Abstract
Background
Limited data exist regarding the impact of human bocavirus (BoV) in hematopoietic cell transplant (HCT) recipients.
Methods
In a longitudinal surveillance study among allogeneic HCT recipients, pre-HCT and weekly post-HCT nasal washes and symptom surveys were collected through day 100, then at least every 3 months through 1 year post-HCT at the Fred Hutchinson Cancer Research Center (2005–2010). Samples were tested by multiplex semiquantitative polymerase chain reaction (PCR) for 12 viruses. Plasma samples from BoV + subjects were analyzed by PCR. Separately, we conducted a retrospective review of HCT recipients with BoV detected in lower respiratory tract specimens.
Results
Among 51 children and 420 adults in the prospective cohort, 21 distinct BoV respiratory tract infections (RTIs) were observed by 1 year post-HCT in 19 patients. Younger age and exposure to children were risk factors for BoV acquisition. Univariable models among patients with BoV RTI showed higher peak viral load in nasal samples (P = .04) and presence of respiratory copathogens (P = .03) were associated with presence of respiratory symptoms, but BoV plasma detection was not. Only watery eyes and rhinorrhea were associated with BoV RTI in adjusted models. With additional chart review, we identified 6 HCT recipients with BoV detected in lower respiratory tract specimens (incidence rate of 0.4% [9/2509] per sample tested). Although all cases presented with hypoxemia, 4 had respiratory copathogens or concomitant conditions that contributed to respiratory compromise.
Conclusions
BoV RTI is infrequent in transplant recipients and associated with mild symptoms. Our studies did not demonstrate convincing evidence that BoV is a serious respiratory pathogen.
Keywords: human bocavirus, bronchoalveolar lavage, respiratory tract infection, hematopoietic cell transplant, surveillance
Bocavirus respiratory tract infection is infrequent and associated with rhinorrhea and watery eyes in transplant recipients. Bocavirus is rarely found in lower respiratory tract specimens and we unable to demonstrate convincing evidence that bocavirus is a serious respiratory pathogen.
Human bocavirus (BoV) is a DNA parvovirus first identified in pediatric respiratory specimens in 2005 [1]. The virus is prevalent among young children with respiratory illness, with a seroprevalence study in Finland demonstrating that all 109 children were seropositive for BoV by age 6 years [2]. Although current research suggests that BoV typically causes a mild syndrome in healthy children, particularly young children with primary infection, and rarely causes disease in adults, the degree to which BoV is directly responsible for respiratory illness has been debated [3, 4]. Other common respiratory viruses are recognized as pathogens contributing to significant morbidity and mortality in an immunocompromised host, with guidelines on management available. In contrast, the impact of BoV in immunocompromised hosts is not well characterized, and systematic, longitudinal data on BoV respiratory tract infection (RTI) are limited [5, 6]. This issue is particularly relevant given increasing widespread use of multiplex polymerase chain reaction (PCR) panels for detection of respiratory viruses.
The objective of this study was to examine the incidence and disease manifestations of BoV RTI in HCT recipients. We conducted analyses of BoV incidence and related symptoms as part of a prospective, surveillance longitudinal study of allogeneic HCT recipients [7–9]. In addition, we performed a retrospective review of HCT recipients with BoV detected in lower respiratory tract samples at the Fred Hutchinson Cancer Research Center.
METHODS
Study Design and Participants
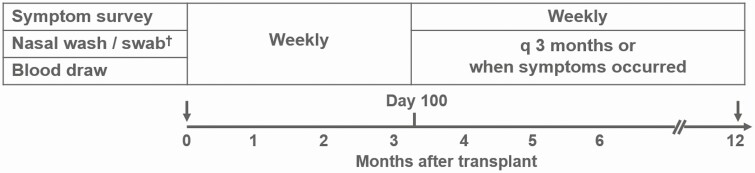
This prospective surveillance study was performed in patients undergoing allogeneic HCT between December 2005 and February 2010 at the Fred Hutchinson Cancer Research Center [7–9] (Figure 1). Altogether, 471 subjects of all ages were enrolled before HCT and followed with longitudinal surveillance for respiratory virus infections for 1 year post-HCT. Nasal wash sampling as well as symptom surveys began pretransplantation and continued weekly regardless of symptoms through 100 days posttransplantation. Virologic and symptom surveillance was performed every 3 months and whenever respiratory symptoms occurred between days 100 and 365 post-HCT. A survey assessed 12 respiratory symptoms (rhinorrhea, sinus congestion, postnasal drip, shortness of breath, cough, wheezing, sputum production, sore throat, sneezing, watery eyes, earache, hoarseness), 4 systemic symptoms (fever, myalgias, diarrhea, and headache), and exposure history.
Figure 1.
