Abstract
Background
We sought to determine if racial differences in influenza vaccination among nursing home (NH) residents during the 2008–2009 influenza season persisted in 2018–2019.
Methods
We conducted a cross-sectional study of NHs certified by the Centers for Medicare & Medicaid Services during the 2018–2019 influenza season in US states with ≥1% Black NH residents and a White–Black gap in influenza vaccination of NH residents (N = 2 233 392) of at least 1 percentage point (N = 40 states). NH residents during 1 October 2018 through 31 March 2019 aged ≥18 years and self-identified as being of Black or White race were included. Residents’ influenza vaccination status (vaccinated, refused, and not offered) was assessed. Multilevel modeling was used to estimate facility-level vaccination status and inequities by state.
Results
The White–Black gap in influenza vaccination was 9.9 percentage points. In adjusted analyses, racial inequities in vaccination were more prominent at the facility level than at the state level. Black residents disproportionately lived in NHs that had a majority of Blacks residents, which generally had the lowest vaccination. Inequities were most concentrated in the Midwestern region, also the most segregated. Not being offered the vaccine was negligible in absolute percentage points between White residents (2.6%) and Black residents (4.8%), whereas refusals were higher among Black (28.7%) than White residents (21.0%).
Conclusions
The increase in the White–Black vaccination gap among NH residents is occurring at the facility level in more states, especially those with the most segregation.
Keywords: influenza, vaccination, nursing homes, White-Black
The persisting White–Black influenza vaccination gap among nursing home residents continues to occur at the facility level in states with at least 1% Black nursing home residents, particularly in states with the most White–Black racial segregation.
Influenza disproportionately burdens the elderly [1], and those in nursing homes (NHs) are at increased risk of hospitalization and death [2]. Since the 2005–2006 influenza season, when the Centers for Medicare & Medicaid Services (CMS) first required that NH residents be offered the vaccine as a requirement for certification, vaccination coverage increased modestly [3]. While the increase from 71.4% in 2005–2006 to 73.0% in 2017–2018 was positive, the gap between White and Black (W–B) residents increased from 7.1 to 9.5 percentage points [3]. In 2008–2009, the national W–B gap was 6.9 percentage points [3], and states with the highest overall vaccination coverage had the smallest W–B inequities [4]. In states with 10 or greater percentage point inequities, differences were greater between NHs than within homes. Furthermore, coverage decreased for White and Black residents as the proportion of Black residents in NHs increased. In states with the greatest inequities, Black residents disproportionately lived in homes with at least 50% Black residents and homes with low vaccination coverage, resulting in state-level inequities. In addition, Black residents were less frequently vaccinated than White residents within the same facilities, though these differences were smaller than between-facility differences.
It is unclear whether racial differences in influenza vaccination among NH residents continue to follow the same patterns. By 2030, 30% of the older adult population in the United States is expected to be racial and ethnic minorities, a trend likely to be mirrored in NHs [5], making it increasingly urgent to understand what drives these disparities and to design more effective interventions. If the W–B gap is increasing in care that is relatively easy to administer, such as the influenza vaccine, it could be a sentinel that the quality of other preventive or healthcare services may also be worsening in minority populations in NHs. For example, a study in California reported increased coronavirus disease 2019 cases and deaths in NHs of lower quality (2-star or lower ratings) and with higher proportions of racial minorities [6].
Therefore, our objective in this study was to determine if the pattern of inequities in influenza vaccination among NH residents reported during the 2008–2009 season persisted in 2018–2019.
METHODS
Study Design
This was a cross-sectional study that used minimum data set (MDS) resident assessments from nursing facilities certified by CMS. Resident assessments are completed using medical records for all residents in CMS-certified facilities by nurses at admission, quarterly thereafter, when any significant change in condition occurs, and at discharge [7].
Study Population and Data Source
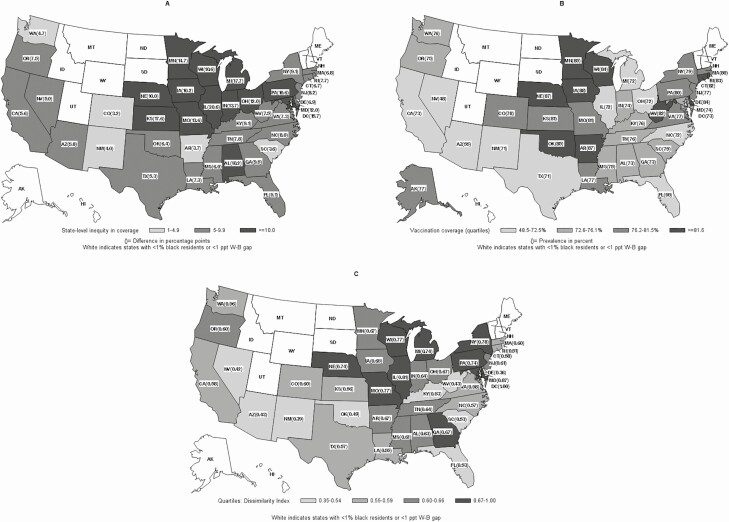
We limited our analysis to the 40 states (39 states and the District of Columbia) that had at least 1% of Black NH residents with at least 1 assessment from 1 October 2018 through 31 March 2019. In addition, only states with a minimally significant inequity, defined as a W–B gap in influenza vaccination of at least 1 percentage point during the 2018–2019 influenza season, were included (Figure 1A). In effect, the same states in the 2018–2019 season were included in the 2008–2009 study [4].
