
| Verbal1back Errors | 3.93 (2.97) | 2.93 (2.78) | −0.93 | 0.36 |
| Verbal2back Hits | 4.18 (2.76) | 4.00 (2.30) | 0.27 | 0.79 |
| Verbal2back Errors | 6.32 (3.49) | 7.2 (3.7) | −0.93 | 0.36 |
| Spatial Span Total | 11.64 (4.39) | 10.1 (2.92) | 1.59 | 0.12 |
| FAS Total | 17.44 (10.03) |
17.52 (7.13) | −0.032 | 0.97 |
| Stroop Effect Score | 251.6 (122.00) | 281.00 (152.66) | −0.71 | 0.48 |
| First Order Theory of Mind (FOT) Index | 0.78 (0.29) | 0.79 (0.22) | −0.11 | 0.92 |
| Second Order Theory of Mind (SOT) Index | 0.34 (0.25) | 0.44 (0.29) | −1.33 | 0.19 |
| Faus Pax (FP) Index | 0.39 (0.17) | 0.39 (0.21) | −0.102 | 0.92 |
| Externalizing Bias (EB) Index | −0.17 (2.8) | 1.1 (2.5) | −1.65 | 0.11 |
| Personalizing Bias (PB) Index | 0.77 (0.34) | 0.78 (0.28) | −0.06 | 0.96 |
| Social Perception (SP) Index | 0.68 (0.14) | 0.61 (0.10) | 1.00 | 0.32 |
| Emotion Recognition (ER) Index | 0.56 (0.16) | 0.57 (0.14) | 0.57 | 0.86 |
Note: All values in cells are mean (SD).
iTBS = intermittent theta burst stimulation.
p is significant at </=0.05
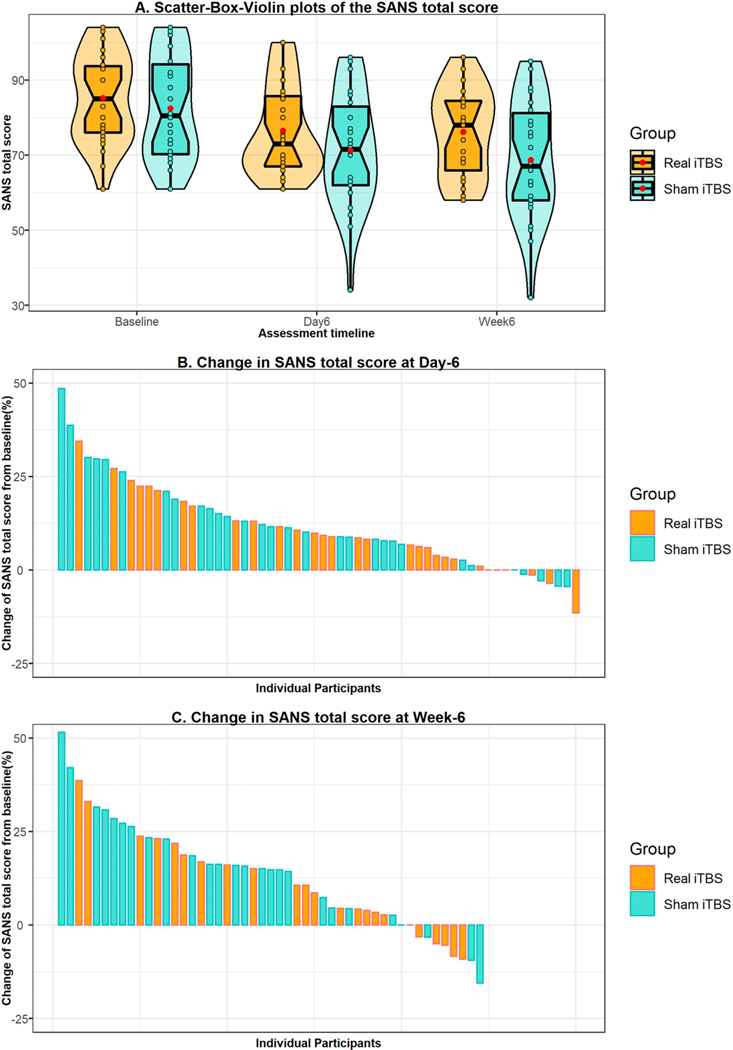
3.2. Mixed Models Repeated Measures analysis
The Akaike’s Information Criterion (AIC) was the least for the AR1 covariance structure type compared to other models confirming a better fit of the AR1 model. There was no significant difference between the two arms in improvement of negative symptoms with Group * Time interaction, F(df) = 0.511(2,110.98), p = 0.602 (Fig. 2, Table 2). The two interventions did not differ significantly from each other on change in other clinical, cognitive, and physical measures (Table 2, Supplemental Tables 2 & 3). However, both the groups showed improvement in negative symptoms, cognitive parameters (except the long-term retention score, Color Trails-A, Verbal 1Back hits, Verbal 2Back errors and the social cognitive variables) over time (Table 2, Supplemental Table 2). Among the physical measures, the diastolic blood pressure, extrapyramidal symptoms score, and the ataxia score decreased over time in both the arms (Supplemental Table 3). Change in global scores of individual symptom domains of the SAPS and SANS was also not significantly different between the two groups (Supplemental Table 4). All the residuals obtained from the linear mixed models satisfied normal distribution. As the real iTBS arm had more treatment resistant participants on clozapine (failure to respond to two adequate trials of antipsychotics), which in turn could confound the response to the intervention despite the similarity in symptom severity at baseline, Group * Time * Clozapine interaction was studied, which was not significantly different for negative symptom severity between the two groups (p = 0.423). The process of blinding was effective as there was no difference between the two groups in the subjective appraisal from participants and the rater, regarding the arm of treatment to which they were assigned (Supplemental Table 5).
Fig. 2.
Comparison of change in negative symptom severity between the 2 groups.
Table 2.
Effect of real iTBS v/s sham iTBS on symptom severity.
| Measure | Timepoint | Real iTBSa Mean (SE) | Sham iTBSa Mean (SE) | F(df) (Group effect) | p (Group effect) | F(df) (Time effect) | p (Time effect) | F(df) (Group * Time effect) | p (Group * Time effect) |
|---|---|---|---|---|---|---|---|---|---|
| Scale for the Assessment of Negative Symptoms (SANS) | Baseline | 86.35 (2.5) | 84.89 (2.93) | 1.144 (1,62.39) | 0.289 | 31.604 (2,110.99) | <0.001 | 0.511 (2,110.98) | 0.602 |
| Day6 | 77.69 (2.5) | 73.72 (2.93) | |||||||
| Week6 | 76.4 (2.7) | 71.97 (3.0) | |||||||
| Scale for the Assessment of Positive Symptoms (SAPS) | Baseline | 5.24 (1.36) | 7.41 (1.6) | 1.246 (1,57.10) | 0.269 | 9.675 (2,105.74) | <0.001 | 0.082 (2,105.73) | 0.921 |
| Day6 | 2.64 (1.36) | 4.69 (1.6) | |||||||
| Week6 | 4.7 (1.45) | 6.2 (1.63) | |||||||
| Calgary Depression Scale in Schizophrenia (CDSS) | Baseline | 1.17 (0.43) | 1.49 (0.49) | 0.074 (1,58.79) | 0.787 | 4.54 (2,106.34) | 0.013 | 1.913 (2,106.33) | 0.153 |
| Day6 | 0.34 (0.43) | 0.59 (0.49) | |||||||
| Week6 | 1.44 (0.48) | 0.48 (0.51) |
df = degrees of freedom, SE = standard error.
iTBS = intermittent theta burst stimulation.
p is significant at </=0.05
Estimated marginal means reported.