Study design of the prospective surveillance study (N = 471).†Polymerase chain reaction: respiratory syncytial virus, influenza, parainfluenza, adenovirus, human metapneumovirus, rhinovirus, coronavirus, bocavirus.
In addition, we reviewed all HCT recipients with BoV detected from lower respiratory tract specimens such as bronchoalveolar lavage (BAL) or lung biopsy at the Fred Hutchinson Cancer Research Center (through December 2018). This examination included patients who were tested for BoV in banked BAL specimens for research purposes (4/26/1988–10/23/2012) [9, 10]. The study was approved by the Fred Hutchinson institutional review board.
Respiratory Samples and Laboratory Testing
For the prospective surveillance cohort, respiratory tract samples included nasal wash (nasopharyngeal or oropharyngeal swab specimens if clinical conditions precluded nasal wash sampling). Clinical samples were also collected at clinicians’ discretion if respiratory symptoms were noted. All respiratory tract samples were tested by multiplex semiquantitative, reverse transcription PCR for respiratory syncytial virus, parainfluenza virus 1–4, influenza A/B, adenovirus, human metapneumovirus, rhinovirus, coronavirus, and BoV [4, 11, 12]. All PCR reactions were performed according to College of American Pathologists standards. The original assay was designed to detect BoV type 1. Following discovery of other bocavirus types (2, 3, and 4), the assay was updated to include all 4 types in August 2011. Both assays are laboratory-developed tests that were validated in our clinical laboratory improvement amendments (CLIA)-certified laboratory. Institutional standard investigation of BAL specimens includes broad diagnostic tests including multiplex PCR for respiratory viruses; conventional cultures for bacteria, fungi, mycobacteria, and viruses; shell vial culture for cytomegalovirus; immunofluorescent antibody staining for Pneumocystis jirovecii; Aspergillus galactomannan enzyme-linked immunosorbent assay; cytopathologic examination; and PCR for Legionella and fungus if applicable. Available plasma samples close to the time of BoV detection in respiratory specimens were tested by PCR to examine BoV viremia [4, 12–14].
Statistical Analysis
Univariable Cox regression analyses were performed to evaluate risk factors for acquisition of BoV in the first 100 days post-HCT using transplant demographics as baseline variables, and exposure history (steroid use, antibiotic use, contact with sick persons), acute graft-versus-host disease, cell counts, and albumin as time-dependent variables. We examined characteristics of patients with BoV infection according to the presence of multiple respiratory symptoms (≥2 respiratory symptoms) as well as BoV viremia. Fisher’s exact or χ 2 tests for categorical variables and Wilcoxon rank sum test for continuous variables were used, as appropriate. Univariable and multivariable logistic regression with generalized estimating equations were used to evaluate longitudinal relationships between occurrences of each symptom during the first 100 days after HCT and BoV detection within the past week, as previously described [7]. Weekly symptom reports were used as binary outcomes in these models and evaluated in relation to a binary risk factor indicator of BoV detection within the past week. We used generalized estimating equations with robust variance estimates to include all episodes in the analyses. The following covariates were selected as potential adjustment factors: presence of other respiratory viruses within the past week, days relative to transplantation, age at transplantation, allergy history, conditioning regimen, donor type, season at time of symptom survey, and grades 2 to 4 acute graft-versus-host disease. Variables with P < .10 in univariable analyses were included as adjustment factors in multivariable models. Rates for occurrence of BoV detection per sample was described using 95% confidence interval (CI).
RESULTS
Demographics of Patients in the Prospective Surveillance Cohort
The prospective study cohort consisted of 471 allogeneic HCT recipients (median age, 51 years; range, 8 months–75 years) as shown in Table 1 [15]. Children accounted for 10.8% of patients (51/471). Overall, the cohort was predominately Caucasian (80%) and male (63.5%). Peripheral blood stem cells were the most common graft source (72.8%) with cord blood the least common (9.6%). Use of T-cell depletion was uncommon in this cohort (4.7%); 343 patients (72.8%) developed grades 2–4 acute graft-versus-host disease.
Table 1.