Figure 1.
A, Inequities in influenza vaccination among White and Black nursing home (NH) residents by state, United States, 2018–2019. B, State influenza vaccination coverage among NH residents, 2018–2019. C, Dissimilarity index or the proportion of Black NH residents who would have to move to another facility to be randomly distributed in the state. White indicates the state has <1% Black residents (ID, MT, WY, ND, SD, ME, VT, NH) or <1 percentage point W–B vaccination gap (AK, UT). Abbreviations: AL, Alabama; AK, Alaska; AZ, Arizona; AR, Arkansas; CA, California; CO, Colorado; CT, Connecticut; DE, Delaware; FL, Florida; GA, Georgia; HI, Hawaii; ID, Idaho; IL, Illinois; IN, Indiana; IA, Iowa; KS, Kansas; KY, Kentucky; LA, Louisiana; ME, Maine; MD, Maryland; MA, Massachusetts; MI, Michigan; MN, Minnesota; MS, Mississippi; MO, Missouri; MT, Montana; NE, Nebraska; NV, Nevada; NH, New Hampshire; NJ, New Jersey; NM, New Mexico; NY, New York; NC, North Carolina; ND, North Dakota; OH, Ohio; OK, Oklahoma; OR, Oregon; PA, Pennsylvania; ppt, percentage points; RI, Rhode Island; SC, South Carolina; SD, South Dakota; TN, Tennessee; TX, Texas; UT, Utah; VT, Vermont; VA, Virginia; W–B, White–Black; WA, Washington; WV, West Virginia; WI, Wisconsin; WY, Wyoming.
We obtained data from MDS resident assessments (version 3.0) [7] completed from 1 October 2018 through 31 March 2019. The dates were selected to be consistent with our previous study, which used a prior version of the MDS (version 2.0) [7]. NH residents without an NH facility identifier were excluded.
Outcome Measure
The immunization supplement to the resident assessment instrument asks, “Did the resident receive the influenza vaccine in this facility for this year’s influenza season?” The next question asks, “If influenza vaccine was not received, state reason: 1) not in facility during this year’s flu season; 2) received outside of this facility; 3) not eligible-medical contraindication; 4) offered and declined; 5) not offered; and 6) inability to obtain vaccine” [7]. Most residents had data from more than 1 assessment for the 2018–2019 influenza season. Vaccination status was determined using the process that was used in our previous study [4]. Vaccination coverage reported here includes only White and Black NH residents in the denominators.
Exposure Measure
Race was the main exposure of interest. The MDS resident assessment instrument has a variable for each race/ethnicity, allowing for multiple affirmative responses. Residents were classified as Black if they reported Black race, regardless of reporting another or Hispanic ethnicity. Similarly, residents were classified as White if they reported White race; however, if they also reported Black race, they were classified as Black. For purposes of comparing our analyses with those from a previous study, we only report results for White and Black NH residents. However, to report the actual proportion of residents in the state or in the nursing home, the denominator included all residents regardless of race. Thus, the “proportion of Blacks” means the proportion out of all residents; “the proportion of Blacks vaccinated” is out of all Black residents, and similarly for Whites.
Covariates
We adjusted for 2 resident-level factors: gender and age. Additionally, we adjusted for 2 facility-level factors: the total number of residents in the NH and the proportion of Black residents in the NH during the influenza season.
Segregation Measure
To assess segregation in NHs across states, we used the dissimilarity index and present it by state. While we could not assess segregation within an NH, we could assess segregation across facilities within states. The index is formulated on the assumption that if there is no segregation, then members of a minority racial group would be distributed randomly throughout the various NHs in the state [8]. The formula gives the proportion of Black NH residents who would have to move to another NH to be randomly distributed in the state.
Statistical Analyses
To assess vaccination differences across states, we produced the following 3 unadjusted, state-level plots: inequity in vaccination among NH residents vs the proportion of NH residents vaccinated; the proportion of NH residents vaccinated vs the proportion of Black NH residents; and the inequity in vaccination coverage among NH residents vs the proportion of Black NH residents. Linear regression was used.
We also fit a 3-level multilevel model with the 40 states as the third level, NHs as the second level, and residents as the first level in order to test for variability between states and to determine if region and the proportion of Black residents in the state were associated with residents’ vaccination, after adjusting for other factors. The null hypothesis for the random effects was variance equal to zero (H0: σ o22 = 0 and H02: σ r22 = 0) at the facility level (2) and (H0: σ o32 = 0 and H0: σ r32 = 0) at the state level (3), where o = offered vaccine and r = refused vaccine, vs vaccinated.
To further assess whether a pattern persisted across states, we calculated the adjusted vaccination coverage for White and Black residents for each NH in a 2-level multilevel model for each state. The outcome of each model was the resident-level vaccination status for White and Black residents in each NH. For each state, medians of vaccination status for White and Black residents are reported for each stratum of NH by the proportion of Black residents. Only residents who self-identified as Black/African American or White were included in the multilevel models, excluding 9.6% (n = 215 144) of the study population who self-identified as other than Black/African American or White. For each state, we estimated the adjusted vaccination coverage across the strata of racially mixed NHs by the distribution of Black residents (0%, 0.1%–4.9%, 5.0%–19.9%, 20.0%–49.9%, and ≥50% Black residents). States were grouped by the degree of inequity in each state (ie, 1–4.9, 5.0–9.9, and ≥10 percentage points). Next, we examined adjusted probability of refusal and not being offered the vaccine for White and Black residents.
