Abstract
Introduction
Although recent guidelines have recommended monitoring vancomycin (VAN) area under the curve (AUC)/minimum inhibitory concentration (MIC) to ensure clinical efficacy and minimize toxicity in methicillin-resistant Staphylococcus aureus (MRSA) for various infections, there are no recommendations regarding complicated skin and soft tissue infections (cSSTIs). We aimed to evaluate the association between VAN AUC and clinical outcomes in MRSA cSSTIs.
Methods
This was a retrospective cohort study of adult patients treated with ≥72 hours of VAN for MRSA cSSTI from 2008 to 2013 at Detroit Medical Center. The primary outcome was timely clinical success (TCS) defined as (1) resolution of signs and symptoms of infection within 72 hours, (2) stabilization and/or reduction in lesion size, (3) alternative agents not required due to VAN failure or toxicity as elected by the prescribing clinician. Classification and regression tree (CART) analysis was performed to determine the AUC associated with TCS in the cohort. Multivariable logistic regression was used to evaluate the association between VAN-AUC and the primary outcome.
Results
A total of 154 patients were included in this analysis. CART identifed an AUC ≥435 mg*hr/L for TCS. Overall, 60.9% of patients experienced TCS; 69.7% in the target-AUC group versus 52.5% in the below-target AUC group, (P = .013). Target-AUC attainment was independently associated with increased odds of TCS (adjusted odds ratio [aOR], 2.208; 95% confidence interval [CI], 1.047–4.659).
Conclusions
In adults treated with VAN for MRSA cSSTI, target-AUC attainment was independently associated with improved clinical outcomes and maybe most warranted for patients at high risk of VAN failure or VAN-associated toxicity.
Keywords: vancomycin, skin and soft tissue, gram-positive infections
In adults treated with Vancomycin (VAN) for methicillin-resistant Staphylococcus aureus Complicated Skin and Soft Tissue Infection, target-Area Under the Curve attainment was independently associated with improved clinical outcomes and maybe most warrated for patients at high risk of VAN-failure or VAN-associated toxicity.
The 2020 consensus guidelines for therapeutic monitoring of vancomycin (VAN) recommends a target VAN area under the concentration curve (AUC) of 400–600 mg*hr/L for serious methicillin-resistant Staphylococcus aureus (MRSA) infections, assuming a minimum inhibitory concentration (MIC) of 1 mg/L and no longer recommends the use of trough monitoring [1]. The AUC can be calculated utilizing a Bayesian-derived approach or first-order pharmacokinetic (PK) analytical equations [1, 2]. The switch to an AUC monitoring method has been associated with significantly less VAN-induced acute kidney injury (AKI) than a trough-based approach [1, 2]. Although an AUC-based monitoring approach has been evaluated in a variety of infections including endocarditis, bacteremia, pneumonia, and bone/joint infections, it had not been well investigated in complicated skin and soft tissue infections (cSSTIs) [3–6]. This may be due to the fact that patients treated for cSSTIs typically receive less intensive VAN monitoring, are switched to oral therapy earlier, and therefore are assumed to have a relatively low observed incidence of VAN-associated AKI [3, 7, 8]. Consequently, patients with cSSTIs have historically been managed in clinical studies using conventional dosing; typically, initially 15mg/kg ABW with doses in the range of 1000–1500 mg every 12 hours and monitoring using a trough only approach. However, VAN-associated AKI in cSSTIs are commonplace, particularly in high-risk patients [7]. Recent data demonstrate that the incidence in cSSTIs can be as high as 9%. In fact, each additional day of VAN therapy beyond day 3 has been shown to increase the odds of VAN-associated AKI by 14.3%. These considerations emphasize the need to identify the optimal AUC target for effectiveness and safety in cSSTIs. In addition, it is particularly important to determine high-risk subjects who would benefit from VAN AUC-based monitoring. The objectives of this study were to identify a target AUC and evaluate the impact of target AUC attainment on clinical outcomes in MRSA cSSTI patients treated with VAN.
METHODS
Study Design and Population
We conducted a retrospective cohort analysis at the Detroit Medical Center (DMC) between 2008 and 2013. The DMC is a single large healthcare system that includes 8 hospitals within Michigan. Patients were screened and eligible for inclusion upon meeting the following criteria: (1) age ≥18 years; (2) MRSA cSSTI [9], (3) ≥72 hours of intravenous VAN, (4) ≥1 VAN trough concentration within the 72 hours of VAN initiation, (5) ≥1 systemic inflammatory response syndrome (SIRS) criteria [10], and (6) ≥2 signs indicating infection involving deep skin and/or soft tissue involvement such as purulent drainage, erythema, swelling, tenderness, warmth, and/or induration [10]. Exclusion criteria included: (1) confirmed or presumed osteomyelitis, infected joints or a cSSTI secondary to burn wounds, (2) acute kidney injury at time of VAN initiation, (3) treatment of current cSSTI with an alternative anti-MRSA agent, (4) undergoing hemodialysis, or (5) having missing data in the electronic medical record. This study was reviewed and approved by the Wayne State University Human Investigational Review Board and the DMC Research Review Committee prior to initiation.
