Abstract
In response to epidermal growth factor (EGF), the EGF receptor is endocytosed and degraded. A substantial lag period exists between endocytosis and degradation, suggesting that endocytosis is more than a simple negative feedback. Phospholipase D (PLD), which has been implicated in vesicle formation in the Golgi, is activated in response to EGF and other growth factors. We report here that EGF receptor endocytosis is dependent upon PLD and the PLD1 regulators, protein kinase C α and RalA. EGF-induced receptor degradation is accelerated by overexpression of either wild-type PLD1 or PLD2 and retarded by overexpression of catalytically inactive mutants of either PLD1 or PLD2. EGF-induced activation of mitogen-activated protein kinase, which is dependent upon receptor endocytosis, is also dependent upon PLD. These data suggest a role for PLD in signaling that facilitates receptor endocytosis.
Phospholipase D (PLD) is a widely distributed enzyme that hydrolyzes phosphatidylcholine, a major phospholipid in the cell membrane, to form phosphatidic acid (PA) and choline. PLD activity, which can be detected in virtually all cell types as well as in most cellular organelles, is believed to play an important role in the regulation of cell physiology by extracellular signals, such as hormones, neurotransmitters, growth factors, and cytokines (8). Multiple PLD activities have been characterized in mammalian cells, and more recently, two mammalian PLD genes (PLD1 and PLD2) have been cloned (6, 11, 18, 22, 30). Recent studies indicate that PLD has many different functions in signal transduction, vesicle trafficking, and cytoskeletal dynamics (21). Vesicle budding in the Golgi network was shown to be mediated in part by Arf family GTPases (35). The discovery that Arf family GTPases regulate PLD activity (3, 5) suggested the possibility that PLD was also involved in vesicle transport. Consistent with this idea, PA formation by PLD-mediated hydrolysis of phosphatidylcholine has been reported to be required for the formation of Golgi vesicles (20) and for the transport of vesicles from the endoplasmic reticulum to the Golgi complex (1). PLD has also been reported to stimulate the release of secretory vesicles from the trans-Golgi network (4). It was therefore proposed that the role that Arf plays in vesicle budding in the Golgi network is to regulate PLD activity and PA production (15, 33, 34). However, there is controversy on this point (2, 40, 41), and it still is not clear how PLD and its primary metabolite, PA, might contribute to vesicle formation.
PLD activity is elevated in response to many extracellular signals (8). Our laboratory has investigated the role PLD plays in the transduction of intracellular signals initiated by epidermal growth factor (EGF) (13, 23, 38). In response to EGF, the EGF receptor is internalized and then degraded (10). The internalization of the EGF receptor is a process that involves endocytic vesicles (10). Since PLD has been implicated in vesicle formation and membrane traffic as discussed above, we hypothesized that PLD might play a role in receptor endocytosis as well.
MATERIALS AND METHODS
Materials.
EGF and Gö6976 were purchased from Calbiochem. 1-Butanol (1-BtOH) and iso-butanol (iso-BtOH) were from Sigma. Monoclonal antibody (LA22) to the EGF receptor was obtained from Upstate Biotechnology. Polyclonal antibody (Y11) raised against the Flu tag was from Santa Cruz Biotechnology. The polyclonal anti-p42/44, anti-phospho-p42/44, anti-MEK1/2, and anti-phospho-MEK1/2 antibodies were from New England Biolabs. The secondary antibodies to rabbit or mouse immunoglobulin G (IgG) conjugated with horseradish peroxidase were from Bio-Rad. The anti-mouse IgG conjugated with rhodamine red-X and the anti-rabbit IgG conjugated with cyanine were from Jackson ImmunoResearch.
Cell lines and culture conditions.
The construction of EGFR cells (3Y1 rat fibroblasts overexpressing the EGF receptor) (13) and the establishment of EGFR cells expressing the wild type and the S28N mutant of RaIA (23) were described previously. EGFR cells expressing human PLD1 (hPLD1), mouse PLD2 (mPLD2), and the catalytically inactive mutants hPLD1-K898R and mPLD2-K758R were obtained by cotransfection with the hygromycin B selection vector pCEP4 (Invitrogen), using Lipofectamine Plus reagent (GIBCO) according to the vendor's instructions. Transfected cultures were selected with hygromycin B (200 μg/ml) for 10 to 14 days at 37°C. At that time antibiotic-resistant colonies were pooled and expanded for further analysis under selective conditions. Plasmid expression vectors for PLD1 (pCGN-hPLD1) (11), PLD2 (pCGN-mPLD2) (6), hPLD1-K898R (pCGN-hPLD1-K898R) (43), and mPLD2-K758R (pCGN-mPLD2-K758R) (42, 43) were the generous gift of Michael Frohman (State University of New York—Stony Brook). All of the PLD proteins expressed were Flu tagged and could be detected using antibody raised against the Flu epitope. All cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 5% bovine calf serum (HyClone) as described previously (13). Cells were grown to confluence and then made quiescent by replacement with fresh medium containing 0.5% bovine calf serum 1 day before experiment.
PLD assays.
PLD activity was assayed as follows. Cells were grown in DMEM supplemented with 5% bovine calf serum (HyClone) in 60-mm culture dishes. Confluent cells were then made quiescent by being shifted into DMEM containing 0.5% bovine calf serum for 1 day and then were prelabeled for 4 h with 3 μCi (40 Ci/mmol) of [3H]myristate in 3 ml of medium. PLD catalyzed transphosphatidylation in the presence of 1% 1-BtOH (or iso-BtOH, when indicated below), and the extraction and characterization of lipids by thin-layer chromatography (TLC) were performed as previously described (39).
Plasma membrane preparation.