3.3. rsfMRI
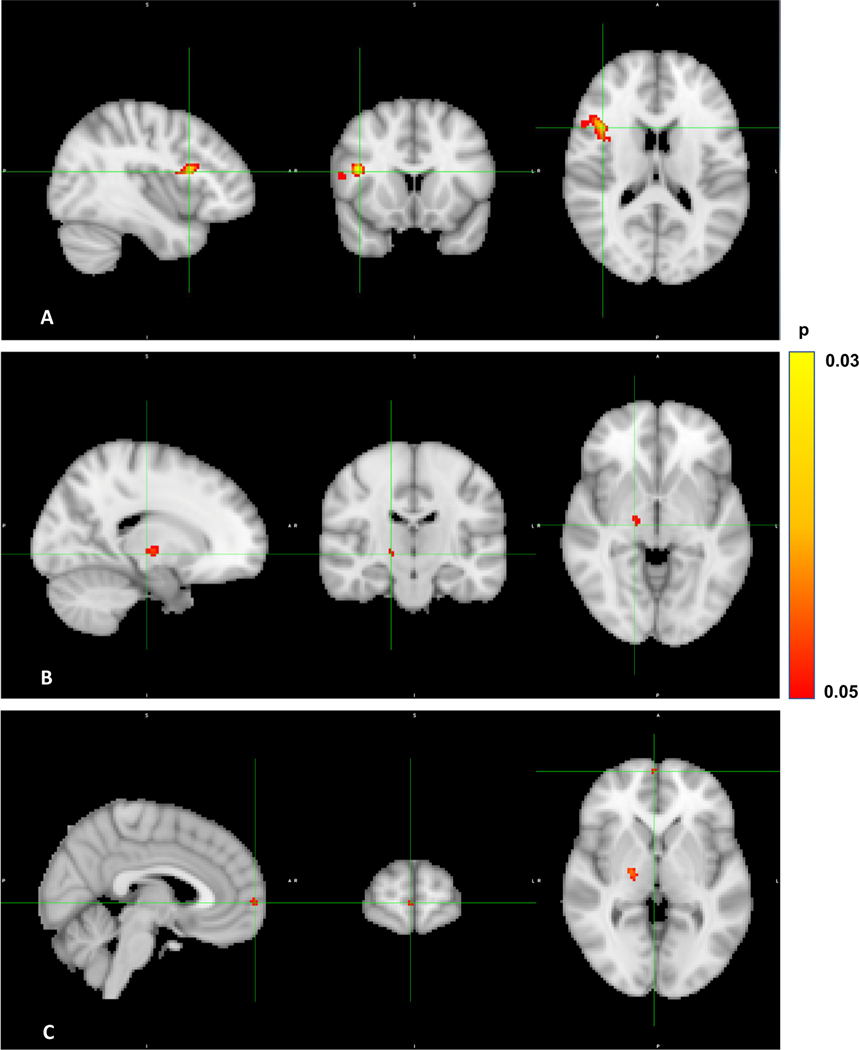
Twenty-five participants in the real iTBS group and 30 in the sham iTBS group had good quality resting state fMRI data available for analysis. GLM comparing the cerebellar vermis area VIIB seed–whole brain connectivity maps between the two groups at baseline (pre-intervention) did not show any statistically significant differences. GLM comparing the connectivity maps before the intervention to that post-intervention in the real iTBS arm showed significantly increased connectivity between the cerebellar vermis area VIIB and areas corresponding to the right inferior frontal gyrus (IFG) (a threshold free enhancement family wise error rate corrected p value (pcluster-FWER) of 0.033, with a size of 278 voxels, with peak MNI coordinates of 44,16, 20), right pallidum (pcluster-FWER = 0.042, size = 42 voxels, peak MNI coordinates of 20, - 12, 0) and right frontal pole (pcluster-FWER = 0.047, size =17 voxels, peak MNI coordinates of 6, 62, 2) (Fig. 3). There was no significant difference observed between the pre and post intervention connectivity maps of the sham iTBS group (pcluster-FWER = 0.179). GLM comparing the subtraction maps (post intervention – pre intervention) of the two groups revealed no significant differences (pcluster-FWER = 0.207). Given that the real iTBS arm had more treatment resistant participants on clozapine, addition of clozapine status as a covariate in the GLM comparing the subtraction maps did not yield any significant difference (pcluster-FWER = 0.126) either. There was no significant association between change in connectivity of the cerebellar area VIIB after TMS and change in negative symptom severity in the whole group (pcluster-FWER = 0.077) or individually in either of the groups (Real iTBS: pcluster-FWER = 0.275, Sham iTBS: pcluster-FWER = 0.286) (Table 3). The baseline functional connectivity maps of the whole group, real and sham iTBS arms are illustrated in Supplemental Fig. 3.
Fig. 3.
Change in resting state functional connectivity in real iTBS arm.
Legend: Sagittal, coronal, and axial (from left to right in each panel) sections of the brain demonstrating increased resting state functional connectivity between cerebellar seed, vermal area VIIB and A. Right inferior frontal gyrus (pcluster-FWER = 0.033), B. right pallidum (pcluster-FWER = 0.042) and C. right frontal pole (pcluster-FWER = 0.047) from baseline to post-intervention in the real iTBS arm. iTBS = intermittent theta burst stimulation, pcluster-FWER = threshold-free cluster enhancement and a family-wise error rate (FWER) corrected cluster significance.
Table 3.
rsfMRI results of different contrasts showing the peak MNI co-ordinates of clusters with the lowest p-value.
| Analysis | Contrast | pcluster-FWER | Cluster size (no. of voxels) | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Difference in connectivity in Real iTBS arm due to intervention | Post-pre | 0.033 | 278 | 44 | 16 | 20 |
| 0.042 | 42 | 20 | −12 | 0 | ||
| 0.047 | 17 | 6 | 62 | 2 | ||
| Pre-post | 0.962 | 3 | 26 | 67 | 17 | |
| Difference in connectivity in Sham iTBS arm due to intervention | Post-pre | 0.179 | 15 | 45 | 48 | 35 |
| Pre-post | 0.647 | 01 | 56 | 15 | 49 | |
| Difference of subtraction connectivity maps (post-pre) of the two groups | Real-sham | 0.207 | 15 | 39 | 31 | 51 |
| Sham-real | 0.849 | 25 | 45 | 48 | 36 | |
| Difference of subtraction connectivity maps (post-pre) of the two groups (with clozapine status as a covariate) | Real-sham | 0.126 | 12 | 40 | 31 | 51 |
| Sham-real | 0.905 | 1 | 77 | 49 | 22 | |
| Relationship between change in connectivity and change in SANS total (whole group) | Direct | 0.077 | 23 | 69 | 37 | 29 |
| 0.077 | 16 | 72 | 33 | 36 | ||
| 0.077 | 09 | 51 | 60 | 31 | ||
| Inverse | 0.975 | 3 | 25 | 62 | 21 | |
| Relationship between change in connectivity and change in SANS total (real iTBS) | Direct | 0.684 | 1 | 39 | 65 | 20 |
| Inverse | 0.275 | 7 | 51 | 77 | 42 | |
| Relationship between change in connectivity and change in SANS total (sham iTBS) | Direct | 0.286 | 1 | 42 | 22 | 16 |
| Inverse | 0.955 | 3 | 40 | 58 | 26 | |
MNI = Montreal Neurological Institute, FWER = family wise error rate, iTBS = intermittent theta burst stimulation, SANS=Scale for Assessment of Negative Symptoms.
p is significant at </=0.05
3.4. Adverse events
Two participants who received real iTBS showed symptoms suggestive of mania/hypomania during the trial whereas none who received sham iTBS showed such symptoms, a report of which has been described elsewhere (Basavaraju et al., 2020). One participant from the real arm reported neck muscle contraction and ensuing tolerable neck pain/discomfort during the TMS sessions, none was reported in the sham arm. No other adverse events were reported.
4. Discussion
In this randomized controlled experimental study, we report increased functional connectivity of the stimulated midline cerebellum with the right inferior frontal gyrus and pallidum in schizophrenia patients with predominant negative symptoms in the real iTBS arm. Both the groups showed significant improvement, with no between-group differences in the primary clinical outcome measure (SANS scores), suggesting a placebo effect. The enhanced functional connectivity within the fronto-pallidal-cerebellar network did not relate to change in negative symptoms.