Data Collection and Study Definitions
The electronic medical record was used to extract patients’ demographics, comorbid conditions, laboratory, clinical, and treatment data, infectious diseases (ID) consultation, and the pursuit of source control. Cultures were processed at the DMC microbiology laboratories according to standard procedures. Variables associated with cSSTI were determined based on clinical notes and microbiological/diagnostic reports. During the study period, VAN was dosed per institution protocol targeting a VAN trough of 10–20 mg/L for cSSTI with the exception of necrotizing fasciitis or concomitant bacteremia where a trough of 15–20 mg/L was targeted. Loading doses were permitted but not mandated.
The initial 24-h AUC day 1 was calculated by using Bayesian simulation in ADAPT5 [11, 12]. This approach was validated to calculate the AUC values with high precision and low bias using VAN trough concentrations only. Using a previously published 2-compartment VAN population PK model, the mean parameter vector and variance-covariance matrix was used as the Bayesian prior in ADAPT5 [13, 14]. The Bayesian procedure in ADAPT5 estimated the Bayesian conditional posterior PK parameters for all patients based on their specific characteristics. The AUC was calculated by utilizing the dosing received by each patient, as well as the Bayesian conditional posterior PK parameter. The predictive performance of the MAP-Bayesian approach was assessed by comparing the estimated VAN concentrations to the measured concentrations. Although the AUC/MIC is driving the parameter for VAN efficacy, we refer to the AUC only assuming an MIC of 1 as recommended by the VAN consensus guideline due to the variability in MIC testing.
Outcome
The primary outcome was timely clinical success (TCS) defined as (1) resolution of signs and symptoms of infection within 72 hours, (2) clinical improvement in lesion healing and/or cessation of lesion growth (stabilization) at 72 hours, and (3) no MRSA alternative agents required due to VAN failure or toxicity. All clinical outcomes were assessed at end of treatment. We were not able to collect abscesses sizes as these are not routinely measured and recorded in the electronic medical record by treating physicians. Other secondary endpoints evaluated included VAN dose, 30-day mortality, 30-day hospital readmission, hospital length of stay, oral step down therapy, discharge home on antibiotic regimen for the same infection, nephrotoxicity, and persistent bloodstream infection (BSI) among patients who developed positive blood culture(s). Secondary outcomes were assessed from VAN start date. VAN maintenance was defined as lack of switching to an alternative anti-MRSA agent due to VAN toxicity and/or failure. Nephrotoxicity was defined as an increase in serum creatinine (0.5 mg/dl or ≥50% increase of Scr from baseline, whichever was greater). Persistent BSI was defined as BSI lasting ≥5 days [15].
Statstical Analysis
Descriptive statistics were used to evaluate patients’ demographics. Nominal data were reported as percentages and frequencies, and continous data were reported as medians and interquartile ranges (IQR). Categorical variables between the TCS and no TCS group were compared by the χ 2 or Fisher exact test, as appropriate and continuous variables were compared by Mann-Whitney U test. Classification and regression tree (CART) analysis was performed to determine the AUC24h breakpoint (BP) that was most predicitve of TCS in the entire cohort. In CART, the minimum parent node was specified at 30 cases, and the terminal node was set at 15 cases. To assess the independent association between AUC24h dichotomized at the CART derived cut-point and TCS, a multivariable logistic regression model was performed. AUC24h at the BP, along with all the variables associated with TCS at a P- value < .2 in bivariate analysis, were entered into the model simultaneously and removed using a backward stepwise approach. Variables with ≤10 subjects overall were not included. Covariates were retained in the model if the P-value for the likelihood ratio test for their removal was <0.1. The variance inflation factor was used to assess the multicollinearity of covariates in the model with values in the range of 1–5 were considered acceptable. The Hosmer-Lemeshow goodness-of-fit test was used to assess the model’s fit. All tests were 2 tailed with P-values ≤ .5 to be considered statistically significant. IBM SPSS software, version 26.0 (SPSS, Inc., Chicago, IL, USA) was used for all calculations.