Plasma membrane was prepared as follows. Cells were grown to confluence in 150-mm culture dishes with 5% bovine calf serum in DMEM and made quiescent by shifting them into medium containing 0.5% bovine calf serum for 1 day. After EGF treatment (30 min), cells were washed twice with phosphate-buffered saline (PBS) (136 mM NaCl, 2.6 mM KCl, 1.4 mM KH2PO4, 4.2 mM Na2HPO4 [pH 7.4]) and collected in buffer A (0.25 M sucrose, 1 mM EDTA, 20 mM Tricine [pH 7.8]). The preparation of the plasma membrane fractions was carried out using a method developed by Smart and colleagues (37). Briefly, cells were homogenized in a Wheaton tissue grinder with 25 to 30 strokes followed by centrifugation at 1,000 × g for 10 min. The post nuclear supernatant was layered on top of 23 ml of 30% Percoll in buffer A and centrifuged at 84,000 × g for 30 min in a Ti 60 rotor (Beckman) at 4°C. The plasma membrane was collected and analyzed. This fraction contained all of the plasma membrane markers but lacked protein markers for cytoplasm and other cell organelles (37).
Western analysis.
Extraction of proteins from cultured cells was performed as previously described (13). Equal amounts of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis using an 8% acrylamide separating gel, transferred to nitrocellulose filters, and blocked at room temperature for 1 h with 5% nonfat dry milk in PBS. The nitrocellulose filters were washed in PBS with 0.05% Tween 20 and then incubated with antibodies as described in the figure legends. Depending upon the origin of the primary antibodies, either anti-mouse or anti-rabbit IgG conjugated with horseradish peroxidase was used for detection with the enhanced chemiluminescence system (Pierce).
Immunofluorescence microscopy.
EGFR cells were plated onto coverslips in six-well plates. Twenty-four hours later the cells were transfected with 1 μg of plasmid DNA using Lipofectamine Plus reagent (GIBCO). One day after transfection, the cells were made quiescent by shifting them into medium containing 0.5% serum for 24 h. The cells were then washed twice with PBS, fixed in 3.7% paraformaldehyde (Sigma) in PBS for 10 min at room temperature, washed with PBS, and then permeabilized by incubation with 0.2% Triton X-100–PBS for 5 min. After being washed with PBS, cells were incubated with 0.2% bovine serum albumin–PBS for 5 min and then subjected to successive incubation with primary and fluorophore-conjugated secondary antibodies. PLD proteins were detected using rabbit polyclonal anti-hemagglutinin antibody (Y11) and anti-rabbit IgG conjugated with cyanine (green). The EGF receptor was detected by using mouse monoclonal anti-EGF receptor antibody (LA22) and anti-mouse IgG conjugated with rhodamine red-X (red). Each antibody incubation was done in 2% bovine serum albumin–PBS at room temperature for 1 h followed by rinsing with PBS. After a final rinse with PBS, coverslips were mounted in 50% glycerol in PBS and visualized using a Nikon Optiphot 2 fluorescent microscope.
RESULTS
EGF-induced receptor degradation is inhibited by primary, but not secondary, alcohol.
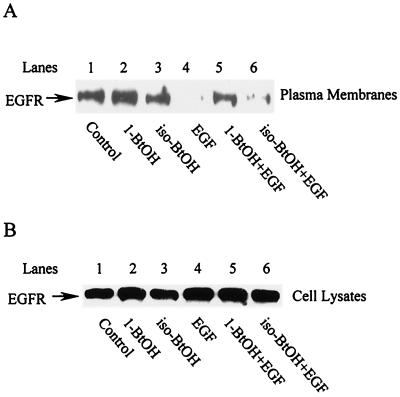
PLD activity is frequently detected by virtue of the transphosphatidylation reaction, whereby the enzyme preferentially utilizes a primary alcohol over water to generate a phosphatidylalcohol instead of PA. Thus, the presence of a primary alcohol inhibits the production of PA by PLD; primary alcohols have been widely used to implicate PLD activity in a variety of cellular functions (28). The reaction is highly specific for primary alcohols; secondary alcohols are not utilized by PLD. In Fig. 1, the activation of PLD activity and PA production by EGFR cells (13) is shown. In response to EGF, an approximately eightfold increase (densitometry quantitation, not shown) in the transphosphatidylation product phosphatidylbutanol (PBt) was seen only when the primary alcohol 1-BtOH was used (Fig. 1, upper panel, lanes 3 and 4). PBt was not detectable when the secondary alcohol iso-BtOH was used (Fig. 1, upper panel, lanes 5 and 6). Consistent with this observation, increased PA was seen when the secondary alcohol was present but not when the primary alcohol was used (Fig. 1, lower panel, compare lanes 4 and 6). Thus, 1-BtOH inhibited EGF-induced PA production, whereas the secondary alcohol iso-BtOH, which did not inhibit EGF-induced PA production, served as a negative control for the effects of 1-BtOH.
FIG. 1.
EGF-induced PA production is inhibited by primary, but not secondary, alcohol. EGFR cells were prelabeled with [3H]myristate for 4 h and then treated with EGF (100 ng/ml) for 10 min as indicated. Where indicated, 1-BtOH (lanes 3 and 4) or iso-BtOH (lanes 5 and 6) was added 5 min prior to EGF treatment to a concentration of 1%. PA and the transphosphatidylation product PBt were separated by TLC and visualized by autoradiography of TLC plates as described in Materials and Methods. The data were from the same TLC chromatography plate; however, the PA bands were exposed longer than the PBt bands. The data presented are representative results of an experiment that was repeated three times.
Degradation of the EGF receptor in response to EGF was detected by monitoring the amount of receptor by using Western blot analysis. As shown in Fig. 2A, the EGF receptor was almost depleted between 4 and 6 h after EGF treatment in the EGFR cells. The loss of the receptor in response to EGF was strongly inhibited by the primary (1-BtOH), but not the secondary (iso-BtOH), alcohol (Fig. 2B). However, 1-BtOH had no effect upon autophosphorylation of the receptor induced by EGF (data not shown), indicating that the inhibitory effect was not due to interference of the alcohol with receptor dimerization or kinase activity. These data suggest a PLD requirement for receptor degradation.
FIG. 2.