The dysfunction of cortical-subcortical-cerebellar circuitry has been implicated to underlie a poor mental coordination or cognitive dysmetria which is proposed to translate into diverse symptoms of schizophrenia (Andreasen et al., 1998, 1996) including negative symptoms (Andreasen et al., 1999; Honey et al., 2005). Many recent large-scale functional connectivity studies also imply this structurally polysynaptic network in the brain as a central substrate underlying key symptoms of schizophrenia (Brady et al., 2019; Chen et al., 2013; Collin et al., 2011; Gao et al., 2019; Guo et al., 2018, 2015; Ji et al., 2019; Lee et al., 2019; Liu et al., 2011; Zhuo et al., 2018). Hence, we utilized this network as a biological target for engagement through an intervention. Target engagement is a method of approach to clinical trials called experimental medicine which has been proposed by the National Institute of Mental Health (NIMH) to search for novel targets and increase Phase III success instead of trials designed to just look for an efficacy signal (Insel, 2015). Brady et al. (2019) adopted a similar approach in a smaller sample and demonstrated that enhancing the network connectivity between midline cerebellum and the right DLPFC through iTBS correlated with improvement in negative symptoms. Our study had a TMS stimulation protocol similar to that of Brady et al. (2019). In our study, cerebellar vermal iTBS enhanced the RSFC in a network comprising the vermis area VIIB, right pallidum, right inferior frontal gyrus and the right frontal pole. Previous studies have shown abnormalities in RSFC between cerebellum and IFG in schizophrenia (Collin et al., 2011; Ji et al., 2019; Tarcijonas et al., 2019). High functioning patients with first episode schizophrenia showed increased functional connectivity of the right pallidum and right IFG compared to low functioning patients (Tarcijonas et al., 2019). Our results represent a novel right-sided tripartite cerebello-subcortical-cortical circuit being activated in schizophrenia with predominant negative symptoms through TMS.
Though we have demonstrated the engagement of a novel network and enhancement of the network’s intrinsic RSFC through vermal iTBS, there was no significant difference between the real and sham arms in improvement of negative and cognitive symptoms. Also, a lack of relationship between change in connectivity of this network and change in negative symptom severity might imply that this network may not be a suitable biological target for engagement to successfully improve negative symptoms but just a mechanism of action of vermal iTBS (Insel, 2015). Alternatively, this trial has many unique participant, trial-design and treatment-protocol related characteristics that might have predisposed to the dissociation between the imaging and the clinical results. In contrast to most other studies testing interventions for negative symptoms (Brady et al., 2019), our trial had a minimum cut-off of symptom severity for inclusion as a score of three or more on each of the five global rating items of SANS (moderate severity) and also, they had to be on stable medications for three months before recruitment in to the trial. Hence our patient group was moderate to severely ill, with predominant negative symptoms and minimal to absent positive symptoms due to stabilized antipsychotic medication (mean total SANS score above 80 and mean total SAPS score around 5), absent to minimal depressive and extrapyramidal symptoms and a longer duration of illness (>8 years). This probably represents a population of patients with enduring primary negative symptoms or deficit syndrome (Carpenter, 1994), with unique biological underpinnings (Voineskos et al., 2013; Wheeler et al., 2015), suggestive of a possible subtype of schizophrenia. On the other hand, in this trial, negative symptoms and cognitive parameters improved over time irrespective of the interventional arm which points towards a high placebo response being one of the reasons contributing to the absence of efficacy. High placebo response in a trial like ours could be due to the general trend of increase in placebo response in trials of psychiatry (Razza et al., 2018; Rutherford et al., 2014; Rutherford and Roose, 2013; Weimer et al., 2015) and schizophrenia (Gopalakrishnan et al., 2020). TMS trials additionally offer a unique challenge in accounting for the placebo effect compared to medication trials. Due to large embedded placebo effects in sham medical devices compared to pill placebo, an efficacy paradox is created, requiring RCTs that are powered higher than regular trials (Burke et al., 2019). Advancement over years in the methodological effectiveness of blinding in rTMS trials (Dollfus et al., 2016; Razza et al., 2018), improvement in rTMS delivery technology like neuronavigation (Dollfus et al., 2016), parallel study over cross-over designs (Dollfus et al., 2016) and placebo-controlled studies over active drug-drug comparator trials (Rutherford et al., 2014; Rutherford and Roose, 2013), all contribute to this phenomenon. The improvement of symptoms despite being severe and treatment-resistant can be due to possible rater bias in baseline inflation of severity which is known to happen in questionnaire-based RCTs in psychiatry (Rutherford and Roose, 2013), enriched therapeutic milieus which these withdrawn and isolated patients are exposed to in the trial compared to treatment as usual (Rutherford and Roose, 2013), and the short duration of the trial with closely spaced assessments which can beget abrupt and transient placebo responses compared to real clinical efficacy that usually has a gradual onset and is persistent (Quitkin et al., 1991). Placebo lead in periods, cross over designs, comparing cerebellar vermal iTBS with an active intervention like DLPFC stimulation or transcranial direct current stimulation (tDCS), matched in terms of durations of treatment and physician-patient contact, centralized rating blind to study inclusion severity-criteria, longer trials with adequately spaced-out assessments are some of the methods we propose to handle the issue of increased placebo response in future similar trials.
Despite a difficult-to-treat severely ill population and also with a higher proportion of treatment-resistant patients on clozapine in the real iTBS group, we have demonstrated modulation of a target network by vermal iTBS pointing towards the ability of this treatment modality in causing a significant neurobiological change in the brain. We observed that two patients belonging to the real iTBS arm showed symptoms of hypomania/mania whereas there were none in the sham arm (Basavaraju et al., 2020). In keeping with these observations, and also given the absence of physical adverse effects, we propose alteration of TMS delivery parameters like the number of sessions, number of pulses per session, and intensity of stimulation to generate improvement beyond the observed placebo response in future trials. The total pulses administered in depression trials with theta burst stimulation is between 60,000 and 90,000(Cole et al., 2020; Williams et al., 2018) compared to just 6000 total pulses in our trial. There exists evidence for a dose-response relationship between the number of pulses of TBS and the strength of functional connectivity of the modulated network (Nettekoven et al., 2014). This potentially implies a direct translation of dose of intervention to magnitudes of biological change and clinical effects yielding a promise of better clinical effects with higher doses.
One of the limitations of the study is that there was no difference in the subtraction connectivity maps between the two groups despite significant difference in modulation of connectivity within individual groups. However, we do emphasize on the within group significant change in functional connectivity of the vermis seed in the real group, since this finding partially replicates observations of Brady et al. (2019) regarding our a priori proposed target engagement. This study used a figure-of-8 coil for the cerebellar vermis which is >3 cm (Hurtado-Puerto et al., 2020) from the scalp surface. In a previous electrical field modeling study the figure-of-8 coil has been demonstrated to induce a supra-threshold electric field at midline cerebellum (Bijsterbosch et al., 2012). It is possible that targeting this deep-seated structure with sub-threshold intensity from a figure-of-8 coil might not have ensured adequate stimulation of the cerebellar vermis, which is difficult to conclude without modeling the electrical field generated in the target tissue. A double-cone coil which has a deeper penetration and hence more suitable for midline cerebellar stimulation (Fernandez et al., 2020) with concomitant electrical field modeling can mitigate this shortcoming.
This evidence of an apparent biological effect in the form of target-engagement of a cortico-subcortical-cerebellar network in a more severely symptomatic population, albeit with null clinical efficacy with the relatively lower stimulation parameters should encourage future studies to engage this target with a high-dose stimulation protocol. Thus, modulation of this newly discovered biological substrate requires replication in experiments with modifications of the various trial parameters as discussed. This would very likely ensure adequate magnetic stimulation of the brain area and aid in translating the target engagement to actual clinical efficacy. Our findings open avenues for exploring promising novel therapeutic modalities by testing the mechanism of action, through target engagement by brain stimulation techniques, for treatment of disabling symptoms of schizophrenia.