Software and Ethics Approval
We used HLM v.8 software (Scientific Software International, Inc, Lincolnwood, IL) to conduct multilevel analyses for each of the 40 states. The study was approved by the Brown University Institutional Review Board.
RESULTS
Our study population included 2 233 392 residents from 14 237 NHs in 40 states, 14.1% of whom self-reported Black race (2.2% of the 14.1% also reported White race). Among NH residents in the 40 states, overall unadjusted vaccination coverage among White residents was 76.2% and among Black residents was 66.3%, a difference of 9.9 percentage points. Among the 40 states with a W–B difference >1 percentage point, vaccination coverage by quartiles was 48.5%–72.5%, 72.6%–76.1%, 76.2%–81.5%, and 81.6%–87.8% (Figure 1A). In Alaska, vaccination coverage among White NH residents was 3.0 percentage points lower than among Black NH residents. An inequity of ≥10 percentage points existed in 14 states, inequity of 5.0–9.9 percentage points existed in 21 states, and inequity of 1–4.9 percentage points existed in 5 states. Inequities were concentrated in the Midwest (Figure 1B). In the adjusted 3-level model, variability in being offered and refusing vs being vaccinated across facilities was statistically significant (σ o2 = 2.49 and σ r2 = 0.54, P < .001, respectively); variability across states was also significant (σ o2 = 0.18 and σ r2 = 0.11, P < .001, respectively) though to a lesser extent.
State-level Differences
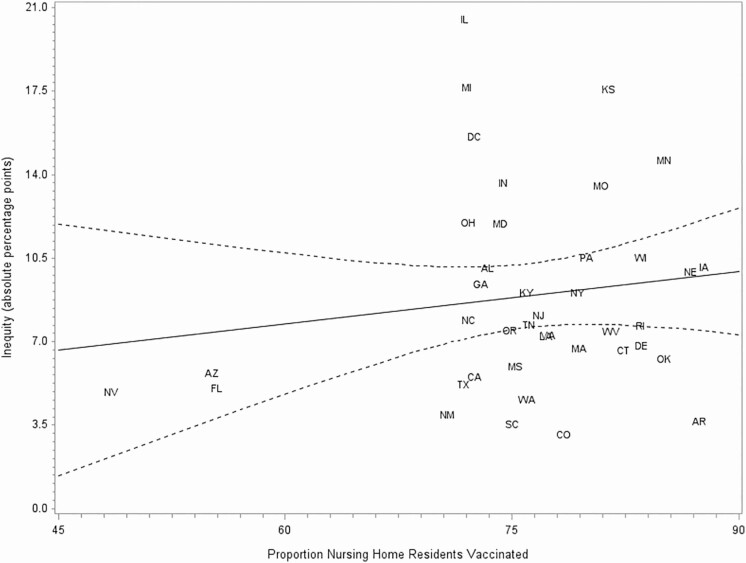
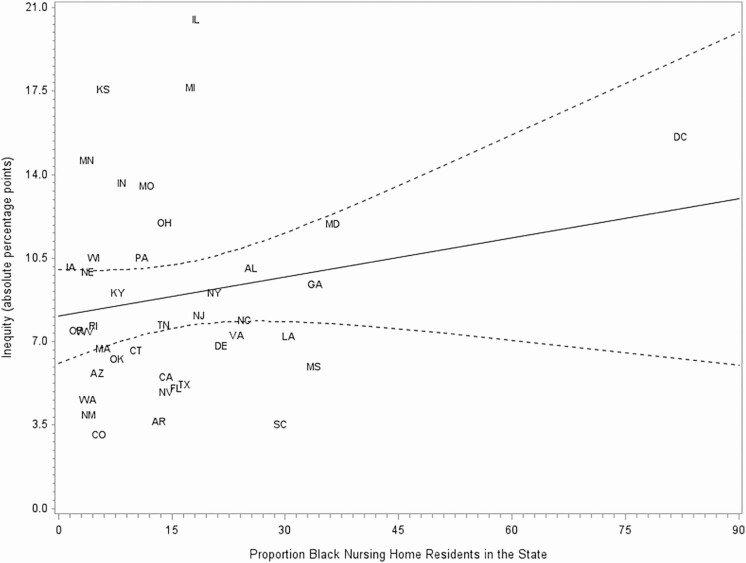
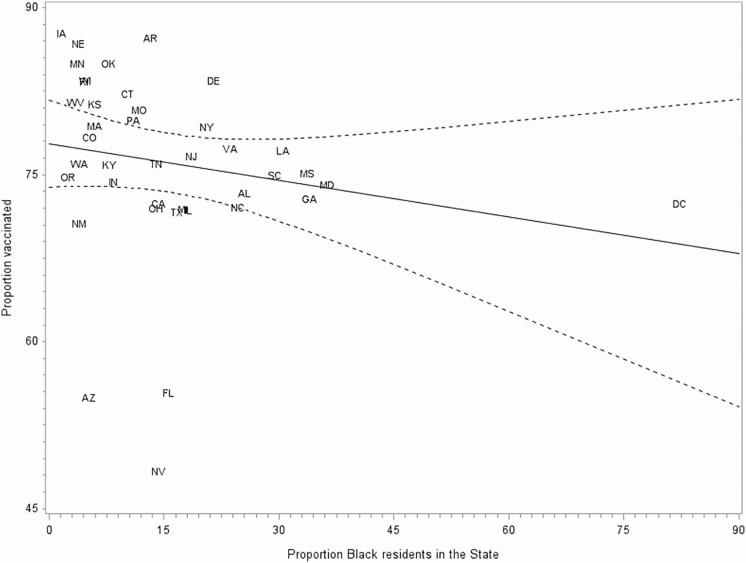
In the adjusted 3-level model, the proportion of Black NH residents in the state was not significantly associated with residents being offered the vaccine vs being vaccinated (P = .42) or between refusing the vaccine and being vaccinated (P = .44). Similarly, in the unadjusted state-level analysis, no significant relationship was found between state-level vaccination coverage among NH residents and W–B inequity in vaccination (β = 0.07; P = .37; Figure 2) or between the proportion of Black NH residents in states and W–B inequity in vaccination (β = 0.05, P = .24; Figure 3). Additionally, no significant relationship was found between the proportion of Black NH residents and vaccination coverage (β = −0.11, P = .23; Figure 4).