EGF-induced receptor degradation is inhibited by primary, but not secondary, alcohol. (A) The EGFR cells were treated with EGF (100 ng/ml) and at the times shown, cells were harvested, lysed, and subjected to Western blot analysis using an anti-EGF receptor antibody (LA22). (B) EGFR cells were treated with EGF for 4 h in the absence or presence of either 1-BtOH (1%) or iso-BtOH (1%) as shown, and then the cell lysates were subjected to Western blot analysis as for panel A. The alcohols were added 5 min prior to EGF treatment. The amount of protein loaded for all lanes was normalized for total protein. The data presented are representative results of an experiment that was repeated three times.
EGF-induced receptor internalization is inhibited by primary, but not secondary, alcohol.
The internalization of the EGF receptor was determined by the disappearance of the EGF receptor from the plasma membranes. Plasma membranes were isolated and examined for the presence of the EGF receptor by Western blot analysis. Within 30 min of EGF treatment, there was almost a complete depletion of the EGF receptor from the plasma membrane fraction (Fig. 3A, compare lanes 1 and 4). In contrast, the plasma membrane protein Na+/K+ ATPase was not lost from the plasma membrane fraction (data not shown). At this time point, there was no detectable loss of the EGF receptor in whole-cell lysates (Fig. 3B, lane 4, and Fig. 2A, lane 2), indicating that the loss was due to internalization, not degradation. The loss of the EGF receptor from the plasma membrane fraction was inhibited by 1-BtOH but not by iso-BtOH (Fig. 3A, compare lanes 5 and 6), suggesting that PLD is a mediator of receptor internalization and that it is at the level of internalization that PLD is critical.
FIG. 3.
EGF-induced receptor internalization is inhibited by primary, but not secondary, alcohol. (A) EGFR cells were treated with EGF (100 ng/ml) for 30 min in the absence or presence of alcohols (1%) as indicated. Alcohols were added 5 min prior to EGF treatment. Cells were harvested and plasma membranes were isolated as described in Materials and Methods. The plasma membrane fractions were then subjected to Western blot analysis using an anti-EGF receptor antibody (LA22). (B) Total EGF receptor levels in the cells from which plasma membranes were isolated were determined as for Fig. 2A. The cells were harvested at the same times as those shown in panel A. The amount of protein loaded for all lanes was normalized for total protein. The data presented are representative results of an experiment that was repeated three times.
EGF-induced receptor internalization and degradation are dependent on RalA.
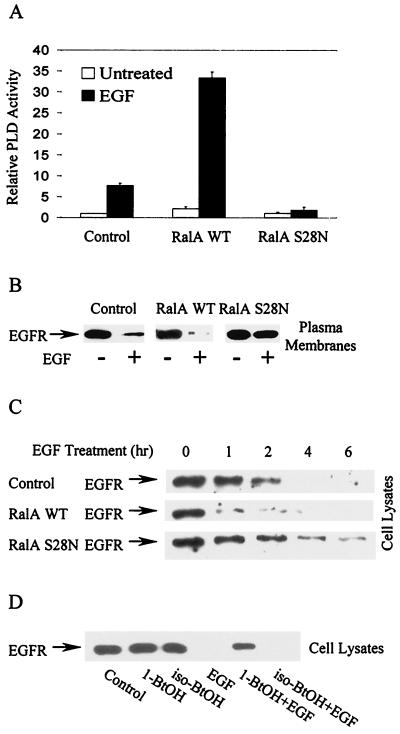
It was recently reported that EGF-induced PLD activity is dependent upon RalA (23, 45), a Ras family GTPase (9) that is associated with PLD1 (14, 25). The generation of EGFR cells that also overexpress either wild-type RalA or a dominant negative allele of RalA (S28N) was described previously (23). The activation of PLD activity in these and the parental cells was examined, and as shown in Fig. 4A, wild-type RalA enhanced and the S28N RalA mutant inhibited EGF-induced PLD activity. We then examined EGF-induced receptor internalization in these cells. As shown in Fig. 4B, overexpression of the wild-type RalA increased the rate of receptor loss from the plasma membrane in response to EGF, whereas overexpression of the dominant negative RalA substantially reduced EGF-induced receptor internalization. Similarly, EGF receptor degradation was substantially increased by wild-type RalA overexpression and was retarded by expression of the dominant negative RalA (Fig. 4C). Thus, higher levels of induced PLD activity seen in the cells overexpressing wild-type RalA corresponded with an increased rate of internalization and degradation of the EGF receptor. Furthermore, the increased turnover of the EGF receptor in the cells overexpressing wild-type RalA was inhibited by 1-BtOH but not by iso-BtOH (Fig. 4D), indicating that the effect of RalA was dependent upon PLD activity. These data suggest that RalA, which is required for EGF-induced PLD activity, was also required for EGF-induced receptor internalization and degradation.
FIG. 4.
EGF-induced receptor internalization and degradation are dependent on RalA. EGFR cells stably expressing elevated levels of either wild-type (WT) or dominant negative (S28N) RalA were characterized previously (23). (A) The induction of PLD by EGF (100 ng/ml for 15 min) in these and the parental EGFR cells was determined as described in Materials and Methods. Values were normalized to the PLD activity in the untreated EGFR cells. Error bars represent the ranges for duplicate results from representatives of three independent experiments. (B) The effect of wild-type and mutant (S28N) RalA on EGF receptor internalization was determined as for Fig. 3A. (C) The effect of wild-type and mutant (S28N) RalA on EGF receptor degradation was determined as for Fig. 2A. (D) EGFR cells overexpressing wild-type RalA were treated with EGF for 2 h in the absence or presence of either 1-BtOH (1%) or iso-BtOH (1%) as shown and then subjected to Western blot analysis using an anti-EGF receptor antibody (LA22). The alcohols were added 5 min prior to EGF treatment. The amount of protein loaded for all lanes was normalized for total protein. The data presented are representative results of an experiment that was repeated at least three times.
EGF-induced receptor internalization and degradation are dependent on PKC α.