Supplementary Material
Acknowledgements
RB acknowledges support from Research Training Fellowship awarded by DBT/Wellcome Trust India Alliance, Grant/Award Number: IA/RTF/15/1/1009 during the study. DI acknowledges support from Research Training Fellowship awarded by DBT/Wellcome Trust India Alliance, Grant/Award Number: IA/RTF/15/1/1004 during the study.
ROB acknowledges current funding support, R01 MH116170.
Role of the funding source
This RCT was the project of the Research Training Fellowship awarded to RB by Wellcome Trust/DBT India Alliance (https://www.indiaalliance.org/) from August 2016 to July 2018. Grant/Award Number: IA/RTF/15/1/1009. DBT/Wellcome Trust India Alliance wholly funded the study. National Institute of Mental Health and Neurosciences (NIMHANS) administered the award to RB and the study was conducted at NIMHANS. Some equipment utilized for the study was supported by DBT/Wellcome Trust India Alliance Early Career Fellowship [IA/E/12/1/500755] awarded to UMM. Neither NIMHANS nor DBT/Wellcome Trust India Alliance had or have the ultimate authority in study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
APL was partly supported by the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R24AG06142, and P01 AG031720), the National Science Foundation, and DARPA. APL is a co-founder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Magstim Inc., Nexstim, and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of noninvasive brain stimulation with electroencephalography and magnetic resonance imaging.
UMM receives an honorarium from Elsevier for serving as Associate Editor for Schizophrenia Research.
RB, DI, MVT, AHR, JT, RPR, ROB, MAH, NRB, MSK and KMD report no financial relationships with commercial interests.
Appendix A.
Cognitive tests
| Domain | Cognitive test | Task description |
|---|---|---|
| Neurocognition (Rao et al, n.d.) | ||
| Verbal Learning & Memory | Auditory Verbal Leaning Test | A 15-word list (A) is presented 5 times verbally followed by a recall. Then, an interference list (B) of 15 words is presented before a recall of list-A. Delayed recall and recognition are also tested |
| Visual Learning & Memory | Complex Figure Test | Participants are asked to draw a complex diagram, initially by copying and then are asked to redraw from memory immediately and at a delayed timepoint |
| Processing Speed | Digit Symbol Substitution Test | The participant is asked to fill up the blanks below numbers with corresponding symbols according to a key provided. |
| Attention and Concentration | Digit Vigilance Test | Participants are asked to strike out particular digits (6&9) on a sheet of paper with multiple lines of single digits. |
| Verbal Working Memory | N-Back Test | Participants are presented a sequence of verbal stimuli. For each stimulus the participant has to decide whether the current stimulus matches the stimulus presented N number of trials back |
| Visuospatial Working Memory | Spatial Span Test | Participants are presented with a spatial sequence of blocks arranged on a board with serially increasing complexity. They are expected to reproduce the spatial sequence as demonstrated. |
| Executive Functions | Color Trails-A&B | Participants are asked to join numbers in sequence (A) and asked to alternate between colors of the circles in which the numbers are printed (B). For example, red 1 followed by a yellow 2, red 3 and so on. |
| Stroop Test | Participants are expected to read a list of words written in incongruent colors followed by mentioning the color of the ink instead of reading the word. | |
| Social cognition | ||
| Theory of Mind/Social perception (Mehta et al., 2011) | First and second order Theory of Mind, Faux Pas, Attribution Bias and Social Cue Perception Test | Story-based tasks in which the participants are required to decipher mental states of others at different complexity levels. Tasks based on videos demonstrating social interaction between characters, where participants are required to pick up the social cues |
| Emotion Recognition (Behere et al., 2008) | Facial emotion recognition test | Static images and dynamic videos of six basic human emotions (happy, anger, sad, fear, surprise and disgust) depicted by trained actors |
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
CRediT authorship contribution statement
RB: Substantial contribution to conception or design of the work; acquisition, analysis, and interpretation of clinical, cognitive and neuroimaging data for the work; drafting the manuscript; revising the manuscript critically for important intellectual content; final approval of the version to be published.
DI: Substantial contribution to executing the work; analysis and interpretation of the neuroimaging data of the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
MVT: Substantial contribution to executing the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
AHR: Substantial contribution to design of the work; revising the manuscript critically for important intellectual content; final approval of the version to be published. JT: Substantial contribution to conception or design of the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
RPR: Substantial contribution to design of the cognitive assessments of the work; acquisition, analysis, and interpretation of cognitive data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published. ROB: Substantial contribution to analysis, and interpretation of neuroimaging data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
MAH: Substantial contribution to analysis, and interpretation of neuroimaging data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
NRB: Substantial contribution to analysis, and interpretation of neuroimaging data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
MSK: Substantial contribution to analysis and interpretation of clinical data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
APL: Substantial contribution to conception or design of the work; analysis and interpretation of clinical and neuroimaging data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
UMM: Substantial contribution to conception or design of the work; analysis, and interpretation of clinical, cognitive and neuroimaging data for the work; drafting the manuscript; revising the manuscript critically for important intellectual content; final approval of the version to be published.
MK: Substantial contribution to conception or design of the work; analysis, and interpretation of clinical and cognitive data for the work; revising the manuscript critically for important intellectual content; final approval of the version to be published.
Appendix B. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2021.10.005.
References
- Addington D, Addington J, Schissel B, 1990. A depression rating scale for schizophrenics. Schizophr. Res 3, 247–251. [DOI] [PubMed] [Google Scholar]