Characteristic of Allogeneic Transplant Recipients (Prospective Cohort)
| Variables | Descriptions | (N = 471) |
|---|---|---|
| Year of transplantation | 2006 | 99 (21.0) |
| 2007 | 114 (24.2) | |
| 2008 | 126 (26.8) | |
| 2009 | 121 (25.7) | |
| 2010 | 11 (2.3) | |
| Age at transplantation (y) | Median (range) | 50.8 (0.7–75.2) |
| Children (<18 y) | Yes | 51 (10.8) |
| Children (<18 y) | Median (interquartile range) | 10.3 (4.0–14.1) |
| Race | Caucasian | 377 (80.0) |
| Gender | Female | 172 (36.5) |
| HLA | Mismatch/unrelated | 247 (52.4) |
| Haplo/related | 22 (4.7) | |
| Matched/related | 157 (33.3) | |
| Cord blood/unrelated | 45 (9.6) | |
| Cell source | Bone marrow | 83 (17.6) |
| Peripheral blood stem cell | 343 (72.8) | |
| Cord blood | 45 (9.6) | |
| Conditioning regimen | Myeloablative + TBI | 129 (27.4) |
| Myeloablative + non-TBI | 145 (30.8) | |
| Nonmyeloablative | 197 (41.8) | |
| Underlying disease | Acute myeloid leukemia | 164 (34.8) |
| Acute lymphocytic leukemia | 58 (12.3) | |
| Myelodysplastic syndrome | 54 (11.5) | |
| Lymphoma | 51 (10.8) | |
| Myeloma | 34 (7.2) | |
| Chronic lymphocytic leukemia | 25 (5.3) | |
| Chronic myeloid leukemia | 20 (4.3) | |
| Other diseases | 65 (13.8) | |
| Underlying disease riska | High | 173 (36.7) |
| Intermediate | 38 (8.1) | |
| Low | 260 (55.2) | |
| T-cell depletion | Yes | 22 (4.7) |
| Smoking status | Current | 30 (6.4) |
| Former | 127 (27.0) | |
| Never | 280 (59.4) | |
| Unknown | 34 (7.2) | |
| History of allergies | Yes | 166 (35.2) |
| No | 293 (62.2) | |
| Unknown | 12 (2.5) | |
| CMV serostatus | D+/R+ | 110 (23.4) |
| D+/R− | 41 (8.7) | |
| D−/R+ | 163 (34.6) | |
| D−/R− | 157 (33.3) | |
| Acute GVHD grades | 0–1 | 128 (27.2) |
| 2 | 279 (59.2) | |
| 3–4 | 64 (13.6) | |
| Chronic GVHD | Yes | 157 (33.3) |
Data are n (%), unless otherwise specified.
Abbreviations: CMV, cytomegalovirus; D, donor; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; R, recipient; TBI, total body irradiation.
aReference [15].
BoV Detection in Respiratory Tract in the Prospective Surveillance Cohort
Overall, BoV was detected in 37 respiratory tract specimens including 1 BAL specimen by 1 year post-HCT in 19 patients (4%; median age, 42 years; range, 9 months–67 years). Because 2 patients (1 years old and 39 years old, respectively) had multiple BoV RTIs over time, a total of 21 distinct BoV RTIs (3 pre-HCT and 18 post-HCT) were observed. The underlying disease in these 2 patients was acute leukemia. Children accounted for 32% (6/19) of the BoV-positive group, a proportion significantly higher than that in the entire study population (11%, 51/471; P = .003). BoV was detected from respiratory tract specimens throughout the year without apparent seasonality (Supplementary Figure 1). BoV was more frequently detected in the latter half of the first 100 days post-HCT (Supplementary Figure 2). The cumulative incidence of BoV RTI is also shown in Supplementary Figure 3.
We examined risk factors for acquisition of BoV in the first 100 days post-HCT in univariable Cox regression models. Significant variables were younger age (P = .003) and exposure to children (P = .009), especially exposure to younger children younger than age 4 years (P < .001). Exposure to any people with cold symptoms was not significant (P = .337). Because of the low number of BoV infections, multivariable models were not feasible.
Five individuals (26%), age range between 9 months and 64 years, demonstrated BoV shedding, defined as multiple positive samples, with no more than a single interim negative. Median shedding duration between the first and last detection among these individuals was 21 days with the longest shedding event lasting 56 days (in the 64 year old).
Characteristics of Patients With BoV Detection in Respiratory Tract Post-HCT in the Prospective Surveillance Cohort
We evaluated clinical factors associated with the presence of respiratory symptoms among 17 patients with BoV detected in the respiratory tract with corresponding symptom surveys available post-HCT (Table 2). Among 9 patients with respiratory symptoms, 5 were found to have respiratory copathogens (4 rhinovirus and 1 coronavirus) during the BoV infection. Univariable models showed higher peak viral load of BoV in nasal samples (mean, 5.6 log10 copies/mL vs 3.4 log10 copies/mL, P = .04), and presence of respiratory copathogens (P = .03) were associated with presence of multiple respiratory symptoms. However, age, BoV detection in plasma, cytopenia, and steroid use were not associated with this outcome.
Table 2.