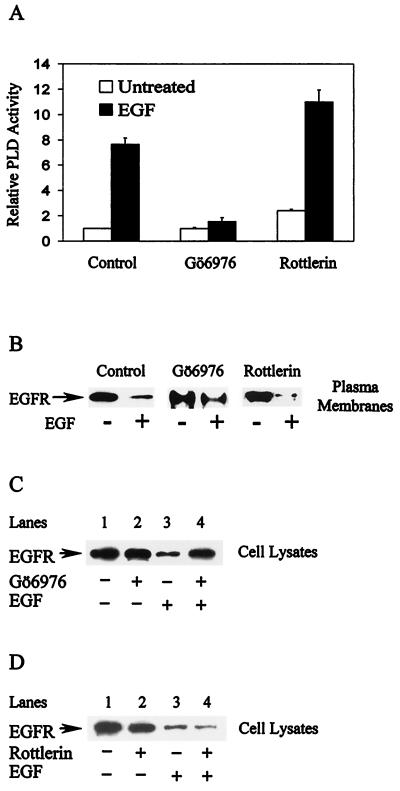
EGF-induced PLD activity is dependent upon protein kinase C α (PKC α) (13). If PLD activity is required for the internalization of the EGF receptor, then the internalization and degradation of the EGF receptor should also be dependent upon PKC α. As shown in Fig. 5A, the PKC α-specific inhibitor Gö6976 inhibited EGF-induced PLD activity, whereas the PKC δ inhibitor rottlerin did not. We then examined the effects of Gö6976 and rottlerin on EGF-induced receptor internalization and degradation. EGF-induced receptor internalization, as measured by the loss of the receptor from the plasma membrane, was inhibited by Gö6976 but not by rottlerin (Fig. 5B). Whereas EGF induced more than an 80% reduction in the EGF receptor in the plasma membrane, in the presence of Gö6976 the receptor level was reduced by only 35%. In the presence of the PKC δ inhibitor the receptor was reduced by more than 90%, consistent with the ability of rottlerin to elevate PLD activity (13). Similarly, Gö6976 (Fig. 5C), but not rottlerin (Fig. 5D), inhibited EGF-induced receptor degradation. Thus, as expected, inhibiting PKC α, which is required for EGF-induced PLD activity, inhibited EGF-induced receptor internalization and degradation.
FIG. 5.
EGF-induced receptor internalization and degradation are dependent on PKCα. (A) The induction of PLD by EGF (100 ng/ml for 15 min) in the EGFR cells was determined in the absence or presence of Gö6976 (0.5 μM) and rottlerin (10 μM) as shown. Gö6976 and rottlerin were added 30 min prior to EGF treatment. Values were normalized to those for the untreated EGFR cells. Error bars represent the range for duplicate results from a representatives of two independent experiments. (B) The effects of Gö6976 and rottlerin on EGF receptor internalization were determined as in Fig. 3A. EGFR cells were incubated in the absence or presence of Gö6976 (0.5 μM) or rottlerin (10 μM), as shown, for 30 min, and then treated with EGF (100 ng/ml) for 30 min. These data are representative results of an experiment that was repeated twice. The effects of Gö6976 (C) and rottlerin (D) on EGF receptor degradation were determined as for Fig. 2A. EGFR cells were incubated in the absence or presence of Gö6976 (0.5 μM) or rottlerin (10 μM), as shown, for 30 min and then treated with EGF (100 ng/ml) for 2 hr or left untreated. The data presented are representative results of an experiment that was repeated three times.
Overexpression of PLD proteins influences receptor degradation.
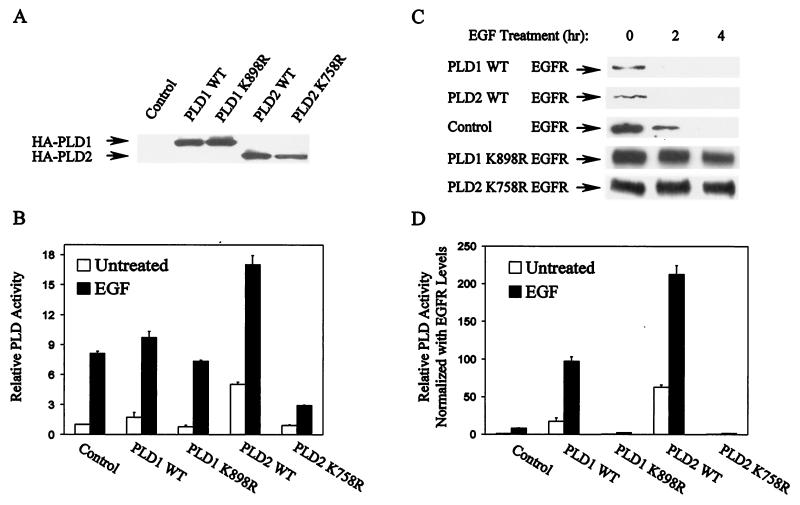
Data presented in Fig. 4 and 5 suggested that elevated PLD activity increased receptor endocytosis and degradation. To test the hypothesis that PLD regulates receptor endocytosis more directly, we examined EGF receptor levels and turnover rate in EGFR cells overexpressing either hPLD1 (11), mPLD2 (6), or catalytically inactive mutants of PLD1 (hPLD1-K898R) (43) and PLD2 (mPLD2-K758R) (42, 43). The transfectants were assayed for expression of the Flu-tagged PLD proteins by Western blot analysis (Fig. 6A). The subcellular distributions of overexpressed PLD1 and PLD2 were examined by cell fractionation and were found to have essentially the same patterns as observed for endogenous PLD1 and PLD2 (not shown).
FIG. 6.
PLD1 and PLD2 overexpression influences receptor degradation. EGFR cells were stably transfected with plasmids encoding wild-type (WT) hPLD1 and mPLD2 and their catalytically inactive mutants hPLD1-K898R and mPLD2-K758R. (A) All of the PLD proteins expressed were tagged with a Flu epitope that allows detection with an anti-Flu antibody (Y11), and the expression of PLD proteins was verified by Western blot analysis. (B) The relative PLD activity in the parental and PLD-expressing cells was determined as for Fig. 4A and 5A. Values were normalized to those for the untreated EGFR cells. Error bars represent the ranges for duplicate results from representatives of three independent experiments. (C) The effect of EGF (100 ng/ml) on EGF receptor levels in the parental EGFR cells and the PLD-overexpressing cell lines was investigated by Western blot analysis. Cells were treated with EGF for the indicated times prior to harvest. Cell lysates were then examined for EGF receptor levels as for Fig. 2A. The data presented are representative results of an experiment that was repeated at least three times. (D) The EGF receptor levels shown in panel C were quantified by densitometry (not shown), and the relative PLD activity shown in panel B was normalized to the basal EGF receptor levels.