- Aleman A, Enriquez-Geppert S, Knegtering H, Dlabac-de Lange JJ, 2018. Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: meta-analysis of controlled trials. Neurosci. Biobehav. Rev 89, 111–118. 10.1016/j.neubiorev.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, Knegtering H, 2017. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr. Res 186, 55–62. 10.1016/j.schres.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Allen G, 1997. Attentional activation of the cerebellum independent of motor involvement. Science 275, 1940–1943. 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, 1989. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 49–58. [PubMed] [Google Scholar]
- Andreasen NC, 1984. Scale for the Assessment of Negative Symptoms/Scale for the Assessment of Positive Symptoms [Manual]. [Google Scholar]
- Andreasen NC, Carpenter WT, Kane JM, Lasser RA, Marder SR, Weinberger DR, 2005. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiatry 162, 441–449. 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M, 1999. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol. Psychiatry 46, 908–920. 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD, 1996. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc. Natl. Acad. Sci. U. S. A 93, 9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS, 1998. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr. Bull 24, 203–218. [DOI] [PubMed] [Google Scholar]
- Basavaraju R, Ithal D, Ramalingaiah AH, Thirthalli J, Mehta UM, Kesavan M, 2020. “Apathetic to hypomanic/manic”: a case series-illustration of emergent mood symptoms during intermittent theta burst stimulation (iTBS) of cerebellar vermis in schizophrenia with predominant negative symptoms. Schizophr. Res 10.1016/j.schres.2020.05.037. [DOI] [PubMed] [Google Scholar]
- Behere RV, Raghunandan V, Venkatasubramanian G, Subbakrishna DK, Jayakumar PN, Gangadhar BN, 2008. Trends-a tool for recognition of emotions in neuropsychiatric disorders. Indian J. Psychol. Med 30, 32. 10.4103/0253-7176.43132. [DOI] [Google Scholar]
- Bhagyavathi HD, Mehta UM, Thirthalli J, Kumar CN, Kumar JK, Subbakrishna DK, Gangadhar BN, 2015. Cascading and combined effects of cognitive deficits and residual symptoms on functional outcome in schizophrenia - a path-analytical approach. Psychiatry Res. 229, 264–271. 10.1016/j.psychres.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Bijsterbosch JD, Barker AT, Lee K-H, Woodruff PWR, 2012. Where does transcranial magnetic stimulation (TMS) stimulate? Modelling of induced field maps for some common cortical and cerebellar targets. Med. Biol. Eng. Comput 50, 671–681. 10.1007/s11517-012-0922-8. [DOI] [PubMed] [Google Scholar]
- Brady RO, Gonsalvez I, Lee I, Ongür D, Seidman LJ, Schmahmann JD, Eack SM, Keshavan MS, Pascual-Leone A, Halko MA, 2019. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatry 10.1176/appi.ajp.2018.18040429appiajp201818040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807–815. 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Burke MJ, Kaptchuk TJ, Pascual-Leone A, 2019. Challenges of differential placebo effects in contemporary medicine: the example of brain stimulation. Ann. Neurol 85, 12–20. 10.1002/ana.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon M, Correll CU, 2014. Thinking and acting beyond the positive: the role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 19 (Suppl. 1), 38–52. 10.1017/S1092852914000601 quiz 35–37, 53. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, 1994. The deficit syndrome. Am. J. Psychiatry 151, 327–329. 10.1176/ajp.151.3.327. [DOI] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K, 2019. Cerebellar modulation of the reward circuitry and social behavior. Science 363, eaav0581. 10.1126/science.aav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, McGrath JJ, Whiteford HA, 2018. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr. Bull 44, 1195–1203. 10.1093/schbul/sby058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-L, Tu P-C, Lee Y-C, Chen Y-S, Li C-T, Su T-P, 2013. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr. Res 149, 26–34. 10.1016/j.schres.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Lawrence Earlbaum Associates, Hillsdale, NJ. [Google Scholar]
- Cole EJ, Stimpson KH, Bentzley BS, Gulser M, Cherian K, Tischler C, Nejad R, Pankow H, Choi E, Aaron H, Espil FM, Pannu J, Xiao X, Duvio D, Solvason HB, Hawkins J, Guerra A, Jo B, Raj KS, Phillips AL, Barmak F, Bishop JH, Coetzee JP, DeBattista C, Keller J, Schatzberg AF, Sudheimer KD, Williams NR, 2020. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am. J. Psychiatry 177, 716–726. 10.1176/appi.ajp.2019.19070720. [DOI] [PubMed] [Google Scholar]
- Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP, 2011. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front. Psychiatry 2, 73. 10.3389/fpsyt.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A, 2010. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr. Res 124, 91–100. 10.1016/j.schres.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N, 2009. A probabilistic MR atlas of the human cerebellum. NeuroImage 46, 39–46. 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Lecardeur L, Morello R, Etard O, 2016. Placebo response in repetitive transcranial magnetic stimulation trials of treatment of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr. Bull 42, 301–308. 10.1093/schbul/sbv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez L, Rogasch NC, Do M, Clark G, Major BP, Teo W-P, Byrne LK, Enticott PG, 2020. Cerebral cortical activity following non-invasive cerebellar stimulation-a systematic review of combined TMS and EEG studies. Cerebellum Lond. Engl 19, 309–335. 10.1007/s12311-019-01093-7. [DOI] [PubMed] [Google Scholar]
- Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A, 2014. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. U. S. A 111, E4367–E4375. 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P, 2015. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr. Bull 41, 892–899. 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Tang X, Wang C, Yu M, Sha W, Wang X, Zhang H, Zhang Xiangrong, Zhang Xiaobin, 2019. Aberrant cerebellar neural activity and cerebro-cerebellar functional connectivity involving executive dysfunction in schizophrenia with primary negative symptoms. Brain Imaging Behav. 10.1007/s11682-018-0032-9. [DOI] [PubMed] [Google Scholar]
- Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N, 2016. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res. 243, 413–420. 10.1016/j.psychres.2016.07.023. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan M, Zhu H, Farchione TR, Mathis M, Mehta M, Uppoor R, Younis I, 2020. The trend of increasing placebo response and decreasing treatment effect in schizophrenia trials continues: an update from the US Food and Drug Administration. J. Clin. Psychiatry 81. 10.4088/JCP.19r12960. [DOI] [PubMed] [Google Scholar]
- Green MF, 2016. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J. Clin. Psychiatry 77 (Suppl. 2), 8–11. 10.4088/JCP.14074su1c.02. [DOI] [PubMed] [Google Scholar]
- Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, Xiao C, Zhao J, 2015. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci. Rep 5, 17275. 10.1038/srep17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zhang F, Liu F, Chen J, Wu R, Chen DQ, Zhang Z, Zhai J, Zhao J, 2018. Cerebellar abnormalities in first-episode, drug-naive schizophrenia at rest. Psychiatry Res. Neuroimaging 276, 73–79. 10.1016/j.pscychresns.2018.03.010. [DOI] [PubMed] [Google Scholar]