Figure 2.
Inequity in influenza vaccination among nursing home (NH) residents by proportion of NH residents vaccinated in the state, 2018–2019 season. Dashed lines indicate 95% confidence interval. Abbreviations: AL, Alabama; AZ, Arizona; AR, Arkansas; CA, California; CO, Colorado; CT, Connecticut; DE, Delaware; FL, Florida; GA, Georgia; HI, Hawaii; IL, Illinois; IN, Indiana; IA, Iowa; KS, Kansas; KY, Kentucky; LA, Louisiana; MD, Maryland; MA, Massachusetts; MI, Michigan; MN, Minnesota; MS, Mississippi; MO, Missouri; NE, Nebraska; NV, Nevada; NJ, New Jersey; NM, New Mexico; NY, New York; NC, North Carolina; OH, Ohio; OK, Oklahoma; OR, Oregon; PA, Pennsylvania; RI, Rhode Island; SC, South Carolina; TN, Tennessee; TX, Texas; VA, Virginia; WA, Washington; WV, West Virginia; WI, Wisconsin.
Figure 3.
Inequity in influenza vaccination among nursing home (NH) residents by proportion of Black NH residents in the state, 2018–2019 season. Dashed lines indicate 95% confidence interval. Abbreviations: AL, Alabama; AZ, Arizona; AR, Arkansas; CA, California; CO, Colorado; CT, Connecticut; DE, Delaware; FL, Florida; GA, Georgia; HI, Hawaii; IL, Illinois; IN, Indiana; IA, Iowa; KS, Kansas; KY, Kentucky; LA, Louisiana; MD, Maryland; MA, Massachusetts; MI, Michigan; MN, Minnesota; MS, Mississippi; MO, Missouri; NE, Nebraska; NV, Nevada; NJ, New Jersey; NM, New Mexico; NY, New York; NC, North Carolina; OH, Ohio; OK, Oklahoma; OR, Oregon; PA, Pennsylvania; RI, Rhode Island; SC, South Carolina; TN, Tennessee; TX, Texas; VA, Virginia; WA, Washington; WV, West Virginia; WI, Wisconsin.
Figure 4.
Proportion of nursing home (NH) residents vaccinated in the state by proportion of Black NH residents in the state, 2018–2019 season. Dashed lines indicate 95% confidence interval. Abbreviations: AL, Alabama; AZ, Arizona; AR, Arkansas; CA, California; CO, Colorado; CT, Connecticut; DE, Delaware; FL, Florida; GA, Georgia; HI, Hawaii; IL, Illinois; IN, Indiana; IA, Iowa; KS, Kansas; KY, Kentucky; LA, Louisiana; MD, Maryland; MA, Massachusetts; MI, Michigan; MN, Minnesota; MS, Mississippi; MO, Missouri; NE, Nebraska; NV, Nevada; NJ, New Jersey; NM, New Mexico; NY, New York; NC, North Carolina; OH, Ohio; OK, Oklahoma; OR, Oregon; PA, Pennsylvania; RI, Rhode Island; SC, South Carolina; TN, Tennessee; TX, Texas; VA, Virginia; WA, Washington; WV, West Virginia; WI, Wisconsin.
Adjusted Vaccination Status Among States and Proportion of Black Residents in Facilities
In the strata with ≥10 percentage points inequity, the median adjusted vaccination rate was highest in facilities with no Black residents (range, 82.4%–90.6%) and lowest among White and Black residents in facilities with ≥50% Black residents (range for White residents, 43.2%–81.5%; range for Black residents, 40.8%–77.8%; Table 1). Compared with states with ≥10 percentage point inequities, in states with vaccination inequities of 5.0–9.9 percentage points, vaccination coverage was generally higher in facilities with ≥5% Black residents; the differences across strata by racial composition of the NH were less marked. Among states with inequities of 1–4.9 percentage points, differences in vaccination were negligent within strata; there was no dose-response relationship between vaccination coverage and racial composition in NHs.
Table 1.