Both the induction of PLD activity and the turnover of the EGF receptor in response to EGF were examined in the cells overexpressing PLD proteins. As shown in Fig. 6B, in response to EGF neither the elevation by wild-type PLD1 nor the inhibition by catalytically inactive mutants of cellular PLD activity was remarkable. Nevertheless, each of these PLD proteins had a dramatic effect on EGF-induced receptor degradation (Fig. 6C). The cells overexpressing either wild-type PLD1 or PLD2 had reduced basal levels of the EGF receptor relative to the parental EGFR cells, whereas the cells overexpressing catalytically inactive PLD mutants enhanced the basal receptor levels (Fig. 6C, compare the EGF receptor levels of the untreated cells). When treated with EGF, more than 90% (densitometry quantitation) of the EGF receptor was depleted before 2 h in the cells overexpressing either wild-type PLD1 or PLD2, whereas in the parental EGFR cells it took about 4 h to reach this level of depletion. In the cells overexpressing the catalytically inactive mutants of PLD1 and PLD2, EGF treatment did not significantly reduce EGF receptor levels by 4 h (Fig. 6C). Since the EGF receptor is essential for cellular response to EGF, we normalized the PLD activity in Fig. 6B with the basal levels of the EGF receptor (densitometry quantitation of data in Fig. 6C [results not shown]). As shown in Fig. 6D, the normalized PLD activity was strongly elevated by both wild-type PLD1 and PLD2 and was strongly inhibited by the catalytically inactive mutants.
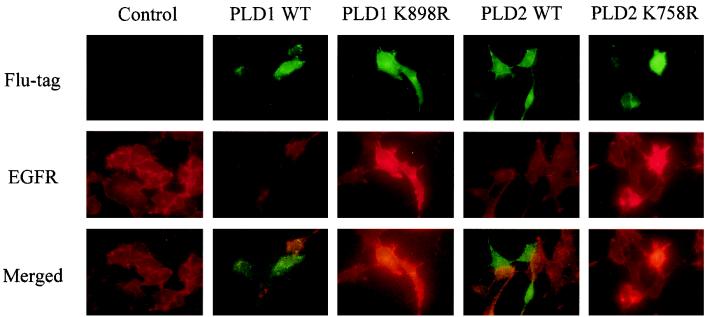
We also examined the effect of PLD expression upon EGF receptor levels using immunofluorescence. PLD expression vectors were transiently transfected into EGFR cells. PLD expression, as seen by green fluorescence (Fig. 7, upper panel), and the level of the EGF receptor, as seen by red fluorescence (Fig. 7, middle panel), showed an interesting pattern of correlation. Cells with a higher expression level of wild-type PLD1 or PLD2 had a lower level of the EGF receptor (compared with adjacent cells), whereas cells with higher levels of catalytically inactive mutants had a higher level of the EGF receptor. When images of the two stainings were merged (Fig. 7, bottom panel), the red and green stains did not overlap on cells transfected with wild-type PLD1 or PLD2, but it was clear that the cells with high levels of PLD mutant expression were the same cells as those with high levels of the EGF receptor. These data are consistent with Fig. 6C, which shows that expression of either PLD1 or PLD2 resulted in reduced basal EGF receptor levels and that expression of the catalytically inactive PLDs resulted in elevated basal receptor levels.
FIG. 7.
Immunofluorescence microscopy study of PLD1 and PLD2 influence on EGF receptor levels. EGFR cells were transiently transfected with plasmids encoding either wild-type (WT) hPLD1 or mPLD2 or their catalytically inactive mutants hPLD1-K898R and mPLD2-K758R and prepared as described in Materials and Methods. Upper panel, the Flu-tagged PLD proteins were stained with green fluorophore; middle panel, the EGF receptor was stained with red fluorophore; bottom panel, the images in upper and middle panels were merged. Images shown are representative results that were observed in a majority of the treated cells in two independent experiments.
EGF-induced MAP kinase activation, which is dependent upon internalization, is dependent upon PLD.
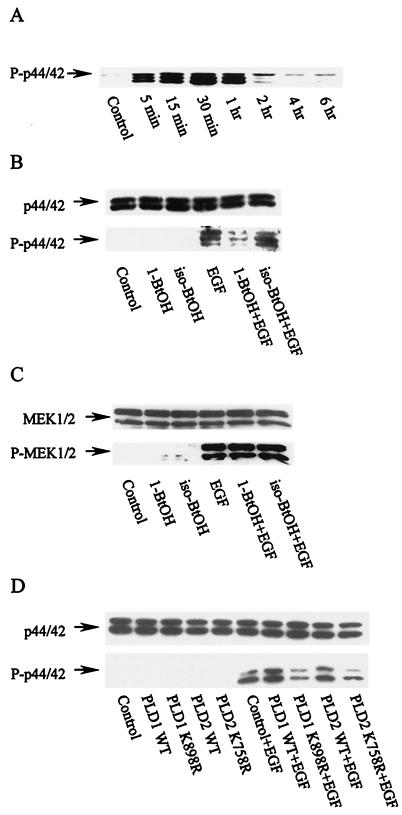
Endocytosis of the EGF receptor has been shown to be required for EGF-induced mitogen-activated protein (MAP) kinase activation (19, 44). We therefore examined the effect of inhibiting PA production on MAP kinase activation by EGF. As shown in Fig. 8A, p42/p44 MAP kinase is phosphorylated within 5 min after EGF treatment. In Fig. 8B (lower panel), it is shown that 1-BtOH, but not iso-BtOH, prevented EGF-induced phosphorylation of MAP kinase. This treatment did not affect the level of MAP kinase (Fig. 8B, upper panel). Kranenburg et al. (19) showed that while MAP kinase phosphorylation was dependent upon EGF receptor internalization, phosphorylation of MAP kinase kinase or MEK was independent of internalization. Consistent with these results, phosphorylation of MEK was unaffected by 1-BtOH (Fig. 8C), indicating that MEK phosphorylation was independent of PA production by PLD. We also examined the effect of overexpressing PLD1 and -2 and the corresponding catalytically inactive mutants on EGF-induced MAP kinase phosphorylation. As shown in Fig. 8D, both wild-type PLD1 and PLD2 enhanced and the catalytically inactive mutants inhibited EGF-induced MAP kinase phosphorylation. Thus, EGF-induced MAP kinase phosphorylation, which is dependent upon EGF receptor internalization, is dependent upon PLD activity.