RESULTS
During the study period, 154 adult patients treated with VAN for MRSA cSSTIs were evaluated. The demographics of these patients are illustrated in Table 1. The cohort was predominately male (64.3%) and Black (70.1%) and had a median (IQR) age of 50.5 (39.6–61.2) years. The median (IQR) actual body weight and ideal body weight were 82.0 (70.0–99.6) kg and 68.5 (59.3–76.0) kg, respectively. At the time of the first VAN concentration level, the median (IQR) creatinine clearance (CrCl) was 88.2 (64.7–111.7) mL/min, and the median (IQR) serum creatinine was 0.9 (0.7–1.1) mg/dL. Thirty percent of patients had a polymicrobial infection. The most common pathogens associated with polymicrobial infections included Pseudomonas aeruginosa (25.5%) and Enterococcus faecalis (17.0%). The most common primary cSSTIs were abscess (57.8%), followed by wounds (27.9%), and diabetic ulcers (7.1%).
Table 1.
Clinical Characteristics and Outcomes of Study Population
| Characteristics | Result (n = 154) |
|---|---|
| Age, years | 50.5 (39.6–61.2) |
| Age 65 years and older | 24 (15.6) |
| Sex, male | 99 (64.3) |
| Race, Black | 108 (70.1) |
| Weight, kg | 82.0 (70.0–99.6) |
| Ideal body weight, kg | 68.5 (59.3–76.0) |
| Body mass index, kg/m2 | 27.1 (23.2–32.8) |
| Obesea | 56 (36.4) |
| Wilson severity-of-illness scoring system | 97.0 (64.0–131.0) |
| Baseline Scr, mg/dL | 1.0 (0.8–1.3) |
| Serum Scr at time level drawn, mg/dL | 0.9 (0.7–1.1) |
| Highest Scr with VAN start, mg/dL | 1 (0.9–1.3) |
| CrCl, mL/minc | 88.2 (64.7–111.7) |
| Intensive care unit | 22 (14.3) |
| Polymicrobial infection | 47 (30.5) |
| Acinetobacter baumannii | 3 (6.4) |
| Escherichia coli | 7 (15.0) |
| Enterococcus faecalis | 8 (17.0) |
| Enterobacter cloacae | 7 (15.0) |
| Klebisella pneumoniae | 6 (12.8) |
| Morganella morganii | 4 (8.5) |
| Pseudomonas aeruginosa | 12 (25.5) |
| Proteus mirabilis | 6 (12.8) |
| Streptococcus agalactiae | 2 (4.3) |
| Streptococcus viridans | 4 (8.7) |
| Otherf | 15 (31.9) |
| Source control | 118 (76.6) |
| Incision and drainage | 107 (90.7) |
| Other measuresd | 11 (9.3) |
| Infectious diseases consultation | 107 (69.5) |
| Concomitant nephrotoxin | 39 (25.3) |
| Aminoglycosides | 4 (2.6) |
| ACEI/ARB | 22 (14.3) |
| Loop diuretics | 7 (4.5) |
| NSAID | 8 (5.2) |
| IV contrast | 9 (5.8) |
| Concomitant antibiotic, any | 108 (70.1) |
| Ampicillin/sulbactam | 29 (26.9) |
| Aztreonam | 2 (1.9) |
| Cefepime | 38 (35.2) |
| Ceftriaxone | 5 (4.6) |
| Ciprofloxacin | 2 (1.9) |
| Ertapenem | 2 (1.9) |
| Metronidazole | 11 (10.2) |
| Meropenem | 3 (2.8) |
| Piperacillin/tazobactam | 28 (25.9) |
| Tobramycin | 3 (2.8) |
| Otherg | 9 (8.3) |
| Primary infectione | |
| Abscess | 89 (57.8) |
| Cellulitis | 6 (3.9) |
| Diabetic ulcer | 11 (7.1) |
| Foreign body | 10 (6.5) |
| Pressure ulcer | 5 (3.2) |
| Any wound | 43 (27.9) |
| Surgical | 22 (14.3) |
| Nonsurgical | 21 (13.6) |
| Bacteremia | 17 (11.0) |
| VAN criteria | |
| Loading dose received | 98 (63.6) |
| Loading dose, mg/kgb | 23.1 (20.5–25.0) |
| Initial daily dose, mg | 3000 (2000–4500) |
| Initial dose, mg | 1250 (1000–1500) |
| Frequency | |
| Every 8 hours | 81 (52.6) |
| Every 12 hours | 43 (27.9) |
| Duration, days | 5 (4–8) |
| VAN level | |
| Trough mg/L | 15 (11–20) |
| Trough 10–15 mg/L | 50 (32.5) |
| Trough 15–20 mg/L | 45 (29.2) |
| Extrapolated trough, mg/L | 13 (9–18) |
| AUC, mg*hr/L | 433 (346–547) |
| Nephrotoxicity | 16 (10.4) |
| Switched to an oral agent | 46 (30.0) |
| Trimethoprim/sulfamethoxazole | 31 (67.4) |
| Clindamycin | 13 (28.2) |
| Linezolid | 2 (4.3) |
| Early clinical response | |
| Resolution of signs and symptoms of infection within 72 hours | 96 (62.3) |
| Improvement in lesion or improvement in growth of lesion | 150 (97.4) |
| Alternative agents not required | 146 (94.8) |
Data displayed as median (interquartile range) or n (percentage).
Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; AUC, area under the concentration time curve; Cr, creatinine; IV, intravenous; MRSA, methicillin resistant Staphylococcus aureus; NSAID, nonsteroidal anti- inflammatory drugs; Scr, serum creatinine; TCS, timely clinical success; VAN, vancomycin.
aObese subjects defined as those with a body mass index of 30 or higher.
bAmong the 64.1% (n = 100) of subjects who received a loading dose.
cAt time of level drawn.
dThese include amputation, excision of necrotic tissue and/or debridement.
eSome patients may have had more than one type of skin infection.
fThese include: Clostridium spp., Corynebacterium spp., Citrobacter koseri, Candida glabrata, Serratia marcescens, Providencia stuarti, Prevotella spp., Peptostreptococcus spp.
gThese include azithromycin, cefoxitin, cephalexin, colistin, cefoxitin, gentamicin, macrobid, micafungin, nafcillin, voriconazole.
Most patients received other antibiotics for at least 1 dose (70.1%), with the most common agents being cefepime (35.2%), followed by ampicillin/sulbactam (26.9%) and piperacillin/tazobactam (25.9%). The majority of patients had an ID team consultation (69.5%) and achieved source control (76.6%). Notably, all patients had source control within 72 hours. A VAN loading dose was administered to 63.6% of patients, and the median duration (IQR) of VAN was 5.0 (4.0–8.0) days. The median (IQR) initial VAN dose was 3000 (2000–4500) mg per day. The most common frequency was every 8 hours followed by every 12 hours: 81 (52.6%) and 43 (27.9%), respectively. Overall, the most common regimen was 1000 mg every 8 hours (14.9%) [16]. Among obese patients (n = 56), the most common regimen was 1500 mg every 8 hours (44.6%) [17]. Thirty-three percent of patients had a trough level between 10 and 15 mg/L, wherease 28.6% had a trough between 15 and 20 mg/L. The median (IQR) VAN level was 15 mg/L [11–15,18–22].
Observed trough and estimated VAN level serum concentration from the Bayesian estimation approach for the total cohort and subgroups are shown in the Supplemental Material. The regression line from all estimated VAN level plot had an r2 = 0.74 (Supplementary Figure 1). The median (IQR) AUC was 433 (346–547) mg*hr/L. Overall, 61.7% experienced TCS where 62.3% experienced resolution of signs and symptoms within 72 hours, 97.4% experienced improvement in lesion size/growth, and 94.8% did not require alternative agents to VAN.
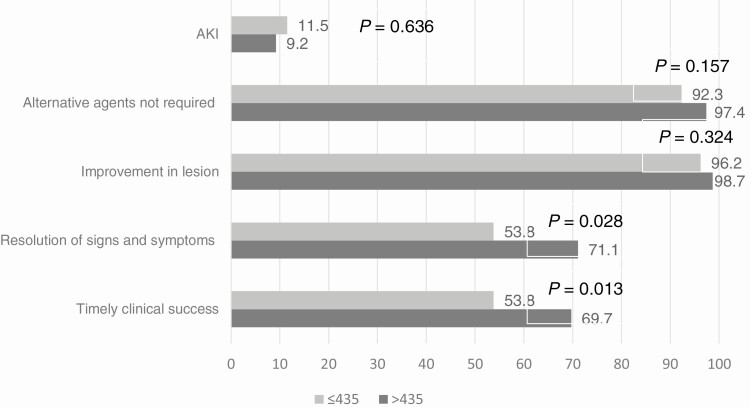
CART modeling identified a calculated AUC breakpoint of 435 mg*hr/L as having the highest correlation with TCS. Patients with VAN target-AUC >435 mg*hr/L had a significantly higher rate of TCS compared with patients below-target-AUC; 53/76 (69.7%) versus 42/78 (53.8%); P = .013). This difference was primarily driven by a greater incidence of timely resolution of signs and symptoms in the target-AUC group compared to below-target-AUC group. The remaining components of TCS were also favorable to the target-AUC group but not statistically significant. Additionally, the incidence of AKI was comparable between the 2 groups (Figure 1).
Figure 1.
Comparing VAN AUC ratio of >435 mg*hr/L and ≤435 mg*hr/L by timely clinical success components and AKI using bivariate analysis. Abbreviations: AKI, acute kidney injury; AUC, area under the concentration-versus-time curve from 0–24 h; VAN, vancomycin.