Characteristics of Patients With Bocavirus Upper Respiratory Tract Infection (Univariable Analysis)
| Respiratory Symptoms | ||||
|---|---|---|---|---|
| Variables | Categories | + (N = 9) | − (N = 8) | P Value |
| Age at transplantation, y | <18 | 1 (11%) | 4 (50%) | .13 |
| Sex | Male | 7 (78%) | 6 (75%) | 1.00 |
| Cell source | BM/cord blood | 1 (11%) | 3 (38%) | .29 |
| PBSC | 8 (89%) | 5 (63%) | ||
| Lowest white cell counts, ×106 cells/La,b | <1000 | 2 (22%) | 0 (0%) | .52 |
| Lowest neutrophil counts, ×106 cells/La,b | <500 | 1 (11%) | 0 (0%) | 1.00 |
| Most recent lymphocyte counts, ×106 cells/La,b | <300 | 2 (22%) | 0 (0%) | .52 |
| Most recent monocyte counts, ×106 cells/La,b | <300 | 2 (22%) | 1 (13%) | 1.00 |
| Nasal steroid usea,c | Yes | 1 (11%) | 1 (13%) | .72 |
| Systemic steroid usea,c | Yes | 6 (67%) | 5 (63%) | .32 |
| Peak bocavirus viral load copies/mL, Log 10a,e | Mean (STD) | 5.6 (2.2) | 3.4 (0.3) | .04 |
| Peak bocavirus viral load, Ct valuea,f | Mean (STD) | 28.3 (7.5) | 38.4 (1.2) | .01 |
| Viremiaa | Positive | 4 (44%) | 4 (50%) | .80 |
| Copathogen during bocavirus infectiond | Yes | 5 (56%) | 0 (0%) | .03 |
| Fever during bocavirus infectiona | Yes | 1 (11%) | 0 (0%) | .33 |
| Myalgia during bocavirus infectiona | Yes | 2 (22%) | 0 (0%) | .08 |
| Headache during bocavirus infectiona | Yes | 3 (33%) | 1 (13%) | .16 |
| Diarrhea during bocavirus infectiona | Yes | 3 (33%) | 3 (38%) | .64 |
Abbreviations: BM, bone marrow; Ct, cycle threshold; PBSC, peripheral blood stem cell; STD, standard deviation.
aMissing data are present.
bIn 2 weeks before bocavirus onset.
cIn 2 weeks before bocavirus onset or during episode.
d4 rhinovirus and 1 coronavirus.
eViral load (copies/mL) are unavailable in 2 patients with respiratory symptoms and 4 patient without respiratory symptoms.
fCt values are unavailable in 2 patients with respiratory symptoms and 1 patient without respiratory symptoms.
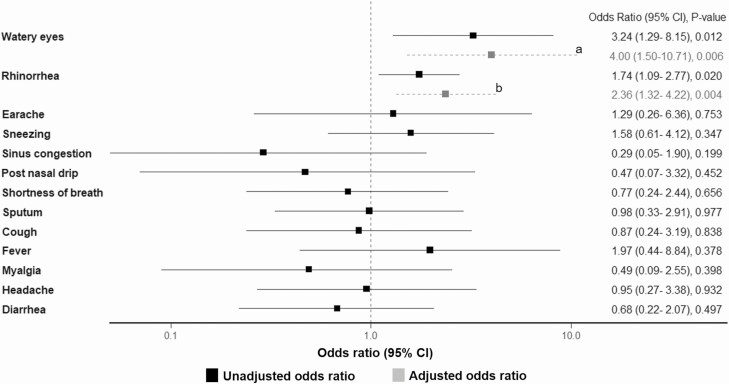
Separately, we also examined whether BoV detection in the upper respiratory tract was associated with specific respiratory or systemic symptoms in the first 100 days post-HCT using longitudinal logistic regression models (BoV occurrence rate, 0.5%; 95% CI, .3–.7). In a univariable model, a positive BoV sample within the past week was significantly associated with rhinorrhea and watery eyes, both of which remained significant in multivariable models (Figure 2). Otherwise, BoV detection was not associated with other symptoms in univariable models. Of note, there were no deaths that appeared to be associated with BoV RTI in this prospective cohort.
Figure 2.
Model estimates for unadjusted and adjusted associations between each symptom and bocavirus detection in the respiratory tract (at the time of symptom or within the prior week). Sore throat, hoarseness, and wheezing were not evaluated because no patients with bocavirus detected had these symptoms at the same time or following week. Abbreviation: CI, confidence interval. aAdjusted for age at transplantation and days relative to transplantation. bAdjusted for history of allergy, donor status, conditioning regimen, days relative to transplantation, and other respiratory viruses within the prior week.
BoV Viremia in the Prospective Surveillance Cohort
Among patients with corresponding serum samples available (30 samples in 18 patients), BoV was detected in 15 serum specimens (9 patients). We also investigated clinical factors associated with the BoV viremia among 16 patients with symptom surveys available post-HCT (Supplementary Table 1). The univariable analyses did not reveal that any variables (transplant demographics, cell counts, systemic steroid use, respiratory copathogens, peak viral load in nasal samples, and systemic symptoms) were associated with the presence of viremia.