FIG. 8.
EGF-induced MAP kinase activation is dependent upon PLD. (A) The EGFR cells were treated with EGF (100 ng/ml). The cells were harvested at the time indicated and subjected to Western blot analysis using an antibody raised against phosphorylated MAP kinase (P-p42/44). (B) The EGFR cells were treated with EGF (100 ng/ml), where indicated, for 30 min in the absence or presence of the indicated alcohols (1%), which were added 5 min prior to EGF treatment. The cells were then harvested and subjected to Western blot analysis using either anti-MAP kinase (p44/42) antibody (upper panel) or anti-phosphorylated MAP kinase (P-p44/42) antibody (lower panel). (C) The samples shown in panel B were also analyzed for MEK1/2 and phosphorylated MEK1/2 by Western blot analysis using antibodies raised against either MEK1/2 (upper panel) or phosphorylated MEK1/2 (lower panel). (D) EGFR cells were transiently transfected with plasmids encoding either wild-type (WT) hPLD1 or mPLD2 or their catalytically inactive mutants hPLD1-K898R and mPLD2-K758R. The effect of EGF on the level of MAP kinase and phosphorylated MAP kinase was examined as for panel B. The data presented are representative results of an experiment that was repeated at least 2 times.
DISCUSSION
Evidence that supports a role for endocytosis and retrograde vesicle movement in signal transduction is growing. The internalization of nerve growth factor and its receptor, TrkA, is required for transmission of nerve growth factor-mediated signals from distal axons to the cell body (31). Endocytosis of the EGF receptor is required for EGF-induced MAP kinase activation (19, 44). This observation is consistent with our data demonstrating that EGF-induced MAP kinase activation is dependent upon PLD activity, which is required for EGF receptor endocytosis. Interestingly, brefeldin A, which prevents PLD1 activation by inhibiting Arf GTP-GDP exchange, inhibited MAP kinase activation and receptor internalization in response to insulin (32), suggesting that PLD activity may be critical for regulating endocytosis of other receptors as well. Thus, the data presented here are consistent with a role for retrograde vesicle movement in the transduction of intracellular signals and a role for PLD in mediating this process.
PLD activity is elevated in response to most, if not all, mitogenic signals. Yet in spite of an apparently ubiquitous involvement of PLD, little is known about what effects PLD and its primary metabolite, PA, have on the transduction of intracellular signals. In this report, we have presented data indicating that a role PLD plays in signaling is to facilitate receptor-mediated endocytosis. PLD has previously been implicated in vesicle budding and trafficking in Golgi membranes (1, 4, 13, 20, 33, 34). We speculated that the role that PLD plays in the transduction of agonistic signals may be similar to the proposed role for PLD in vesicle transport from Golgi membranes—that being the stimulation of vesicle formation for receptor endocytosis.
How PLD might influence membrane topology and vesicle formation is not clear. PLD converts phosphatidylcholine to PA, which could act as a second messenger and could activate specific enzymes, such as phosphatidylinositol-4-phosphate-5-kinase (12, 27), that would lead to the production of phosphatidylinositol-4,5-bisphosphate (PIP2). PIP2 could then indirectly regulate the effects of PLD activity and PA production and thus provide a positive feedback. Both PA and PIP2 could bind to coat proteins and facilitate vesicle budding (15, 20, 33). The generation of PA by PLD results in a significant change in both charge and pH at the membrane. The lower pH could result in the protonation of proteins so that they might be attracted to the negative charge of the phosphate on PA. The association of proteins with the PA-enriched membrane regions could initiate the changes in membrane topology that could ultimately result in the generation of an endocytic vesicle. The EGF receptor is present in caveolae in quiescent cells, but upon stimulation with EGF the receptor migrates out of the caveolar membranes prior to being internalized (26). Thus, it is possible that PLD activity and PA may facilitate translocation of the receptor to a membrane domain where the vesicle-forming machinery can assemble.
The activation of PLD by EGF is dependent upon RalA (23, 45), as was the EGF receptor endocytosis reported here. Consistent with these data, RalA was recently reported to be required for EGF-induced receptor endocytosis (29). RalA is a downstream target of Ras signaling (9) that associates directly with PLD1 (14, 25). Thus, the requirement of RalA for endocytosis of the EGF receptor suggests the involvement of PLD1. The data presented in Fig. 6 and 7 suggest that both PLD1 and PLD2 are required for EGF receptor downregulation. PLD2 is localized mostly in caveolin-enriched light membrane fractions (7), and we have found that the EGF-induced PLD activity is restricted to these light membrane fractions (46). PLD1 is also present in the caveolin-enriched light membrane fractions (17, 46), and upon activation by PKC, PLD1 is phosphorylated only in these fractions (16, 17). Thus, it is possible that PLD1 and PLD2 work together to stimulate receptor endocytosis. Whether PLD1 activation might lead to PLD2 activation or vice versa is not known; however, since increased PLD activity in response to EGF is dependent upon RalA, we would suspect that PLD1 might be activated first. We have found that RalA is highly enriched in the caveolin-enriched light membrane fractions (our unpublished data), where the EGF receptor localized prior to ligand activation. Thus, stimulation of RalA and subsequent activation of PLD1 likely occur here. However, activated RalA is not sufficient to activate PLD, and therefore it is likely that other factors are involved. It may also be of interest that PLD2 can become phosphorylated on tyrosine in response to EGF (36), suggesting that the regulation of PLD activity in response to EGF may be complex and involve both PLD1 and PLD2.