A bivariate comparison of clinical and microbiological characteristics between TCS and non-TCS are displayed in (Table 2). Both groups were similar in most characteristics. Notable statistically significant differences between the 2 groups included age, Wilson severity-of-illness scoring system (WSISS) [18], intensive care unit (ICU) encounter, ID consultation, concomitant antibiotic therapy, and the presence of concomitant bacteremia. Independent predictors of TCS sought by logistic regression included age, race, WSISS, concomitant bacteremia, actual trough level, extrapolated trough level, polymicrobial infection, ID consultation, source control, wound infections, combination antibiotic therapy, bacteremia as a complication, ICU encounter, and an AUC at target (Table 3). Based on the final variables retained in the model, target-AUC was independently associated with TCS (adjusted odds ratio [aOR], 2.263; 95% confidence interval [CI], 1.058–4.841).
Table 2.
Clinical Characteristics and Outcomes Between Patients With Timely Clinical Success and Those With No Timely Clinical Success
| Covariate | TCS (n = 95) | No TCS (n = 59) | P-value |
|---|---|---|---|
| Age, years | 47.0 (37.0–59.00) | 54.0 (44.5–63.0) | .025 |
| Age 65 years and older | 15 (15.8) | 9 (15.3) | .929 |
| Sex, male | 59 (62.1) | 37 (62.7) | .940 |
| Race, Black | 63 (66.3) | 45 (76.3) | .189 |
| Weight, kg | 82.0 (68.5–98.0) | 81.0 (71.0–104.8) | .453 |
| Obesity | 35 (36.8) | 21 (35.9) | .876 |
| Ideal body weight, kg | 68.5 (59.3–77.7) | 66.2 (57.0–73.1) | .276 |
| Wilson severity-of-illness scoring system | 82.0 (56.0–110.0) | 114.0 (87.0–146.0) | <.0001 |
| Baseline serum creatinine, mg/L | 1.0 (0.8–1.2) | 1.1 (0.8–1.3) | .199 |
| Serum creatinine at time level drawn, mg/L | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | .445 |
| Highest Scr with VAN start, mg/L | 1.0 (0.8–1.2) | 1.1 (0.9–1.4) | .329 |
| CrCl, mL/min a | 87.2 (69.7–110.7) | 89.8 (57.0–112.6) | .646 |
| Intensive care unit | 6 (6.3) | 16 (27.1) | <.0001 |
| Polymicrobial infection | 25 (26.3) | 22 (37.3) | .151 |
| Source control | 69 (72.6) | 49 (83.1) | .137 |
| Infectious diseases consult | 61 (64.2) | 48 (78.0) | .072 |
| Concomitant nephrotoxin | 24 (25.3%) | 15 (25.3%) | .982 |
| Concomitant antibiotic, any | 59 (62.1) | 49 (83.1) | .006 |
| Primary infection | |||
| Abscess | 57 (60.0) | 32 (54.2) | .481 |
| Cellulitis | 6 (6.3) | 0 (0.0) | .100 |
| Diabetic ulcer | 6 (6.3) | 5 (8.5) | .613 |
| Foreign body | 4 (4.2) | 6 (10.2) | .145 |
| Pressure ulcer | 3 (3.2) | 2 (3.4) | .937 |
| Any wound | 23 (24.2) | 20 (33.9) | .193 |
| Surgical | 12 (12.6) | 10 (16.9) | .457 |
| Bacteremia | 6 (6.3) | 11 (18.6) | .018 |
| VAN criteria | |||
| Loading dose received | 62 (65.3) | 36 (61.0) | .594 |
| Initial daily dose, mg/kg | 23.1 (20.1–25.0) | 23.3 (20.9–25.7) | .237 |
| VAN level | |||
| Trough, mg/L | 15.5 (11.6–19.0) | 13.2 (9.9–19.9) | .179 |
| Trough 10–15 mg/L | 34 (35.8) | 22 (37.3) | .851 |
| Ex-trough, mg/L | 14.2 (10.5–18.3) | 12.2 (9.1–18.3s) | .094 |
| AUC, mg*hr/L | 452.9 (352.9–558.6) | 415.1 (339.9–531.9) | .339 |
Data displayed as median (interquartile range) or n (percentage).
Abbreviations: AUC, area under the concentration time curve; MRSA, methicillin resistant Staphylococcus aureus; Scr, serum creatinine; TCS, timely clinical success; VAN, vancomycin.
aAt time of level drawn.
bTarget AUC is defined as an AUC >435 mg*hr/L.
Table 3.