Characteristics of Patients With BoV Detected in Lower Respiratory Tract Samples in the Retrospective Cohort
A total of 2509 lower respiratory tract specimens were tested for BoV (4/1988–12/2018), which included residual BAL specimens tested for research purpose in previous publications [9, 10] (Table 3). Among these, 6 allogeneic HCT recipients (range, 1–64 years old) had BoV detected in lower respiratory tract specimens with a low incidence rate per sample tested (9/2509, 0.4%; 95% CI, .2–.7). BoV lower respiratory tract infection (LRTI) occurred 18–805 days after HCT, including 1 case in which BoV was detected in the lung at autopsy. Although all 6 cases presented with hypoxemia, 4 patients had significant respiratory copathogens or concomitant pulmonary conditions that contributed to respiratory compromise. In 1 subject (case 3) with a history of diffuse alveolar hemorrhage, initial BAL revealed BoV and pulmonary hemorrhage. After the diagnosis of recurrent diffuse alveolar hemorrhage, this patient’s dose of systemic steroids was increased and other immunosuppressive agents were started. After initial improvement, the patient’s respiratory condition worsened. A subsequent BAL did not reveal any potential pulmonary pathogens other than BoV. BoV was not detected in serum in 2 residual serum samples (5 days and 4 days after initial and subsequent BALs, respectively), which suggests the detection of BoV in BAL samples was from the replication of BoV in the respiratory tract rather than contaminated blood in the setting of pulmonary hemorrhage. The patient died with respiratory failure 29 days after initial BAL procedure. An autopsy was not performed, and it remains unclear the degree to which BoV infection contributed to the death of this patient.
Table 3.
Clinical Manifestation of Transplant Recipients With Bocavirus Detected in Lower Respiratory Tract Samples (Retrospective Cohort)
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Year | 2008 | 2011 | 2015 | 2018 | 1993 | 1993 |
| Age, y | 64 | 1 | 20 | 23 | 11 | 52 |
| Gender | Male | Female | Male | Male | Female | Male |
| Underlying disorder | Lymphoma | Hemophagocytic lymphohistiocytosis | Acute lymphoblastic leukemia | Acute myeloid leukemia | Acute lymphoblastic leukemia | Chronic myeloid leukemia |
| Donor type | Matched/unrelated | Matched/unrelated | Cord/unrelated | Matched/unrelated | Matched/unrelated | Matched/related |
| Donor source | PBSC | BM | Cord blood | PBSC | BM | BM |
| Immunosuppressant before LRTI | FK | FK, MTX, steroid 0.9 mg/kg | FK, steroid 0.9 mg/kg | FK, cytarabine, steroid 0.5 mg/kg | CyA, steroid 1 mg/kg | CyA, steroid > 1 mg/kg |
| Days of LRTI diagnosis (posttransplant) | 231 | 18 | 106 | 805 | 126 | 78 |
| Preceding BoV detection in nose | 69 days before LRTI | 16 days before LRTI | ||||
| Findings in chest imaging | Pleural effusion, tree in bud opacities | Atelectasis | Patchy GGO, nodules | GGO, centrilobular nodules, mycetoma | Pleural effusion, consolidation | Pulmonary nodules |
| Ct values of BoV in initial and subsequent BALs | Unknown | Unknown → not detected (4 days after) | 29.5 → 32.6 (11 days after) | 35.4 (lung autopsy) | 26.4 → 20.3 (6 days after) | 16.9 → 31 (11 days after) |
| Copathogens in BALs | HRV | AdV, HRV | None | Fusarium, Aspergillus fumigatus (lung autopsy) | KI polyomavirus | Aspergillus fumigatus grew from sputum culture |
| Respiratory support following LRTI diagnosis | Supplemental oxygen | Mechanical ventilator (airway protection) | Mechanical ventilator | Supplemental oxygen | Supplemental oxygen | Supplemental oxygen |
| Concomitant illness | Rib fracture, fluid overload, aspiration | Bleeding from mucositis, fluid overload | Diffuse alveolar hemorrhage | Septic shock, relapse, GVHD | None | None |
| Clinical diagnosis at death (within 90 d after LRTI) | N/A | N/A | MOF, respiratory failure (29 days after) | MOF, respiratory failure (same day) | Arrest, seizure (47 days after) | Sepsis (resistant enterococcus), respiratory failure (23 days after) |
Abbreviations: AdV, adenovirus; BAL, bronchoalveolar lavage; BM, bone marrow; BoV, human bocavirus; Ct, cycle threshold; CyA, cyclosporine; FK, tacrolimus; GGO, ground glass opacities; HRV, human rhinovirus; LRTI, lower respiratory tract infection; MOF, multiorgan failure; MTX, methotrexate; N/A, not applicable; PBSC, peripheral blood stem cell.