Adjusted Probability Vaccination Status Stratified by Percent Black Residents in Nursing Homes by State—Minimum Data Set, United States, 2018–2019
| Facilities Grouped by Percent Black Residents | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0% Blacks | 0.1% –4.9% Blacks | 5%–19.9% Blacks | 20%–49.9% Blacks | ≥50% Blacks | |||||||
| Vaccinated | |||||||||||
| Reporting Area | White–Black Gap | % Black Residents in State | White | White | Black | White | Black | White | Black | White | Black |
| States with ≥10 percentage point influenza vaccination inequities | |||||||||||
| Illinois | 20.6 | 17.2 | 85.9 | 79.3 | 75.3 | 70.2 | 64.9 | 68.6 | 63.2 | 48.8 | 42.9 |
| Michigan | 17.7 | 17.0 | 85.8 | 78.8 | 73.9 | 72.1 | 67.8 | 56.9 | 51.4 | 43.2 | 40.8 |
| Kansas | 17.6 | 6.2 | 89.5 | 86.8 | 83.1 | 68.1 | 60.6 | 49.7 | 46.1 | – | – |
| District of Columbia | 15.7 | 77.8 | – | – | – | – | – | – | – | 65.9 | 64.0 |
| Minnesota | 14.7 | 3.4 | 88.9 | 82.6 | 76.2 | 73.9 | 66.3 | 78.6 | 71.7 | – | – |
| Indiana | 13.7 | 8.2 | 83.2 | 80.7 | 76.9 | 68.2 | 62.6 | 61.1 | 54.6 | 63.1 | 59.5 |
| Missouri | 13.6 | 11.5 | 85.9 | 84.4 | 80.9 | 84.0 | 80.4 | 74.4 | 69.9 | 67.3 | 60.1 |
| Ohio | 12.0 | 13.8 | 82.4 | 78.0 | 73.6 | 70.5 | 65.2 | 61.8 | 55.6 | 64.3 | 58.5 |
| Maryland | 12.0 | 35.0 | 90.5 | 84.2 | 84.6 | 85.6 | 84.5 | 77.1 | 76.4 | 56.5 | 54.7 |
| Pennsylvania | 10.6 | 11.0 | 87.0 | 80.2 | 76.9 | 72.8 | 69.2 | 71.7 | 67.8 | 72.0 | 68.4 |
| Wisconsin | 10.6 | 4.6 | 86.3 | 81.0 | 77.3 | 66.9 | 61.8 | 66.1 | 61.1 | 81.5 | 77.8 |
| Iowa | 10.2 | 1.8 | 90.6 | 85.4 | 83.8 | 80.8 | 77.4 | – | – | – | – |
| Alabama | 10.2 | 25.4 | 88.6 | 78.0 | 73.8 | 69.7 | 64.3 | 71.3 | 65.5 | 66.6 | 60.3 |
| Nebraska | 10.0 | 3.6 | 90.3 | 86.2 | 84.0 | 87.7 | 85.1 | 74.6 | 71.1 | – | – |
| Median | 11.3 | 87.0 | 81.0 | 76.9 | 72.1 | 66.3 | 70.0 | 64.4 | 65.1 | 59.8 | |
| States with 5–9.9 percentage point influenza vaccination inequities | |||||||||||
| Georgia | 9.5 | 33.1 | 80.1 | 78.0 | 74.7 | 80.8 | 77.8 | 71.5 | 68.6 | 72.5 | 67.8 |
| Kentucky | 9.1 | 7.7 | 83.9 | 75.6 | 71.4 | 78.8 | 75.0 | 83.8 | 80.6 | – | – |
| New York | 9.1 | 17.8 | 92.4 | 84.3 | 82.2 | 71.4 | 68.6 | 72.5 | 69.2 | 77.5 | 75.0 |
| New Jersey | 8.2 | 16.4 | 92.7 | 78.0 | 75.9 | 75.3 | 73.3 | 68.0 | 65.4 | 76.9 | 74.6 |
| North Carolina | 8.0 | 24.0 | 87.6 | 86.8 | 85.3 | 78.1 | 75.4 | 64.8 | 61.8 | 66.9 | 67.3 |
| Tennessee | 7.8 | 14.1 | 82.6 | 80.4 | 78.1 | 79.0 | 77.5 | 83.4 | 81.6 | 63.2 | 60.8 |
| Rhode Island | 7.7 | 4.3 | 90.9 | 83.5 | 82.3 | 79.2 | 77.3 | – | – | – | – |
| Oregon | 7.5 | 2.1 | 76.7 | 78.5 | 74.6 | 75.2 | 70.2 | – | – | – | – |
| West Virginia | 7.5 | 3.5 | 85.8 | 83.8 | 79.3 | 79.0 | 74.7 | – | – | – | – |
| Virginia | 7.3 | 22.6 | 90.9 | 84.1 | 80.0 | 80.8 | 76.4 | 72.4 | 68.5 | 71.2 | 67.5 |
| Louisiana | 7.3 | 30.5 | – | 86.6 | 84.1 | 80.9 | 77.5 | 79.1 | 75.3 | 69.5 | 65.1 |
| Delaware | 6.9 | 20.9 | – | 92.9 | 90.5 | 85.8 | 81.4 | 83.8 | 78.0 | – | – |
| Massachusetts | 6.8 | 5.7 | 86.8 | 81.0 | 78.6 | 78.0 | 75.1 | 75.9 | 73.2 | 85.1 | 83.0 |
| Connecticut | 6.7 | 9.6 | 90.9 | 84.0 | 81.9 | 77.3 | 75.2 | 82.2 | 79.9 | – | – |
| Oklahoma | 6.4 | 7.1 | 89.4 | 88.2 | 85.5 | 85.1 | 82.1 | 80.7 | 76.4 | – | – |
| Mississippi | 6.0 | 33.3 | – | 76.5 | 73.6 | 84.6 | 82.7 | 84.4 | 82.1 | 70.7 | 67.4 |
| Arizona | 5.8 | 4.2 | 69.1 | 60.2 | 61.4 | 78.4 | 79.3 | – | – | – | – |
| California | 5.6 | 10.1 | 88.6 | 74.5 | 70.9 | 72.2 | 68.4 | 69.4 | 65.4 | 71.2 | 67.4 |
| Texas | 5.3 | 12.9 | 83.1 | 77.7 | 76.2 | 73.6 | 71.9 | 69.8 | 68.1 | 51.4 | 49.4 |
| Florida | 5.1 | 13.7 | 89.0 | 57.1 | 55.7 | 50.2 | 49.4 | 46.8 | 45.3 | 64.6 | 63.2 |
| Nevada | 5.0 | 10.7 | 70.9 | 58.3 | 60.2 | 50.4 | 52.1 | 39.9 | 41.3 | – | – |
| Median | 12.9 | 87.2 | 80.4 | 78.1 | 78.4 | 75.2 | 72.5 | 69.2 | 71.0 | 67.4 | |
| States with 1–4.9 percentage point influenza vaccination inequities | |||||||||||
| South Carolina | 4.7 | 28.4 | – | 85.0 | 84.7 | 87.2 | 87.0 | 78.5 | 78.2 | 69.6 | 69.1 |
| Washington | 4.0 | 3.6 | 80.8 | 75.6 | 72.8 | 78.4 | 75.9 | 73.5 | 70.8 | – | – |