Multivariable Logistic Regression for Factors independently Associated With Timely Clinical Success
| Variable | OR | 95% CI | P-value | Adjusted OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Target AUC, mg*hr/La | 2.056 | .916–4.612 | .081 | 2.263 | 1.058–4.841 | .035 |
| Wilson Severity of Illness | 0.984 | .971–.997 | .020 | 0.984 | .975–.994 | .002 |
| Age, years | 1.002 | .970–1.036 | .893 | |||
| Race, Black | 0.673 | .274–1.651 | .387 | |||
| Baseline Scr, mg/L | 0.999 | .794–1.258 | .994 | |||
| Source Control | 0.437 | .157–1.220 | .114 | 0.389 | .146–1.040 | .060 |
| ID Consult | 0.441 | .278–1.746 | .441 | |||
| Poly-microbial | 1.810 | .690–4.752 | .228 | |||
| Other antibioticsb | 0.359 | .136–.953 | .040 | 0.430 | .172–1.071 | .070 |
| ICU patient | 0.267 | .086–.832 | .023 | 0.278 | .094–.823 | .021 |
| Bacteremia complication | 0.337 | .094–1.203 | .094 | 0.359 | .108–1.191 | .094 |
| Trough, mg/L | 0.994 | .892–1.106 | .906 | |||
| Extrapolated Trough, mg/L | 1.062 | .943–1.196 | .323 | |||
| Wound | 0.648 | .252–1.664 | .367 |
Hosmer-Lemeshow goodness-of-fit test P = .320; variance inflation factor 1–5 for all variables included at model entry.
Abbreviations: AUC, area under the concentration time curve; CI, confidence interval; ICU, intensive care unit; ID, infectious diseases; OR, odds ratio; Scr, serum creatinine.
aTarget AUC is defined as an AUC > 435 mg*hr/L.
bThese include ceftriaxone, ceftriaxone, colistin, ertapenem, piperacillin/tazobactam, naficillin, trimethoprim-sulfamethoxazole.
Thirty-day and 90-day mortality was comparable in the target-AUC and below-target-AUC group, 2.6% versus 1.3% (P = .545) and 5.3% and 3.8% (P = .673), respectively. Thirty-day readmission rates were also comparable (15.8% vs 13.0% in the target-AUC and below-target-AUC group, respectively [P = .621]). In the target-AUC group, 86.8% of patients were discharged home on antibiotics for the same infection, compared to 84.6% in the below-target-AUC group (P = .693). The most common infection type in target-AUC and below-target-AUC groups was abscess: 55.3% and 60.3%, respectively. The second most common infection type in target-AUC and below-target-AUC groups was surgical wounds: 15.8% and 12.8%, respectively. Slightly more patients in the target-AUC group were switched to an oral agent (56.6%) compared to the below-target-AUC group (50.0%); however, this was not statistically significant (P = .413). The median (IQR) hospital length of stay after VAN start was longer but not statistically significant in the below-target-AUC compared to the target-AUC group 8 [4–10] and 6 (4–8.75) days, respectively; (P = .065).
The prevalence of nephrotoxicity in the entire cohort was 10.4%. The rate was comparable in the target-AUC and the below-target-AUC: 9.2% and 11.5%, respectively (P = .636). Twenty-five percent of patients in our cohort received at least 1 nephrotoxic agent. Among patients with nephrotoxicity, 56.3% received a nephrotoxic agent. The proportion of patients who received a nephrotoxic agent in the target-AUC and below-target-AUC was 71.4% and 44.4%, respectively (P = .280). Details of subjects who had nephrotoxicity are displayed in Table 4.
Table 4.
Clinical Criteria of Patients Who Experienced Vancomycin-associated Nephrotoxicity
| Variable | Target-AUCa (n = 7) | Below-Target-AUC (n = 9) |
|---|---|---|
| Nephrotoxin agentb | 5 (71.4) | 4 (44.4) |
| Trough | 17.4 (12.8–24.8) | 15.3 (9.7–18.6) |
| Trough level, mg/L | ||
| <15 | 2 (28.6) | 4 (44.4) |
| 15–20 | 3 (42.9) | 5 (55.6) |
| ≥20 | 2 (28.6) | 0 (0.0) |
| Extrapolated trough level, mg/L | 14.7 (12.3–18.5) | 10.2 (7.4–15.7) |
| AUC, mg*hr/L | 678.4 (562.1–929.8) | 303.2 (282.8–369.5) |
| AUC level, mg*hr/L | ||
| <300 | NA | 4 (44.4) |
| 300–435 | NA | 5 (55.6) |
| 435–600 | 2 (28.6) | NA |
| ≥600 | 5 (71.4) | NA |
Abbreviations: AUC, area under the concentration time curve.
aTarget AUC is defined as an AUC >435 mg*hr/L
bThese include aminoglycosides, angiotensin-converting-enzyme inhibitors/ angiotensin II receptor blockers, loop diuretics, nonsteroidal anti-inflammatory Drugs, intravenous contrast.
Because patients with polymicrobial infections constituted over a third of our cohort, we conducted a subgroup analysis of patients with only MRSA (n = 107). Overall, 65.4% experienced TCS. Patients with target-AUC attainment experienced TCS significantly more frequently than patients below-target-AUC attainment: 74.5% versus 55.8%, respectively (P = .041).