- He H, Lu J, Yang L, Zheng J, Gao F, Zhai Y, Feng J, Fan Y, Ma X, 2017. Repetitive transcranial magnetic stimulation for treating the symptoms of schizophrenia: a PRISMA compliant meta-analysis. Clin. Neurophysiol 128, 716–724. 10.1016/j.clinph.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Heath RG, Franklin DE, Walker CF, Keating JW, 1982. Cerebellar vermal atrophy in psychiatric patients. Biol. Psychiatry 17, 569–583. [PubMed] [Google Scholar]
- Helfer B, Samara MT, Huhn M, Klupp E, Leucht C, Zhu Y, Engel RR, Leucht S, 2016. Efficacy and safety of antidepressants added to antipsychotics for schizophrenia: a systematic review and meta-analysis. Am. J. Psychiatry 173, 876–886. 10.1176/appi.ajp.2016.15081035. [DOI] [PubMed] [Google Scholar]
- Honey GD, Pomarol-Clotet E, Corlett PR, Honey RAE, McKenna PJ, Bullmore ET, Fletcher PC, 2005. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain J. Neurol 128, 2597–2611. 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005a. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005b. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hurtado-Puerto AM, Nestor K, Eldaief M, Camprodon JA, 2020. Safety considerations for cerebellar theta burst stimulation. Clin. Ther 42, 1169–1190.e1. 10.1016/j.clinthera.2020.06.001. [DOI] [PubMed] [Google Scholar]
- Insel TR, 2015. The NIMH experimental medicine initiative. World Psychiatry 14, 151–153. 10.1002/wps.20227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A, Abdollahpour I, Abdulkader RS, Abebe Z, Abera SF, Abil OZ, Abraha HN, Abu-Raddad LJ, Abu-Rmeileh NME, Accrombessi MMK, Acharya D, Acharya P, Ackerman IN, Adamu AA, Adebayo OM, Adekanmbi V, Adetokunboh OO, Adib MG, Adsuar JC, Afanvi KA, Afarideh M, Afshin A, Agarwal G, Agesa KM, Aggarwal R, Aghayan SA, Agrawal S, Ahmadi A, Ahmadi M, Ahmadieh H, Ahmed MB, Aichour AN, Aichour I, Aichour MTE, Akinyemiju T, Akseer N, Al-Aly Z, Al-Eyadhy A, Al-Mekhlafi HM, Al-Raddadi RM, Alahdab F, Alam K, Alam T, Alashi A, Alavian SM, Alene KA, Alijanzadeh M, Alizadeh-Navaei R, Aljunid SM, Alkerwi A, Alla F, Allebeck P, Alouani MML, Altirkawi K, Alvis-Guzman N, Amare AT, Aminde LN, Ammar W, Amoako YA, Anber NH, Andrei CL, Androudi S, Animut MD, Anjomshoa M, Ansha MG, Antonio CAT, Anwari P, Arabloo J, Arauz A, Aremu O, Ariani F, Armoon B, Arnl¨ ov J, Arora A, Artaman A, Aryal KK, Asayesh H, Asghar RJ, Ataro Z, ¨ Atre SR, Ausloos M, Avila-Burgos L, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Ayer R, Azzopardi PS, Babazadeh A, Badali H, Badawi A, Bali AG, Ballesteros KE, Ballew SH, Banach M, Banoub JAM, Banstola A, Barac A, Barboza MA, Barker-Collo SL, Barnighausen TW, Barrero LH, ¨ Baune BT, Bazargan-Hejazi S, Bedi N, Beghi E, Behzadifar Masoud, Behzadifar Meysam, Bejot Y, Belachew AB, Belay YA, Bell ML, Bello AK, Bensenor IM, Bernabe E, Bernstein RS, Beuran M, Beyranvand T, Bhala N, Bhattarai S, Bhaumik S, Bhutta ZA, Biadgo B, Bijani A, Bikbov B, Bilano V, Bililign N, Sayeed MSB, Bisanzio D, Blacker BF, Blyth FM, Bou-Orm IR, Boufous S, Bourne R, Brady OJ, Brainin M, Brant LC, Brazinova A, Breitborde NJK, Brenner H, Briant PS, Briggs AM, Briko AN, Britton G, Brugha T, Buchbinder R, Busse R, Butt ZA, Cahuana-Hurtado L, Cano J, Cardenas R, Carrero JJ, Carter A, Carvalho F, Castaneda-Orjuela CA, Rivas J˜C, Castro F, Catala-L opez F, Cercy KM, Cerin E, Chaiah Y, Chang AR, Chang H-Y, Chang J-C, Charlson FJ, Chattopadhyay A, Chattu VK, Chaturvedi P, Chiang PP-C, Chin KL, Chitheer A, Choi J-YJ, Chowdhury R, Christensen H, Christopher DJ, Cicuttini FM, Ciobanu LG, Cirillo M, Claro RM, Collado-Mateo D, Cooper C, Coresh J, Cortesi PA, Cortinovis M, Costa M, Cousin E, Criqui MH, Cromwell EA, Cross M, Crump JA, Dadi AF, Dandona L, Dandona R, Dargan PI, Daryani A, Gupta RD, Neves JD, Dasa TT, Davey G, Davis AC, Davitoiu DV, Courten BD, Hoz FPDL, Leo DD, Neve J-WD, Degefa MG, Degenhardt L, Deiparine S, Dellavalle RP, Demoz GT, Deribe K, Dervenis N, Jarlais DCD, Dessie GA, Dey S, Dharmaratne SD, Dinberu MT, Dirac MA, Djalalinia S, Doan L, Dokova K, Doku DT, Dorsey ER, Doyle KE, Driscoll TR, Dubey M, Dubljanin E, Duken EE, Duncan BB, Duraes AR, Ebrahimi H, Ebrahimpour S, Echko MM, Edvardsson D, Effiong A, Ehrlich JR, Bcheraoui CE, Zaki MES, El-Khatib Z, Elkout H, Elyazar IRF, Enayati A, Endries AY, Er B, Erskine HE, Eshrati B, Eskandarieh S, Esteghamati A, Esteghamati S, Fakhim H, Omrani VF, Faramarzi M, Fareed M, Farhadi F, Farid TA, Farinha C.S.E.sa, Farioli A, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fentahun N, Fereshtehnejad S-M, Fernandes E, Fernandes JC, Ferrari AJ, Feyissa GT, Filip I, Fischer F, Fitzmaurice C, Foigt NA, Foreman KJ, Fox J, Frank TD, Fukumoto T, Fullman N, Fürst T, Furtado JM, Futran ND, Gall S, Ganji M, Gankpe FG, Garcia-Basteiro AL, Gardner WM, Gebre AK, Gebremedhin AT, Gebremichael TG, Gelano TF, Geleijnse JM, Genova-Maleras R, Geramo YCD, Gething PW, Gezae KE, Ghadiri K, Falavarjani KG, Ghasemi-Kasman M, Ghimire M, Ghosh R, Ghoshal AG, Giampaoli S, Gill PS, Gill TK, Ginawi IA, Giussani G, Gnedovskaya EV, Goldberg EM, Goli S, Gomez-Dantes H, Gona PN, Gopalani SV, Gorman TM, Goulart AC, Goulart BNG, Grada A, Grams ME, Grosso G, Gugnani HC, Guo Y, Gupta PC, Gupta Rahul, Gupta Rajeev, Gupta T, Gyawali B, Haagsma JA, Hachinski V, Hafezi-Nejad N, Bidgoli HH, Hagos TB, Hailu GB, Haj-Mirzaian Arvin, Haj-Mirzaian Arya, Hamadeh RR, Hamidi S, Handal AJ, Hankey GJ, Hao Y, Harb HL, Harikrishnan S, Haro JM, Hasan M, Hassankhani H, Hassen HY, Havmoeller R, Hawley CN, Hay RJ, Hay SI, Hedayatizadeh-Omran A, Heibati B, Hendrie D, Henok A, Herteliu C, Heydarpour S, Hibstu DT, Hoang HT, Hoek HW, Hoffman HJ, Hole MK, Rad EH, Hoogar P, Hosgood HD, Hosseini SM, Hosseinzadeh M, Hostiuc M, Hostiuc S, Hotez PJ, Hoy DG, Hsairi M, Htet AS, Hu G, Huang JJ, Huynh CK, Iburg KM, Ikeda CT, Ileanu B, Ilesanmi OS, Iqbal U, Irvani SSN, Irvine CMS, Islam SMS, Islami F, Jacobsen KH, Jahangiry L, Jahanmehr N, Jain SK, Jakovljevic M, Javanbakht M, Jayatilleke AU, Jeemon P, Jha RP, Jha V, Ji JS, Johnson CO, Jonas JB, Jozwiak JJ, Jungari SB, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kalani R, Kanchan T, Karami M, Matin BK, Karch A, Karema C, Karimi N, Karimi SM, Kasaeian A, Kassa DH, Kassa GM, Kassa TD, Kassebaum NJ, Katikireddi SV, Kawakami N, Karyani AK, Keighobadi MM, Keiyoro PN, Kemmer L, Kemp GR, Kengne AP, Keren A, Khader YS, Khafaei B, Khafaie MA, Khajavi A, Khalil IA, Khan EA, Khan MS, Khan MA, Khang Y-H, Khazaei M, Khoja AT, Khosravi A, Khosravi MH, Kiadaliri AA, Kiirithio DN, Kim C-I, Kim D, Kim P, Kim Y-E, Kim YJ, Kimokoti RW, Kinfu Y, Kisa A, Kissimova-Skarbek K, Kivimaki M, Knudsen AKS, Kocarnik JM, Kochhar S, Kokubo Y, Kolola T, ¨ Kopec JA, Kosen S, Kotsakis GA, Koul PA, Koyanagi A, Kravchenko MA, Krishan K, Krohn KJ, Defo BK, Bicer BK, Kumar GA, Kumar M, Kyu HH, Lad DP, Lad SD, Lafranconi A, Lalloo R, Lallukka T, Lami FH, Lansingh VC, Latifi A, Lau KM-M, Lazarus JV, Leasher JL, Ledesma