| Colorado | 3.7 | 4.4 | 85.8 | 74.4 | 74.5 | 74.2 | 74.4 | 62.8 | 63.1 | – | – |
| Arkansas | 3.6 | 13.0 | 85.0 | 89.5 | 86.7 | 88.6 | 85.6 | 88.3 | 85.2 | 86.6 | 83.2 |
| New Mexico | 3.2 | 2.1 | 71.5 | 65.6 | 65.6 | 83.8 | 80.3 | – | – | – | – |
| Median | 4.4 | 82.9 | 75.6 | 74.5 | 83.8 | 80.3 | 76.0 | 74.5 | 78.1 | 76.2 | |
| Overall Median | 13.6 | 87.2 | 80.3 | 77.3 | 74.2 | 70.6 | 70.5 | 67.0 | 66.9 | 62.6 | |
Includes anyone who lived in a nursing home for at least 1 day between 1 October 2018 and 31 March 2019.
Results from multilevel models for each state including sex, age, race, total number residents in the facility during influenza season, and proportion Black residents in the facility. Vaccination coverage in nursing homes with 0% Black residents and ≥50% Black residents is bolded to show the differences in the most disparate strata. Dash (–) indicates strata with <5 facilities.
When comparing adjusted differences of proportions of coverage for White and Black residents within each racially mixed NH in the 40 states (n = 11 326 NHs), differences were small (difference: median, 2.3 percentage points; data not shown).
Compared with states where the W–B inequity was less than 10 percentage points, in states with ≥10 percentage points inequity, Black residents disproportionately lived in NHs with ≥50% Black residents (49.2%) compared with the other states (states with 5.0–9.9 percentage point inequities, 32.5%; states with 1–4.9 percentage point inequities, 27.0%; data not shown).
The overall proportion of residents not offered the vaccine was 3%. Compared with being vaccinated, being offered the vaccine did not vary across the 4 regions in the 3-level model (P = .57, P = .09, and P = .38). Nevertheless, facility median probabilities were highest in the states with ≥10 percentage point inequities among Black and White residents in the 2 strata of NHs with ≥20% Black residents (20%–49.9%: 1.2%–1.3% and ≥ 50%: 2.0%–2.1% for White and Black residents, respectively).
Vaccination Refusals
Overall, Black residents refused vaccination (28.7%) more frequently than White residents (21.0%). State median probabilities of refusing vaccination in states with ≥10 percentage point inequities ranged from 9.9% among White residents in facilities with no Black residents to 22.7% among Black residents in facilities with ≥50% Black residents. State median probabilities of refusing vaccination in states with 5–9.9 percentage point inequities ranged from 10.1% among White residents in facilities with no Black residents to 20.7% among Black residents in facilities with ≥50% Black residents. In states with ≥5% inequities, facility median refusals were 2–3 percentage points higher among Black residents compared with White residents in all strata of facilities. Compared with being vaccinated, refusing the vaccine was significantly higher in the western region compared with the Midwest in the 3-level model (P < .01).
DISCUSSION
The results of this national cross-sectional study suggest that in states with at least 1% Black NH residents, racial inequities in receiving the influenza vaccine during the 2018–2019 influenza season were mostly at the facility level. Black residents disproportionately lived in NHs with 50% or more Black residents, which generally had the lowest coverage. Additionally, inequities were most concentrated in the Midwestern region, which was also the most segregated. Inequities across states with at least 1% Black NH residents were not associated with vaccination or proportion of Black NH residents in the state. Moreover, vaccination (at the state as well as individual level) was not associated with the proportion of Black NH residents in the state. At the national level, it is important to note that of the 10 states excluded from our study because they did not have at least 1% Black NH residents, 5 had very high vaccination coverage (>82%) [3]. Thus, nationally, state does play a vital role in racial differences in vaccination of NH residents; however, among states with at least 1% Black NH residents, racial inequities were mostly at the facility level.