We speculated previously that PLD contributes to the formation of signaling vesicles that transduce intracellular signals after being endocytosed from the plasma membrane (24). The data presented here suggest a role for PLD in receptor-mediated endocytosis. These data, along with recent data implicating endocytosis in the transduction of signals mediated by the EGF receptor (19, 44) and TrkA (31), strengthen an emerging hypothesis that receptor internalization is not merely a negative feedback pathway leading to receptor degradation. It is likely that receptor-mediated endocytosis is an important aspect of intracellular signal transduction and that PLD may play a critical role in this process. It will be important to evaluate a role for PLD in the ligand-induced endocytosis of other receptors where PLD activity is also elevated.
ACKNOWLEDGMENTS
We thank Michael Frohman and Andrew Morris of the State University of New York—Stony Brook for generously providing plasmids encoding Flu-tagged PLD1, PLD2, and catalytically inactive mutants of PLD1 and PLD2 (K898R and K758R, respectively).
This investigation was supported by National Institutes of Health grant CA46677. Research Centers in Minority Institutions award RR-03037 from the National Center for Research Resources of the National Institutes of Health, which supports infrastructure and instrumentation in the Biological Sciences Department at Hunter College, is also acknowledged.
REFERENCES
- 1.Bi K, Roth M G, Ktistakis N T. Phosphatidic acid formation by phospholipase D is required for transport from the endoplasmic reticulum to the Golgi complex. Curr Biol. 1997;7:301–307. doi: 10.1016/s0960-9822(06)00153-9. [DOI] [PubMed] [Google Scholar]
- 2.Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes C A, Sollner T H, Rothman J E, Wieland F T. Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 3.Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y G, Siddhanta A, Austin C D, Hammond S M, Sung T C, Frohman M A, Morris A J, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockcroft S, Thomas G M, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty N F, Truong O, Hsuan J J. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 6.Colley W C, Sung T C, Roll R, Jenco J, Hammond S M, Altshuller Y, Bar-Sagi D, Morris A J, Frohman M A. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 7.Czarny M, Lavie Y, Fiucci G, Liscovitch M. Localization of phospholipase D in detergent-insoluble, caveolin-rich membrane domains. Modulation by caveolin-1 expression and caveolin-182-101. J Biol Chem. 1999;274:2717–2724. doi: 10.1074/jbc.274.5.2717. [DOI] [PubMed] [Google Scholar]
- 8.Exton J H. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 9.Feig L A, Urano T, Cantor S. Evidence for a Ras/Ral signaling cascade. Trends Biochem Sci. 1996;21:438–441. doi: 10.1016/s0968-0004(96)10058-x. [DOI] [PubMed] [Google Scholar]
- 10.Haigler H, Ash J F, Singer S J, Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci USA. 1978;75:3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammond S M, Altshuller Y M, Sung T C, Rudge S A, Rose K, Engebrecht J, Morris A J, Frohman M A. Human ADP-ribosylation factor-activated phosphatidylcholine-specific phospholipase D defines a new and highly conserved gene family. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 12.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris A J, Frohman M A, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 13.Hornia A, Lu Z, Sukezane T, Zhong M, Joseph T, Frankel P, Foster D A. Antagonistic effects of protein kinase C α and δ on both transformation and phospholipase D activity mediated by the epidermal growth factor receptor. Mol Cell Biol. 1999;19:7672–7680. doi: 10.1128/mcb.19.11.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Luo J Q, Urano T, Frankel P, Lu Z, Foster D A, Feig L A. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995;378:409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- 15.Jones D, Morgan C, Cockcroft S. Phospholipase D and membrane traffic. Potential roles in regulated exocytosis, membrane delivery and vesicle budding. Biochim Biophys Acta. 1999;1439:229–244. doi: 10.1016/s1388-1981(99)00097-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Han J M, Han B R, Lee K A, Kim J H, Lee B D, Jang I H, Suh P G, Ryu S H. Phospholipase D1 is phosphorylated and activated by protein kinase C in caveolin-enriched microdomains within the plasma membrane. J Biol Chem. 2000;275:13621–13627. doi: 10.1074/jbc.275.18.13621. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, Han J M, Park J B, Lee S D, Oh Y S, Chung C, Lee T G, Kim J H, Park S K, Yoo J S, Suh P G, Ryu S H. Phosphorylation and activation of phospholipase D1 by protein kinase C in vivo: determination of multiple phosphorylation sites. Biochemistry. 1999;38:10344–10351. doi: 10.1021/bi990579h. [DOI] [PubMed] [Google Scholar]
- 18.Kodaki T, Yamashita S. Cloning, expression, and characterization of a novel phospholipase D complementary DNA from rat brain. J Biol Chem. 1997;272:11408–11413. doi: 10.1074/jbc.272.17.11408. [DOI] [PubMed] [Google Scholar]
- 19.Kranenburg O, Verlaan I, Moolenaar W H. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 20.Ktistakis N T, Brown H A, Waters M G, Sternweis P C, Roth M G. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liscovitch M, Czarny M, Fiucci G, Lavie Y, Tang X. Localization and possible functions of phospholipase D isozymes. Biochim Biophys Acta. 1999;1439:245–263. doi: 10.1016/s1388-1981(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 22.Lopez I, Arnold R S, Lambeth J D. Cloning and initial characterization of a human phospholipase D2 (hPLD2). ADP-ribosylation factor regulates hPLD2. J Biol Chem. 1998;273:12846–12852. doi: 10.1074/jbc.273.21.12846. [DOI] [PubMed] [Google Scholar]