JR, Lee PH, Leigh J, Leung J, Levi M, Lewycka S, Li S, Li Y, Liao Y, Liben ML, Lim L-L, Lim SS, Liu S, Lodha R, Looker KJ, Lopez AD, Lorkowski S, Lotufo PA, Low N, Lozano R, Lucas TCD, Lucchesi LR, Lunevicius R, Lyons RA, Ma S, Macarayan ERK, Mackay MT, Madotto F, Razek HMAE, Razek MMAE, Maghavani DP, Mahotra NB, Mai HT, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malta DC, Mamun AA, Manda A-L, Manguerra H, Manhertz T, Mansournia MA, Mantovani LG, Mapoma CC, Maravilla JC, Marcenes W, Marks A, Martins-Melo FR, Martopullo I, Marz W, Marzan MB, Mashamba-Thompson TP, Massenburg BB, Mathur MR, Matsushita K, Maulik PK, Mazidi M, McAlinden C, McGrath JJ, McKee M, Mehndiratta MM, Mehrotra R, Mehta KM, Mehta V, Mejia-Rodriguez F, Mekonen T, Melese A, Melku M, Meltzer M, Memiah PTN, Memish ZA, Mendoza W, Mengistu DT, Mengistu G, Mensah GA, Mereta ST, Meretoja A, Meretoja TJ, Mestrovic T, Mezerji NMG, Miazgowski B, Miazgowski T, Millear AI, Miller TR, Miltz B, Mini GK, Mirarefin M, Mirrakhimov EM, Misganaw AT, Mitchell PB, Mitiku H, Moazen B, Mohajer B, Mohammad KA, Mohammadifard N, Mohammadnia-Afrouzi M, Mohammed MA, Mohammed S, Mohebi F, Moitra M, Mokdad AH, Molokhia M, Monasta L, Moodley Y, Moosazadeh M, Moradi G, Moradi-Lakeh M, Moradinazar M, Moraga P, Morawska L, Velasquez IM, Morgado-Da-Costa J, Morrison SD, Moschos MM, Mountjoy-Venning WC, Mousavi SM, Mruts KB, Muche AA, Muchie KF, Mueller UO, Muhammed OS, Mukhopadhyay S, Muller K, Mumford JE, Murhekar M, Musa J, Musa KI, Mustafa G, Nabhan AF, Nagata C, Naghavi M, Naheed A, Nahvijou A, Naik G, Naik N, Najafi F, Naldi L, Nam HS, Nangia V, Nansseu JR, Nascimento BR, Natarajan G, Neamati N, Negoi I, Negoi RI, Neupane S, Newton CRJ, Ngunjiri JW, Nguyen AQ, Nguyen Ha Thu, Nguyen HLT, Nguyen Huong Thanh, Nguyen LH, Nguyen M, Nguyen NB, Nguyen SH, Nichols E, Ningrum DNA, Nixon MR, Nolutshungu N, Nomura S, Norheim OF, Noroozi M, Norrving B, Noubiap JJ, Nouri HR, Shiadeh MN, Nowroozi MR, Nsoesie EO, Nyasulu PS, Odell CM, Ofori-Asenso R, Ogbo FA, Oh I-H, Oladimeji O, Olagunju AT, Olagunju TO, Olivares PR, Olsen HE, Olusanya BO, Ong KL, Ong SK, Oren E, Ortiz A, Ota E, Otstavnov SS, Øverland S, Owolabi MO, Pacella R, Pakpour AH, Pana A, Panda-Jonas S, Parisi A, Park E-K, Parry CDH, Patel S, Pati S, Patil ST, Patle A, Patton GC, Paturi VR, Paulson KR, Pearce N, Pereira DM, Perico N, A MP, Pesudovs K, Pham HQ, Phillips MR, Pigott DM, Pillay JD, Piradov MA, Pirsaheb M, Pishgar F, Plana-Ripoll O, Plass D, Polinder S, Popova S, Postma MJ, Pourshams A, Poustchi H, Prabhakaran D, Prakash S, Prakash V, Purcell CA, Purwar MB, Qorbani M, Quistberg DA, Radfar A, Rafay A, Rafiei A, Rahim F, Rahimi K, Rahimi-Movaghar A, Rahimi-Movaghar V, Rahman M, Rahman M.H.ur, Rahman MA, Rahman SU, Rai RK, Rajati F, Ram U, Ranjan P, Ranta A, Rao PC, Rawaf DL, Rawaf S, Reddy KS, Reiner RC, Reinig N, Reitsma MB, Remuzzi G, Renzaho AMN, Resnikoff S, Rezaei S, Rezai MS, Ribeiro ALP, Roberts NLS, Robinson SR, Roever L, Ronfani L, Roshandel G, Rostami A, Roth GA, Roy A, Rubagotti E, Sachdev PS, Sadat N, Saddik B, Sadeghi E, Moghaddam SS, Safari H, Safari Y, Safari-Faramani R, Safdarian M, Safi S, Safiri S, Sagar R, Sahebkar A, Sahraian MA, Sajadi HS, Salam N, Salama JS, Salamati P, Saleem K, Saleem Z, Salimi Y, Salomon JA, Salvi SS, Salz I, Samy AM, Sanabria J, Sang Y, Santomauro DF, Santos IS, Santos JV, Milicevic MMS, Jose BPS, Sardana M, Sarker AR, Sarrafzadegan N, Sartorius B, Sarvi S, Sathian B, Satpathy M, Sawant AR, Sawhney M, Saxena S, Saylan M, Schaeffner E, Schmidt MI, Schneider IJC, Schottker B, Schwebel DC, Schwendicke F, Scott JG, Sekerija M, Sepanlou S¨G, Servan-Mori E, Seyedmousavi S, Shabaninejad H, Shafieesabet A, Shahbazi M, Shaheen AA, Shaikh MA, Shams-Beyranvand M, Shamsi M, Shamsizadeh M, Sharafi H, Sharafi K, Sharif M, Sharif-Alhoseini M, Sharma M, Sharma R, She J, Sheikh A, Shi P, Shibuya K, Shigematsu M, Shiri R, Shirkoohi R, Shishani K, Shiue I, Shokraneh F, Shoman H, Shrime MG, Si S, Siabani S, Siddiqi TJ, Sigfusdottir ID, Sigurvinsdottir R, Silva JP, Silveira DGA, Singam NSV, Singh JA, Singh NP, Singh V, Sinha DN, Skiadaresi E, Slepak ELN, Sliwa K, Smith DL, Smith M, Filho AMS, Sobaih BH, Sobhani S, Sobngwi E, Soneji SS, Soofi M, Soosaraei M, Sorensen RJD, Soriano JB, Soyiri IN, Sposato LA, Sreeramareddy CT, Srinivasan V, Stanaway JD, Stein DJ, Steiner C, Steiner TJ, Stokes MA, Stovner LJ, Subart ML, Sudaryanto A, Sufiyan MB, Sunguya BF, Sur PJ, Sutradhar I, Sykes BL, Sylte DO, Tabares-Seisdedos R, Tadakamadla SK, Tadesse BT, Tandon N, Tassew SG, Tavakkoli M, Taveira N, Taylor HR, Tehrani-Banihashemi A, Tekalign TG, Tekelemedhin SW, Tekle MG, Temesgen H, Temsah M-H, Temsah O, Terkawi AS, Teweldemedhin M, Thankappan KR, Thomas N, Tilahun B, To QG, Tonelli M, Topor-Madry R, Topouzis F, Torre AE, Tortajada-Girbes M, Touvier M, Tovani-Palone MR, Towbin JA, Tran BX, Tran KB, Troeger CE, Truelsen TC, Tsilimbaris MK, Tsoi D, Car LT, Tuzcu EM, Ukwaja KN, Ullah I, Undurraga EA, Unutzer J, Updike RL, Usman MS, Uthman OA, Vaduganathan M, Vaezi A, Valdez PR, Varughese S, Vasankari TJ, Venketasubramanian N, Villafaina S, Violante FS, Vladimirov SK, Vlassov V, Vollset SE, Vosoughi K, Vujcic IS, Wagnew FS, Waheed Y, Waller SG, Wang Y, Wang Y-P, Weiderpass E, Weintraub RG, Weiss DJ, Weldegebreal F, Weldegwergs KG, Werdecker A, West TE, Whiteford HA, Widecka J, Wijeratne T, Wilner LB, Wilson S, Winkler AS, Wiyeh AB, Wiysonge CS, Wolfe CDA, Woolf AD, Wu S, Wu Y-C, Wyper GMA, Xavier D, Xu G, Yadgir S, Yadollahpour A, Jabbari SHY, Yamada T, Yan LL, Yano Y, Yaseri M, Yasin YJ, Yeshaneh A, Yimer EM, Yip P, Yisma E, Yonemoto N, Yoon S-J, Yotebieng M, Younis MZ, Yousefifard M, Yu C, Zadnik V, Zaidi Z, Zaman SB, Zamani M, Zare Z, Zeleke AJ, Zenebe ZM, Zhang K, Zhao Z, Zhou M, Zodpey S, Zucker I, Vos T, Murray CJL, 2018. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 392, 1789–1858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JL, Diehl C, Schleifer C, Tamminga CA, Keshavan MS, Sweeney JA, Clementz BA, Hill SK, Pearlson G, Yang G, Creatura G, Krystal JH, Repovs G, Murray J, Winkler A, Anticevic A, 2019. Schizophrenia exhibits bi-directional brain-wide alterations in cortico-striato-cerebellar circuits. Cereb. Cortex N. Y. N 1991 (29), 4463–4487. 10.1093/cercor/bhy306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy NI, Lee WH, Frangou S, 2018. Efficacy of non-invasive brain stimulation on the symptom dimensions of schizophrenia: a meta-analysis of randomized controlled trials. Eur. Psychiatry 49, 69–77. 10.1016/j.eurpsy.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Lee K-H, Oh H, Suh J-HS, Cho KIK, Yoon YB, Shin W-G, Lee TY, Kwon JS, 2019. Functional and structural connectivity of the cerebellar nuclei with the striatum and cerebral cortex in first-episode psychosis. J. Neuropsychiatry Clin. Neurosci 31, 143–151. 10.1176/appi.neuropsych.17110276. [DOI] [PubMed] [Google Scholar]