DISCUSSION
This prospective surveillance study showed BoV was infrequently detected in the respiratory tract after allogeneic HCT (0.5%; 95% CI, .3–.7). BoV detection in the respiratory tract was associated with watery eyes and rhinorrhea in adjusted models. Our study did not demonstrate convincing evidence that BoV was a common or generally serious respiratory pathogen in transplant recipients.
Since BoV was first isolated from pediatric respiratory specimens in 2005, there has been debate as to whether BoV is a true respiratory pathogen or an innocent bystander. Even fewer data exist regarding the role of BoV as a respiratory pathogen in immunocompromised patients [5]. This is one of the largest prospective surveillance studies to evaluate the clinical manifestation of respiratory BoV infection in HCT recipients. Overall, 19 patients (4% of the entire cohort) had BoV detected and BoV was the least common respiratory virus detected. Risk factors for acquisition of BoV during HCT are of interest but remain uncertain [16, 17]. Previous studies have demonstrated that symptomatic or asymptomatic BoV shedding is very common in young children and in daycare settings [4, 13]. Exposure to young children with or without cold symptoms (eg, child care situations) may potentially be a risk factor for BoV infection [2]. This current study did not demonstrate any apparent seasonality, and BoV was more frequently detected in the latter half of the first 100 days post-HCT. Univariable models indicated younger age and exposure to younger children are risk factors for acquisition of BoV in the respiratory tract. Interestingly, contact with persons with cold symptoms was not associated with BoV acquisition. These observations suggest that transplant recipients may be at risk of acquiring BoV after exposure to asymptomatic or symptomatic children following engraftment (immediate post-HCT period).
We evaluated the clinical characteristics among patients with BoV detected in the respiratory tract post-HCT based on multiple respiratory symptoms and BoV viremia in univariable models. The frequent detection of respiratory copathogens, especially in children, (coinfection range, 18%–90% [5, 16, 18]) makes understanding the clinical impact of BoV difficult. In our transplant cohort, the presence of respiratory copathogens was also frequent (5/18, 28%) and associated with the presence of multiple respiratory symptoms. Although BoV is often detected in healthy asymptomatic children, previous studies suggest that higher viral load of BoV in the nasopharynx is associated with presence of respiratory symptoms [13, 19–22]. This was also demonstrated in our study. The presence of viremia has been reported to correlate with higher viral load in the respiratory tract as well as respiratory symptoms [19]. In our study, BoV detection in plasma was neither associated with clinical characteristics nor peak viral load in nasal samples although the sample size was limited for this analysis. These results may imply a complex mechanism of viremia and demonstrate the challenges in interpreting its significance.
By using weekly nasal wash sampling and symptom surveys through 100 days post-HCT in a large cohort, we performed analyses to investigate whether any specific symptoms were associated with recent detection of BoV in the respiratory tract. BoV detection was associated with watery eyes and rhinorrhea in adjusted models, suggesting that BoV may be the etiology for these localized symptoms in allogeneic HCT recipients. Watery eyes is a relatively uncommon symptom in the setting of respiratory virus infections in general, but BoV has been isolated from conjunctiva, and conjunctivitis has been reported as present in patients with BoV infection [4, 18, 23]. Another interesting finding in this current study is that no patients with BoV detected in the respiratory tract had wheezing present. In healthy children, high viral load in nasopharynx and viremia are associated with wheezing, a sign of lower respiratory tract involvement [21, 22]. Although the presence of wheezing would have been mainly identified by self-report in this surveillance study, our finding infers the low propensity of this virus to lower respiratory tract involvement in the HCT population. Our finding is also consistent with BoV being exclusively detected in upper respiratory tract specimens in patients undergoing lung transplantation [24, 25]. Because few overall symptoms are associated with BoV detection even in immunocompromised hosts, the issue of whether BoV should be included in multiplex respiratory viral PCR panels is of interest [26–28], especially if platforms have limited availability for adding novel and more clinically important viruses, such as severe acute respiratory syndrome coronavirus-2.
Life-threatening LRTI resulting from BoV has been described in case reports, where it appears to be the result of primary BoV infection mainly in young children with prematurity or immunocompromised hosts [29–36]. However, few prospective or systematic studies have examined the role of BoV as a serious respiratory pathogen. To ensure that we had not overlooked invasive BoV disease, we separately conducted a retrospective review to describe HCT recipients with BoV detected in BAL or lung biopsy. Only 6 allogeneic HCT recipients (0.4% of samples tested over 3 decades) had BoV detected in the lower respiratory tract. All 6 patients presented with hypoxemia, and respiratory copathogens or concomitant pulmonary conditions were common. One patient, who underwent cord blood transplantation and presented with alveolar hemorrhage, died with respiratory failure. BoV has not been reported as a potential infectious trigger for diffuse alveolar hemorrhage. Approaches to the care of HCT patients with BoV detected in the lower respiratory tract have not been studied and the significance remains undetermined with the rare incidence of BoV LRTI. Until further evidence is available, close monitoring may be warranted, particularly in those patients with immature antigen specific memory T cells.