Due to the noteworthy proportion of obese patients in our cohort (n = 56), we conducted a subgroup analysis in this population. Overall, 62.5% experienced TCS. Patients with target-AUC attainment experienced TCS numerically more frequently than patients below-target-AUC attainment: 70.8% versus 56.3%, respectively (P = .265). Thirty-day and 90-day mortality was comparable in the target-AUC and below-target-AUC group, 1.9% versus 0% (P = .244) and 4.2% and 0% (P = .244), respectively.
DISCUSSION
The updated consensus guideline for VAN monitoring and dosing provide no specific recommendations on VAN monitoring as it related to AUC/MIC targets in patients with skin and soft tissue infections due to lack of research in this area. Here we present a study evaluating the optimum VAN AUC targets in cSSTI patients. VAN AUC was estimated using Bayesian software, albeit more complex; it provides a dynamic mathematical algorithm that provides patient specific pharmacokinetic estimates using a single VAN level [1, 3]. Scant data are available on the vancomycin PK/PD required for efficacy in patients with skin infections. Although many institutions have adopted a flat or conventional dosing approach in the absence of data, we found that AUC values > 435 mg*hr/L were independently associated with best clinical response. This is in concordance with the AUC targets endorsed in the 2020 VAN monitoring guideline for MRSA infections (400–600 mg*hr/L) but is indeed different than targets identified in observational studies of other infections such as endocarditis (≥600 mg*hr/L), and bacteremia (≥515 mg*hr/L) [1, 4, 19]. Nevertheless, these studies should be interpreted with caution due to the heterogeneity in their design, clinical outcome definitions, and variability in MIC testing. Albeit not statistically significant, an important finding in our cohort is the trend toward shorter hospital length of stay in patients at target-AUC compared to those below-target AUC [20]. Although the cost of VAN AUC monitoring is of concern to some clinicians due to additional training and additional required VAN blood sampling in non-Bayesian methodologies, our results may suggest facilitating early discharge particularly in high-risk patients cSSTI.
VAN-associated AKI is well documented and can range from 5% up to 40% depending on patients’ indications and specific risk factors [21, 22]. In our cohort, the overall incidence of AKI was 10%, which is similar to other real-world observations in cSSTI patients [7]. Over half of patients who experienced AKI were on concomitant nephrotoxic drugs (56.3%). Interestingly, our target-AUC estimate (ie, an AUC >435 mg*hr/L) was not associated with higher incidence of VAN-associated AKI compared to below-target-AUC. The majority (71.4%) of subjects who experienced VAN-associated AKI in the target-AUC group had AUCs higher than 600 mg*hr/L, which is beyond the recommended targets and exceeds the AUC cutoff for nephrotoxicity and were on nephrotoxic agents (71.4%) [19, 23, 24]. However, there were few patients in the target-AUC group to make any statistical inferences.
Of interest, most patients received relatively aggressive dosing with approximately 63.6% receiving a vancomycin loading dose followed by a median maintenance dose of 3 grams per day. This practice of high aggressive dosing undoubtedly resulted in higher than anticipated AUCs. Nearly 30% had trough concentrations between 15 and 20 mg/L, and 20.1% exceeded a trough of 20 mg/L. It is not clear why doses were as high in this study, although most patients had a moderate to high WSISS and over a third were obese.
Our study has several important limitations. First, the DMC is a single healthcare system that includes several hospitals and therefore challenges the generalizability of the study population. Second, the retrospective nature of this study has inherent limitations with this study design. Even though it was not possible to collect patients past the reported year as our institution has enforced a 2-level AUC monitoring policy for most MRSA infection excluding cSSTI in January of 2015, we believe our data help to fill the current gap in literature and the unaddressed issue of VAN dosing in cSSTI in the consensus guideline. Despite the significant proportion of obese patients and/or with polymicrobial infections, a subgroup analysis in these 2 patient populations had been consistent with the primary findings. Additionally, our study was restricted to cSSTI patients and excluded those with ESRD, infected joints, and burn-associated cSSTI. These subjects have more complex VAN pharmacokinetic simulations and pharmacodynamic analysis; therefore, our metrics may not be suited for this patient population. Finally, the precision of the Bayesian estimation is lower than projected from previous studies despite using the same validated method for AUC estimation from trough-only pharmacokinetic sampling [12, 16, 17, 23, 25]. This may be due, in part, to the large proportion of obese subjects in our study. Data indicate that 2 rather than 1 VAN concentrations per dose interval increases the accuracy of Bayesian estimation in this population [16]. Although the resulting AUC threshold needs to be interpreted with caution and requires further validation, the findings are important as they establish VAN AUC response in cSSTI and represent the best current estimate of VAN exposure needed for clinical success.