- Liu H, Fan G, Xu K, Wang F, 2011. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J. Magn. Reson. Imaging 34, 1430–1438. 10.1002/jmri.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual-Leone A, 2011. Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol. Psychiatry 70, 19–27. 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta UM, Thirthalli J, Naveen Kumar C, Mahadevaiah M, Rao K, Subbakrishna DK, Gangadhar BN, Keshavan MS, 2011. Validation of social cognition rating tools in indian setting (SOCRATIS): a new test-battery to assess social cognition. Asian J. Psychiatry 4, 203–209. 10.1016/j.ajp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool E-M, Rehme AK, Eickhoff SB, Fink GR, Grefkes C, 2014. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J. Neurosci 34, 6849–6859. 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK, 2004. Identification of separable cognitive factors in schizophrenia. Schizophr. Res 72, 29–39. 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Osoegawa C, Gomes JS, Grigolon RB, Brietzke E, Gadelha A, Lacerda ALT, Dias AM, Cordeiro Q, Laranjeira R, de Jesus D, Daskalakis ZJ, Brunelin J, Cordes J, Trevizol AP, 2018. Non-invasive brain stimulation for negative symptoms in schizophrenia: an updated systematic review and meta-analysis. Schizophr. Res 197, 34–44. 10.1016/j.schres.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, Bashir S, Vernet M, Shafi M, Westover B, Vahabzadeh-Hagh AM, Rotenberg A, 2011. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 24, 302–315. 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitkin FM, McGrath PJ, Rabkin JG, Stewart JW, Harrison W, Ross DC, Tricamo E, Fleiss J, Markowitz J, Klein DF, 1991. Different types of placebo response in patients receiving antidepressants. Am. J. Psychiatry 148, 197–203. 10.1176/ajp.148.2.197. [DOI] [PubMed] [Google Scholar]
- Rao SL, Subbukrishna DK, Gopukuar K, 2004. NIMHANS Neuropsychology Battery. NIMHANS Publications, Bangalore. [Google Scholar]
- Rao et al. , n.d., SL Rao DK Subbukrishna K Gopukuar n.d. NIMHANS Neuropsychology Battery, 2004th ed. NIMHANS Publications. [Google Scholar]
- Razza LB, Moffa AH, Moreno ML, Carvalho AF, Padberg F, Fregni F, Brunoni AR, 2018. A systematic review and meta-analysis on placebo response to repetitive transcranial magnetic stimulation for depression trials. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 81, 105–113. 10.1016/j.pnpbp.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, 2011. Screening questionnaire before TMS: an update. Clin. Neurophysiol 122, 1686. 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol 120, 2008–2039. 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lücking CH, 1994. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol 91, 79–92. [DOI] [PubMed] [Google Scholar]
- Rüsch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G, 2007. Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr. Res 93, 79–89. 10.1016/j.schres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Pott E, Tandler JM, Wall MM, Roose SP, Lieberman JA, 2014. Placebo response in antipsychotic clinical trials: a meta-analysis. JAMA Psychiatry 71, 1409–1421. 10.1001/jamapsychiatry.2014.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Roose SP, 2013. A model of placebo response in antidepressant clinical trials. Am. J. Psychiatry 170, 723–733. 10.1176/appi.ajp.2012.12040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Velez C, Yusim A, Anbarasan D, Lindenmayer J-P, 2009. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J. Clin. Psychiatry 70, 104–112. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, 2019. The cerebellum and cognition. Neurosci. Lett 688, 62–75. 10.1016/j.neulet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M, 1999. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. NeuroImage 10, 233–260. 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Shafi MM, Westover MB, Fox MD, Pascual-Leone A, 2012. Exploration and modulation of brain network interactions with noninvasive brain stimulation in combination with neuroimaging. Eur. J. Neurosci 35, 805–825. 10.1111/j.1460-9568.2012.08035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 22–33 quiz 34–57. [PubMed] [Google Scholar]
- Simpson GM, Angus JW, 1970. A rating scale for extrapyramidal side effects. Acta Psychiatr. Scand. Suppl 212, 11–19. [DOI] [PubMed] [Google Scholar]
- Singh SP, Singh V, 2011. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs 25, 859–885. 10.2165/11586650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98. 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Strassnig MT, Raykov T, O’Gorman C, Bowie CR, Sabbag S, Durand D, Patterson TL, Pinkham A, Penn DL, Harvey PD, 2015. Determinants of different aspects of everyday outcome in schizophrenia: the roles of negative symptoms, cognition, and functional capacity. Schizophr. Res 165, 76–82. 10.1016/j.schres.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarcijonas G, Foran W, Haas GL, Luna B, Sarpal DK, 2019. Intrinsic connectivity of the globus pallidus: an uncharted marker of functional prognosis in people with first-episode schizophrenia. Schizophr. Bull 10.1093/schbul/sbz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B, 1997. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. the ataxia neuropharmacology Committee of the World Federation of neurology. J. Neurol. Sci 145, 205–211. [DOI] [PubMed] [Google Scholar]
- Tsapakis EM, Dimopoulou T, Tarazi FI, 2015. Clinical management of negative symptoms of schizophrenia: an update. Pharmacol. Ther 153, 135–147. 10.1016/j.pharmthera.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Foussias G, Lerch J, Felsky D, Remington G, Rajji TK, Lobaugh N, Pollock BG, Mulsant BH, 2013. Neuroimaging evidence for the deficit subtype of schizophrenia. JAMA Psychiatry 70, 472–480. 10.1001/jamapsychiatry.2013.786. [DOI] [PubMed] [Google Scholar]
- Weimer K, Colloca L, Enck P, 2015. Placebo eff ects in psychiatry: mediators and moderators. Lancet Psychiatry 2, 246–257. 10.1016/S2215-0366(14)00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AL, Wessa M, Szeszko PR, Foussias G, Chakravarty MM, Lerch JP, DeRosse P, Remington G, Mulsant BH, Linke J, Malhotra AK, Voineskos AN, 2015. Further neuroimaging evidence for the deficit subtype of schizophrenia: a cortical connectomics analysis. JAMA Psychiatry 72, 446–455. 10.1001/jamapsychiatry.2014.3020. [DOI] [PubMed] [Google Scholar]
- Williams NR, Sudheimer KD, Bentzley BS, Pannu J, Stimpson KH, Duvio D, Cherian K, Hawkins J, Scherrer KH, Vyssoki B, DeSouza D, Raj KS, Keller J, Schatzberg AF, 2018. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain 141, e18. 10.1093/brain/awx379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. NeuroImage 92, 381–397. 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J, 1992. Negative symptoms and hypofrontality in chronic schizophrenia. Arch. Gen. Psychiatry 49, 959–965. 10.1001/archpsyc.1992.01820120047007. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM, 2001. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage 14, 1370–1386. 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Zhuo C, Wang C, Wang L, Guo X, Xu Q, Liu Y, Zhu J, 2018. Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging Behav. 12, 383–389. 10.1007/s11682-017-9704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.