- 23.Lu Z, Hornia A, Joseph T, Sukezane T, Frankel P, Zhong M, Bychenok S, Xu L, Feig L A, Foster D A. Phospholipase D and RalA cooperate with the epidermal growth factor receptor to transform 3Y1 rat fibroblasts. Mol Cell Biol. 2000;20:462–467. doi: 10.1128/mcb.20.2.462-467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo J Q, Liu X, Frankel P, Rotunda T, Ramos M, Flom J, Jiang H, Feig L A, Morris A J, Kahn R A, Foster D A. Functional association between Arf and RalA in active phospholipase D complex. Proc Natl Acad Sci USA. 1998;95:3632–3637. doi: 10.1073/pnas.95.7.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo J Q, Liu X, Hammond S M, Colley W C, Feig L A, Frohman M A, Morris A J, Foster D A. RalA interacts directly with the Arf-responsive, PlP2-dependent phospholipase D1. Biochem Biophys Res Commun. 1997;235:854–859. doi: 10.1006/bbrc.1997.6793. [DOI] [PubMed] [Google Scholar]
- 26.Mineo C, Gill G N, Anderson R G. Regulated migration of epidermal growth factor receptor from caveolae. J Biol Chem. 1999;274:30636–30643. doi: 10.1074/jbc.274.43.30636. [DOI] [PubMed] [Google Scholar]
- 27.Moritz A, De Graan P N, Gispen W H, Wirtz K W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992;267:7207–7210. [PubMed] [Google Scholar]
- 28.Morris A J, Frohman M A, Engebrecht J. Measurement of phospholipase D activity. Anal Biochem. 1997;252:1–9. doi: 10.1006/abio.1997.2299. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, Okawa K, Iwamatsu A, Kishida S, Kikuchi A. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 1999;18:3629–3642. doi: 10.1093/emboj/18.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S K, Provost J J, Bae C D, Ho W T, Exton J H. Cloning and characterization of phospholipase D from rat brain. J Biol Chem. 1997;272:29263–29271. doi: 10.1074/jbc.272.46.29263. [DOI] [PubMed] [Google Scholar]
- 31.Riccio A, Pierchala B A, Ciarallo C L, Ginty D D. An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science. 1997;277:1097–1100. doi: 10.1126/science.277.5329.1097. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo M A, Shome K, Vasudevan C, Stolz D B, Sung T C, Frohman M A, Watkins S C, Romero G. Phospholipase D and its product, phosphatidic acid, mediate agonist-dependent raf-1 translocation to the plasma membrane and the activation of the mitogen-activated protein kinase pathway. J Biol Chem. 1999;274:1131–1139. doi: 10.1074/jbc.274.2.1131. [DOI] [PubMed] [Google Scholar]
- 33.Roth M G, Bi K, Ktistakis N T, Yu S. Phospholipase D as an effector for ADP-ribosylation factor in the regulation of vesicular traffic. Chem Phys Lipids. 1999;98:141–152. doi: 10.1016/s0009-3084(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 34.Roth M G, Sternweis P C. The role of lipid signaling in constitutive membrane traffic. Curr Opin Cell Biol. 1997;9:519–526. doi: 10.1016/s0955-0674(97)80028-2. [DOI] [PubMed] [Google Scholar]
- 35.Rothman J E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 36.Slaaby R, Jensen T, Hansen H S, Frohman M A, Seedorf K. PLD2 complexes with the EGF receptor and undergoes tyrosine phosphorylation at a single site upon agonist stimulation. J Biol Chem. 1998;273:33722–33727. doi: 10.1074/jbc.273.50.33722. [DOI] [PubMed] [Google Scholar]
- 37.Smart E J, Ying Y S, Mineo C, Anderson R G. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song J, Jiang Y W, Foster D A. Epidermal growth factor induces the production of biologically distinguishable diglyceride species from phosphatidylinositol and phosphatidylcholine via the independent activation of type C and type D phospholipases. Cell Growth Differ. 1994;5:79–85. [PubMed] [Google Scholar]
- 39.Song J, Pfeffer L M, Foster D A. v-Src increases diacylglycerol levels via a type D phospholipase-mediated hydrolysis of phosphatidylcholine. Mol Cell Biol. 1991;11:4903–4908. doi: 10.1128/mcb.11.10.4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Coatomer, Arf1p, and nucleotide are required to bud coat protein complex l-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stamnes M, Schiavo G, Stenbeck G, Sollner T H, Rothman J E. ADP-ribosylation factor and phosphatidic acid levels in Golgi membranes during budding of coatomer-coated vesicles. Proc Natl Acad Sci USA. 1998;95:13676–13680. doi: 10.1073/pnas.95.23.13676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung T C, Altshuller Y M, Morris A J, Frohman M A. Molecular analysis of mammalian phospholipase D2. J Biol Chem. 1999;274:494–502. doi: 10.1074/jbc.274.1.494. [DOI] [PubMed] [Google Scholar]
- 43.Sung T C, Roper R L, Zhang Y, Rudge S A, Temel R, Hammond S M, Morris A J, Moss B, Engebrecht J, Frohman M A. Mutagenesis of phospholipase D defines a superfamily including a trans-Golgi viral protein required for poxvirus pathogenicity. EMBO J. 1997;16:4519–4530. doi: 10.1093/emboj/16.15.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vieira A V, Lamaze C, Schmid S L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 45.Voß M, Weernink P A, Haupenthal S, Moller U, Cool R H, Bauer B, Camonis J H, Jakobs K H, Schmidt M. Phospholipase D stimulation by receptor tyrosine kinases mediated by protein kinase C and a Ras/Ral signaling cascade. J Biol Chem. 1999;274:34691–34698. doi: 10.1074/jbc.274.49.34691. [DOI] [PubMed] [Google Scholar]
- 46.Xu L, Shen Y, Joseph T, Bryant A, Luo J Q, Frankel P, Rotunda T, Foster D A. Mitogenic phospholipase D activity is restricted to caveolin-enriched membrane microdomains. Biochem Biophys Res Commun. 2000;273:77–83. doi: 10.1006/bbrc.2000.2907. [DOI] [PubMed] [Google Scholar]