Consistent with the 2008–2009 influenza season [4], the same 40 states with inequities had at least a 1 percentage point difference in 2018–2019, with an increase in the unadjusted W–B gap from 8.1 to 9.9 percentage points. Comparing 2018–2019 to 2008–2009 [4], 40.0% more states had ≥10 percentage point inequities, 23.5% more states had 5%–9.9% inequities, and the W–B median gap between facilities with no Black residents and facilities with ≥50% Black residents increased in all strata of states. Furthermore, a larger proportion of Black NH residents lived in homes with ≥50% Black residents in the 35 states with ≥5 percentage point inequities than previously [4]. The proportion of residents not being offered the vaccine dropped dramatically since 2008–2009, from as high as 24.1% among Black residents in facilities with ≥50% Black residents [4] to 2.1% in 2018–2019. Conversely, refusing the vaccine was reported more frequently among White and Black residents in all states compared with 2008–2009 [4]. Notably, 4 states with a large proportion (20.9%–33.3%) of Black NH residents had fewer than 5 facilities with no Black residents during the 2018–2019 influenza season.
Similar to the 2008–2009 influenza season, differences were greater between NHs than within NHs in states with ≥10 percentage point inequities. In the 2008–2009 study, the patterns of racial differences in vaccination were primarily in the NHs rather than due to regional- or state-level characteristics [4]. During that influenza season, the states with the greatest inequities were also the states with the lowest vaccination coverage. In addition, they were the states with the greatest proportion of Black NH residents in homes with ≥50% Black residents. There was a dose-response decrease in vaccination coverage by racial composition of NHs. However, in the current study, inequities were larger, and larger in more states, but not primarily in the states with the lowest vaccination coverage as was the case in 2008–2009. In addition, more Black NH residents lived in homes with ≥50% Black residents in all strata of states. While vaccination coverage varied significantly across the 40 states in the adjusted 3-level model, it varied to a larger extent between facilities. Region was not significantly associated with vaccination or being offered the vaccine but was associated with refusing vaccine, more so in the western states than in the Midwest.
It is unknown if our results are due to a persisting two-tiered system of NH care, with racial composition differing by private vs public funding [9]; a result of an increase in admissions to NHs from hospitals [10]; an increase in postacute stay residents [11]; or some combination. It is also unknown if there has been a change in vaccination policies such as standing orders for vaccinations since 2004, when about 40% of US NHs had such policies, since standing orders for vaccinations are associated with higher vaccination coverage and lower racial inequities [12]. It is noteworthy that the overall pattern of inequities in 2018–2019 was similar to the pattern in 2008–2009. However, in the previous season not being offered the vaccine was quite high in facilities with ≥50% Black residents (24.1%), whereas now the difference is due to refusals in that strata (22.7%). One intervention study found that NHs that adopted a policy to document residents’ refusal of influenza vaccine resulted in 10% or greater increase in vaccination [13].
Although the Advisory Committee for Immunization Practices recommends annual influenza vaccination for all NH residents [14], CMS requires that all certified NHs offer vaccination to all residents, and Medicare funds vaccination [15], Influenza vaccination coverage among NH residents at the national level decreased from 77.5% in the 2008–2009 influenza season to 73.1% in the 2018–2019 season [3], below the Healthy People 2020 goal of 90% [16]. In spite of suboptimal vaccination and persistent racial inequities, influenza immunization of NH residents is no longer a health indicator in Healthy People 2030 [17].
A limitation of this study is that reporting of race and ethnicity changed since our 2008–2009 study. This could excessively increase the proportion of Black residents if self-identification was more likely when given more than 1 choice of race and/or ethnicity. However, the proportion of Black NH residents in all US states was 11.5% in 2008-2009 [18] compared with 14.1% in the 40 states with >1% Black NH residents in 2018–2019. An increase of 2.6 percentage points is reasonable given the increase in racial minorities among the aging population [19, 20]. A strength of our study is that we examined each state separately, holding state constant, controlling for state-specific variability in access to healthcare, and accounting for variability between NHs in each state with multilevel models.
In conclusion, the increase in the W–B influenza vaccination gap among NH residents is likely occurring at the facility level in states with the most W–B racial segregation. Increased racial inequities in receiving the influenza vaccine suggests that interventions to increase vaccination while narrowing racial differences, such as standing orders for vaccinations [12], should be considered.
Notes
Author contributions. B. H. B., S. G., and A. R. Z. conceived and designed the study. B. H. B., R. R. B., E. B., and A. R. Z. drafted the manuscript. J. O. obtained the data. All authors revised the manuscript for important intellectual content and contributed to the literature search. B. H. B. conducted statistical analyses. R. v A., A. C., M. L., and A. R. Z. provided administrative, technical, and material support.
Disclaimer. This work was supported by Sanofi Pasteur, which provided a research grant to the author’s institution (A. R. Z.) as part of a larger project aiming to estimate the burden of infections in long-term care. The author’s institution retained the right to publish and publicly present all results. Sanofi Pasteur was not involved in establishing the scope of the study, creating the initial protocol, designing the study, or performing analysis but was involved in suggesting edits to the final study protocol and reviewing the final manuscript. Incorporation of any edits suggested by Sanofi Pasteur was not compulsory.