A strength of this study is the large surveillance prospective cohort that collected longitudinal symptom surveys and upper respiratory tract specimens. We used a well-established multiplex semiquantitative PCR to evaluate the role of BoV RTI in transplant recipients, regardless of symptoms. In addition, we attempted to overcome other confounding effects in multivariable models that included other respiratory viruses as adjustment factors. We also examined the impact of BoV viremia using saved serum samples. One limitation of our study was the relatively low incidence rates of BoV RTI, which limited our ability to perform multivariable analyses to evaluate risk factors for BoV acquisition and presence of multiple respiratory symptoms. Despite our attempt to use multivariable models to assess the associations between BoV detection and each symptom, the possibility of unrecognized confounders cannot be eliminated. Although this is likely the largest case series of BoV LRTI in this high-risk population, the significance of BoV as a serious respiratory pathogen is still undetermined because of the very low incidence of BoV in lower respiratory tract specimens and frequent presence of copathogens. Last, although we detected DNAemia, we did not perform messenger RNA detection assay, which in conjunction with detection of viral replication can be a better target for the diagnosis of active BoV infection [37].
In conclusion, we demonstrated that BoV RTI is infrequent in allogeneic HCT recipients and associated with mild symptoms including rhinorrhea and watery eyes. BoV detection in the lower respiratory tract was rare and generally associated with clinically significant copathogens. Overall, our data demonstrate that BoV is unlikely to be a serious respiratory pathogen in allogeneic HCT recipients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank Jane Kuypers, Arun Nalla, and Terry Stevens-Ayers for laboratory studies, and Chris Davis for database services.
Financial support . This work was supported by the National Institutes of Health (grant numbers R01HL081595, K24HL093294, HL081595 to M. B., grant number K23AI139385 to C.O., grant number K23HL091059 to A. P. C., grant number K23AI114844 to A. W., grant number CA18029) to W. L., grant number CA15704 to H. X.; the Fred Hutchinson Cancer Research Center Vaccine and Infectious Disease Division (biorepository); and the Pediatric Infectious Diseases Society Fellowship Award funded by Horizon Pharma to C. O.
Potential conflicts of interest. M. B.: Kyorin (consultant), Gilead (research support, consultant), ReViral (consultant), Janssen (research support, consultant), Ansun (research support, consultant), Moderna (consultant), Vir Bio (research support, consultant), GSK (consultant), Pulmocide (consultant), VB Tech (research support), Amazon (research support), Bavarian Nordic (consultant), and Allovir (consultant), all outside of the submitted work. J. A. E.: GlaxoSmithKline (research support), AstraZeneca (research support), Merck (research support), Novavax (research support), Chimerix (research support), Sanofi Pasteur (consultant), and Meissa Vaccines (consultant), all outside of the submitted work. A. W.: Kyorin (consultant), Ansun (research support), VB Tech (research support), and Amazon (research support), all outside of the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 2005; 102:12891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meriluoto M, Hedman L, Tanner L, et al. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 2012; 18:264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guido M, Tumolo MR, Verri T, et al. Human bocavirus: current knowledge and future challenges. World J Gastroenterol 2016; 22:8684–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin ET, Kuypers J, McRoberts JP, Englund JA, Zerr DM. Human bocavirus 1 primary infection and shedding in infants. J Infect Dis 2015; 212:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piñana JL, Madrid S, Pérez A, et al. Epidemiologic and clinical characteristics of coronavirus and bocavirus respiratory infections after allogeneic stem cell transplantation: a prospective single-center study. Biol Blood Marrow Transplant 2018; 24:563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vliora C, Papadakis V, Doganis D, et al. A prospective study on the epidemiology and clinical significance of viral respiratory infections among pediatric oncology patients. Pediatr Hematol Oncol 2019; 36:173–86. [DOI] [PubMed] [Google Scholar]
- 7. Milano F, Campbell AP, Guthrie KA, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 2010; 115:2088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campbell AP, Guthrie KA, Englund JA, et al. Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clin Infect Dis 2015; 61:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuypers J, Campbell AP, Guthrie KA, et al. WU and KI polyomaviruses in respiratory samples from allogeneic hematopoietic cell transplant recipients. Emerg Infect Dis 2012; 18:1580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seo S, Renaud C, Kuypers JM, et al. Idiopathic pneumonia syndrome after hematopoietic cell transplantation: evidence of occult infectious etiologies. Blood 2015; 125:3789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuypers J, Wright N, Ferrenberg J, et al. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol 2006; 44:2382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin ET, Taylor J, Kuypers J, et al. Detection of bocavirus in saliva of children with and without respiratory illness. J Clin Microbiol 2009; 47:4131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin ET, Fairchok MP, Kuypers J, et al. Frequent and prolonged shedding of bocavirus in young children attending daycare. J Infect Dis 2010; 201:1625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foulongne V, Olejnik Y, Perez V, Elaerts S, Rodière M, Segondy M. Human bocavirus in French children. Emerg Infect Dis 2006; 12:1251–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green ML, Leisenring WM, Xie H, et al. CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia. Blood 2013; 122:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schildgen O, Müller A, Allander T, et al. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev 2008; 21:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ. Feigin and Cherry’s textbook of pediatric infectious diseases. 8th ed. Philadelphia, PA: Elsevier, 2019. [Google Scholar]
- 18. Esposito S, Bosis S, Niesters HG, et al. Impact of human bocavirus on children and their families. J Clin Microbiol 2008; 46:1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christensen A, Nordbø SA, Krokstad S, Rognlien AG, Døllner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J Clin Virol 2010; 49:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonvicini F, Manaresi E, Gentilomi GA, et al. Evidence of human bocavirus viremia in healthy blood donors. Diagn Microbiol Infect Dis 2011; 71:460–2. [DOI] [PubMed] [Google Scholar]
- 21. Deng Y, Gu X, Zhao X, et al. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One 2012; 7:e34353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007; 44:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnold JC, Singh KK, Spector SA, Sawyer MH. Human bocavirus: prevalence and clinical spectrum at a children’s hospital. Clin Infect Dis 2006; 43:283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soccal PM, Aubert JD, Bridevaux PO, et al. Upper and lower respiratory tract viral infections and acute graft rejection in lung transplant recipients. Clin Infect Dis 2010; 51:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyakis S, van Hal SJ, Barratt J, Stark D, Marriott D, Harkness J. Absence of human Bocavirus in bronchoalveolar lavage fluid of lung transplant patients. J Clin Virol 2009; 44:179–80. [DOI] [PubMed] [Google Scholar]
- 26. Kim HK, Oh SH, Yun KA, Sung H, Kim MN. Comparison of Anyplex II RV16 with the xTAG respiratory viral panel and Seeplex RV15 for detection of respiratory viruses. J Clin Microbiol 2013; 51:1137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salez N, Vabret A, Leruez-Ville M, et al. Evaluation of four commercial multiplex molecular tests for the diagnosis of acute respiratory infections. PLoS One 2015; 10:e0130378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Costa E, Rodríguez-Domínguez M, Clari MÁ, Giménez E, Galán JC, Navarro D. Comparison of the performance of 2 commercial multiplex PCR platforms for detection of respiratory viruses in upper and lower tract respiratory specimens. Diagn Microbiol Infect Dis 2015; 82:40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ursic T, Steyer A, Kopriva S, Kalan G, Krivec U, Petrovec M. Human bocavirus as the cause of a life-threatening infection. J Clin Microbiol 2011; 49:1179–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edner N, Castillo-Rodas P, Falk L, Hedman K, Söderlund-Venermo M, Allander T. Life-threatening respiratory tract disease with human bocavirus-1 infection in a 4-year-old child. J Clin Microbiol 2012; 50:531–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tabatabai J, Fakhiri J, Meyburg J, et al. Severe human bocavirus 1 respiratory tract infection in an immunodeficient child with fatal outcome. Pediatr Infect Dis J 2019; 38:e219–22. [DOI] [PubMed] [Google Scholar]
- 32. Brebion A, Vanlieferinghen P, Déchelotte P, Boutry M, Peigue-Lafeuille H, Henquell C. Fatal subacute myocarditis associated with human bocavirus 2 in a 13-month-old child. J Clin Microbiol 2014; 52:1006–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Uršič T, Krivec U, Kalan G, Petrovec M. Fatal human bocavirus infection in an 18-month-old child with chronic lung disease of prematurity. Pediatr Infect Dis J 2015; 34:111–2. [DOI] [PubMed] [Google Scholar]
- 34. Sadeghi M, Kantola K, Finnegan DP, et al. Possible involvement of human bocavirus 1 in the death of a middle-aged immunosuppressed patient. J Clin Microbiol 2013; 51:3461–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishiguro T, Hirota S, Kobayashi Y, et al. Fatal primary human bocavirus pneumonia in an immunocompetent adult. Intern Med 2020; 59:421–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schenk T, Strahm B, Kontny U, Hufnagel M, Neumann-Haefelin D, Falcone V. Disseminated bocavirus infection after stem cell transplant. Emerg Infect Dis 2007; 13:1425–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schlaberg R, Ampofo K, Tardif KD, et al. Human bocavirus capsid messenger RNA detection in children with pneumonia. J Infect Dis 2017; 216:688–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.