In conclusion, VAN AUC attainment of >435 mg*hr/L in patients with MRSA cSSTI was associated with TCS. In order to truly measure the optimum VAN exposure for best clinical outcomes in cSSTI, large multicenter prospective clinical trials are necessary. Nevertheless, our results suggest that VAN AUC >435 mg*hr/L can be utilized to assure timely clinical outcomes in patients with MRSA cSSTI. It would also seem prudent to obtain VAN AUC exposure in patients with cSSTI who are at high risk of VAN failure or developing VAN-associated AKI. More studies are needed to define the criteria of these patients at risk for MRSA cSSTI specifically.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This study has been presented, in part, at Infectious Diseases Society of America (IDSA) Meeting 2015; 7–11 October, 2015, San Diego, CA (abstract 800).
Financial support. No funding or sponsorship was received for this study or publication of this article.
Potential conflicts of interest. M. J. R. reports research support, consultant or speaker for Allergan, Melinta, Merck, Motif, Nabriva, Paratek, Tetraphase, and Shionogi and is partially supported by NIAID AI121400. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Rybak MJ, Le J, Lodise TP, et al. . Therapeutic monitoring of vancomycin: a revised consensus guideline and review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society and the Society of Infectious Diseases Pharmacists. In Clin Infect Dis 2020; 71:1361-4. doi: 10.1093/cid/ciaa303. [DOI] [PubMed] [Google Scholar]
- 2. Finch NA, Zasowski EJ, Murray KP, et al. . A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother 2017; 61:e01293-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heil EL, Claeys KC, Mynatt RP, et al. . Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm 2018; 75:1986–95. [DOI] [PubMed] [Google Scholar]
- 4. Casapao AM, Lodise TP, Davis SL, et al. . Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother 2015; 59:2978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moise PA, Forrest A, Bhavnani SM, Birmingham MC, Schentag JJ. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am J Health Syst Pharm 2000; 57 Suppl 2:S4–9. [DOI] [PubMed] [Google Scholar]
- 6. Gawronski KM, Goff DA, Brown J, Khadem TM, Bauer KA. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin Ther 2013; 35: 772–9. [DOI] [PubMed] [Google Scholar]
- 7. Jorgensen SCJ, Murray KP, Lagnf AM, Melvin S, Bhatia S, Shamim MD, et al. . A multicenter evaluation of vancomycin-associated acute kidney injury in hospitalized patients with acute bacterial skin and skin structure infections. Infect Dis Ther 2020; 9:89-106. doi: 10.1007/s40121-019-00278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 9. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–32. [DOI] [PubMed] [Google Scholar]
- 10. Stevens DL, Bisno AL, Chambers HF, et al. ; Infectious Diseases Society of America . Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014; 59:e10–52. [DOI] [PubMed] [Google Scholar]
- 11. Neely MN, Youn G, Jones B, et al. . Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 2014; 58 :309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lodise TP, Drusano GL, Zasowski E, et al. . Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: how much is enough? Clin Infect Dis 2014; 59:666–75. [DOI] [PubMed] [Google Scholar]
- 13. Rodvold KA, Pryka RD, Garrison M, Rotschafer JC. Evaluation of a two-compartment Bayesian forecasting program for predicting vancomycin concentrations. Ther Drug Monit 1989; 11:269–75. [DOI] [PubMed] [Google Scholar]
- 14. Pryka RD, Rodvold KA, Garrison M, Rotschafer JC. Individualizing vancomycin dosage regimens: one- versus two-compartment Bayesian models. Ther Drug Monit 1989; 11:450–4. [PubMed] [Google Scholar]
- 15. Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis 2010; 67: 228–33. [DOI] [PubMed] [Google Scholar]
- 16. Carreno JJ, Lomaestro B, Tietjan J, Lodise TP. Pilot study of a Bayesian approach to estimate vancomycin exposure in obese patients with limited pharmacokinetic sampling. Antimicrob Agents Chemother 2017; 61:e02478-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casapao AM, Lodise TP, Davis SL, et al. . Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother 2015; 59:2978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson SE, Solomkin JS, Le V, Cammarata SK, Bruss JB. A severity score for complicated skin and soft tissue infections derived from phase III studies of linezolid. Am J Surg 2003; 185:369–75. [DOI] [PubMed] [Google Scholar]
- 19. Lodise TP, Rosenkranz SL, Finnemeyer M, et al. . The emperor’s new clothes: PRospective Observational evaluation of the association between initial VancomycIn exposure and failure rates among aDult hospitalizEd patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis 2020; 70:1536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jeffres MN. The whole price of vancomycin: toxicities, troughs, and time. Drugs 2017; 77:1143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 2013; 57:734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. Int J Antimicrob Agents 2011; 37:95–101. [DOI] [PubMed] [Google Scholar]
- 23. Zasowski EJ, Murray KP, Trinh TD, et al. . Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother 2018; 62:e01684-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC(0–24) threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2017; 61:e02535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neely MN, Kato L, Youn G, et al. . Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother 2018; 62:e02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.