Financial support. A. R. Z. was supported, in part, by the National Institute on Aging (grant R21AG061632) and the National Institute of General Medical Sciences (grant U54GM115677). In addition, A. R. Z. is supported by a US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Health Services Research and Development.
Potential conflicts of interests. R. v A., M. L. and A. C. are employees of the pharmaceutical company Sanofi Pasteur, which produces vaccines. A. R. Z. reports conflicts with vaccine manufacturer Sanofi related to grants. S. G. reports conflicts with vaccine manufacturers Sanofi, Seqirus, Pfizer related to grants, consulting, and speaking engagements. S. G. also consults with other pharmaceutical companies including Longevoron, Janssen, and Merck and has grants with Sunovion and Essity. J. B. S. reports grants from Sanofi Pasteur outside the submitted work. P. M. reports Pilot grant funding under the National Institutions of Health (NIH; grant P20GM125507) from Rhode Island Hospital, Faculty Research Award from Brown University, Interagency Personnel Agreement (under VA grant IIR 17–231) from Providence Veterans Administration, Collaborator on 5UH3AG049619 from NIH, and Collaborator on HHSA290201500002I from the Agency for Healthcare Research and Quality outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Iuliano AD, Roguski KM, Chang HH, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network . Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet 2018; 391:1285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaillat J, Chidiac C, Fagnani F, et al. . Morbidity and mortality associated with influenza exposure in long-term care facilities for dependent elderly people. Eur J Clin Microbiol 2009; 28:1077–86. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. 2005–06 through 2018–19 influenza seasons nursing home vaccination coverage trend report, 2020. https://www.cdc.gov/flu/fluvaxview/nursinghome/trends/index.html. Accessed 09/10/2020.
- 4. Bardenheier B, Wortley P, Shefer A, McCauley MM, Gravenstein S. Racial inequities in receipt of influenza vaccination among nursing home residents in the United States, 2008-2009: a pattern of low overall coverage in facilities in which most residents are black. J Am Med Dir Assoc 2012; 13:470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller VJ, Hamler T. A value-critical policy analysis of the Nursing Home Reform Act: a focus on care of African American and Latino residents. Soc Work Health Care 2019; 58:471–93. [DOI] [PubMed] [Google Scholar]
- 6. He M, Li Y, Fang F. Is there a link between nursing home reported quality and COVID-19 cases? Evidence from California skilled nursing facilities. J Am Med Dir Assoc 2020; 21:905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Medicare & Medicaid Services. MDS 3.0 RAI manual, 2020. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual. Published 2020. Accessed 04/22/2020.
- 8. Cortese CF, Falk RF, Cohen JK. Further considerations on methodological analysis of segregation indexes. Am Sociol Rev 1976; 41:630–7. [Google Scholar]
- 9. Mor V, Zinn J, Angelelli J, Teno JM, Miller SC. Driven to tiers: socioeconomic and racial disparities in the quality of nursing home care. Milbank Q 2004; 82:227–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fashaw SA, Thomas KS, McCreedy E, Mor V. Thirty-year trends in nursing home composition and quality since the passage of the Omnibus Reconciliation Act. J Am Med Dir Assoc 2020; 21:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abrahamson K, Shippee TP, Henning-Smith C, Cooke V. Does the volume of post-acute care affect quality of life in nursing homes? J Appl Gerontol 2017; 36:1272–86. [DOI] [PubMed] [Google Scholar]
- 12. Bardenheier B, Shefer A, Ahmed F, Remsburg R, Rowland Hogue CJ, Gravenstein S. Do vaccination strategies implemented by nursing homes narrow the racial gap in receipt of influenza vaccination in the United States? J Am Geriatr Soc 2011; 59:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bardenheier BH, Shefer A, McKibben L, Roberts H, Rhew D, Bratzler D. Factors predictive of increased influenza and pneumococcal vaccination coverage in long-term care facilities: the CMS-CDC Standing Orders Program project. J Am Med Dir Assoc 2005; 6:291–9. [DOI] [PubMed] [Google Scholar]
- 14. Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—United States, 2019-20 influenza season. MMWR Recomm Rep 2019; 68:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; condition of participation: immunization standard for long term care facilities; final rule. Federal Register Part III, 2005. https://www.govinfo.gov/content/pkg/FR-2005-10-07/pdf/05-19987.pdf. Accessed 04/28/2020. [PubMed]
- 16. Office of Disease Prevention and Health Promotion. Healthy People 2020: Immunization and Infectious Diseases Department of Health and Human Services, 2018. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Published 2018. Accessed 04/28/2020.
- 17. National Academies of Sciences Engineering, and Medicine. Criteria for selecting the leading health indicators for Healthy People 2030. Washington, D.C.: National Academies Press, 2019. [PubMed] [Google Scholar]
- 18. Black CL, Williams WW, Arbeloa I, et al. . Trends in influenza and pneumococcal vaccination among US nursing home residents, 2006–2014. J Am Med Dir Assoc 2017; 18:735 e731–e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. US Census Bureau. The Black alone population in the United States: 2008.https://www.census.gov/data/tables/2008/demo/race/ppl-ba08.html. Accessed 04/22/2020.
- 20. US Census Bureau. Population 65 years and over in the United States, 2019. https://data.census.gov/cedsci/table?q=2018%20population%20by%20age%20and%20race&hidePreview=false&tid=ACSST1Y2018.S0103&t=Age%20and%20Sex&y=2018&vintage=2018. Accessed 04/22/2020.