Abstract
Background
Parainfluenza virus (PIV) is a leading cause of lower respiratory tract infections. Although there are several distinct PIV serotypes, few studies have compared the clinical characteristics and severity of infection among the individual PIV serotypes and between PIV and other pathogens in patients with community-acquired pneumonia.
Methods
We conducted active population-based surveillance for radiographically confirmed community-acquired pneumonia hospitalizations among children and adults in 8 US hospitals with systematic collection of clinical data and respiratory, blood, and serological specimens for pathogen detection. We compared clinical features of PIV-associated pneumonia among individual serotypes 1, 2, and 3 and among all PIV infections with other viral, atypical, and bacterial pneumonias. We also compared in-hospital disease severity among groups employing an ordinal scale (mild, moderate, severe) using multivariable proportional odds regression.
Results
PIV was more commonly detected in children (155/2354; 6.6%) than in adults (66/2297; 2.9%) (P < .001). Other pathogens were commonly co-detected among PIV cases (110/221; 50%). Clinical features of PIV-1, PIV-2, and PIV-3 infections were similar to one another in both children and adults with pneumonia. In multivariable analysis, children with PIV-associated pneumonia exhibited similar severity to children with other nonbacterial pneumonia, whereas children with bacterial pneumonia exhibited increased severity (odds ratio, 8.42; 95% confidence interval, 1.88–37.80). In adults, PIV-associated pneumonia exhibited similar severity to other pneumonia pathogens.
Conclusions
Clinical features did not distinguish among infection with individual PIV serotypes in patients hospitalized with community-acquired pneumonia. However, in children, PIV pneumonia was less severe than bacterial pneumonia.
Keywords: parainfluenza virus, community-acquired pneumonia, viral pneumonia
Clinical features did not reliably distinguish pneumonia with different parainfluenza virus (PIV) serotypes. Among children, PIV pneumonia was less severe than bacterial pneumonia, but in adults the severity of PIV pneumonia did not significantly differ from other pathogens.
Parainfluenza virus (PIV) is an important respiratory pathogen in all age groups, with clinical manifestations ranging from mild upper respiratory tract symptoms to complicated lower respiratory tract disease and symptoms typically more severe in infants and young children than in older children and adults. In hospital-based [1–4] and community-based [5–7] surveillance studies, PIV has been identified commonly in infants and young children with acute respiratory illness (ARI), while PIV prevalence is lower in adults [8]. Older adults, immunocompromised patients, and individuals with underlying comorbidities, particularly lung and stem cell transplants, may experience life-threatening respiratory infections with PIV [6, 9–15].
Human PIV is classified in 4 major serotypes [1–4], and each serotype may be associated with specific clinical syndromes, although these have mainly been described in children. PIV-3 is typically the most prevalent [16] and is often associated with more severe disease, including pneumonia and bronchiolitis [17]. PIV-1 and PIV-2 are often associated with laryngotracheobronchitis (“croup”), particularly in young children. Due to difficulties in isolation and its typical association with mild symptoms, PIV-4 has often been excluded from surveillance studies [18–20]. Individual serotypes also have distinct circulation patterns, with biennial peaks of PIV-1 and PIV-2 in the fall of odd and even years, respectively, and the highest prevalence of PIV-3 typically occurring annually in the summer months [16, 21, 22]. Despite the recognized role of PIV in respiratory disease, data are limited regarding the clinical features of individual PIV serotype infections in children and adults with radiographically confirmed community-acquired pneumonia (CAP).
The Centers for Disease Control and Prevention (CDC) Etiology of Pneumonia in the Community (EPIC) study was a prospective, multicenter, population-based active surveillance study designed to determine the etiology of CAP in hospitalized children and adults [23, 24]. We sought to define the clinical features and outcomes associated with PIV-associated pneumonia (PIV-1, -2, and -3; PIV-4 detections were not assessed) in children and adults with radiographically confirmed CAP and to compare these among PIV serotypes 1, 2, and 3, as well as pneumonia caused by other viruses, atypical bacterial, and typical bacterial pathogens.
METHODS
Patient Enrollment and Data Collection
The EPIC study enrolled children (<18 years) and adults from January 2010 through June 2012 at 8 hospitals in Chicago, Illinois; Memphis, Tennessee; Nashville, Tennessee; and Salt Lake City, Utah [23, 24]. Patients were eligible if they were admitted to a study hospital, resided in a defined study catchment area, had acute respiratory symptoms, and had radiographic findings compatible with pneumonia confirmed by independent study radiologists. Patients were excluded if they were recently hospitalized or had severe immunosuppression (human immunodeficiency virus [HIV] infection with CD4 count <200/mm3 or <14%, solid or hematopoietic stem cell transplantation within 90 days or graft-versus-host disease or bronchiolitis obliterans, or cancer with absolute neutrophil count <500/mm3). Detailed inclusion/exclusion criteria are described elsewhere [23, 24]. A convenience sample of asymptomatic children and adults without respiratory symptoms in the prior 14 days were enrolled as controls. They were excluded if they developed respiratory symptoms within 14 days postenrollment [25]. The study was approved by the institutional review boards at each institution and CDC.
Sample Collection and Laboratory Testing
In CAP cases, sera, blood, and nasopharyngeal/oropharyngeal (NP/OP) swabs were routinely obtained as soon as possible after enrollment. Convalescent sera were collected 3–10 weeks later when possible. Serology for PIV-1, -2, and -3; adenovirus (AdV); human metapneumovirus (hMPV); influenza A/B; and respiratory syncytial virus (RSV) was performed using CDC-developed indirect immunoglobulin G (IgG) enzyme immunoassays on acute and convalescent sera [23, 24, 26]. Due to antigenic cross-reactivity among PIV 1–3, serological data from all 3 serotypes were analyzed in aggregate [26]. The NP/OP swabs were tested by a CDC-developed polymerase chain reaction (PCR) assay [27] for detection of PIV-1, -2, and -3; hMPV; AdV; coronaviruses 229E, HKU1, NL63, and OC43 (CoV); human rhinovirus (HRV); influenza A/B viruses; RSV; Chlamydophila pneumoniae; and Mycoplasma pneumoniae in cases and controls. We did not test for PIV-4 by serology or PCR.
Pleural fluid (PF), endotracheal (ET) aspirates, and bronchoalveolar (BAL) specimens, if obtained for clinical care, were also evaluated. Urine was collected from adults, and sputum was obtained in patients with productive cough. Only samples collected 72 hours or earlier after admission were included, except for PF (up to 7 days after admission) [23, 24]. Blood, PF, sputum, ET, and BAL specimens were cultured using standard techniques. Bacterial real-time PCR was performed on PF for all ages [28] and on whole blood for Streptococcus pneumoniae and Streptococcus pyogenes in children [29]. For adults, Legionella pneumophila and S. pneumoniae urinary antigen testing was performed. Legionella PCR was performed on sputum [24].
Pneumonia Definitions
Parainfluenza virus–associated pneumonia was defined as pneumonia with PIV virus detection by NP/OP swab PCR and/or a 4-fold or greater increase in PIV antibody titer between acute and convalescent sera. Some patients with PIV-associated pneumonia also had co-detections with other pathogens. PIV-only pneumonia was defined as detection of only the single virus by either PCR or serology.
Other viral pneumonia was defined as pneumonia with at least 1 other virus detected (hMPV, RSV, AdV, CoV, influenza, or HRV; inclusive of co-detections of >1 of these viruses) by NP/OP swab PCR and/or a 4-fold or greater increase in antibody titer between acute and convalescent serum specimens without co-detection of PIV or typical or atypical bacteria.
Atypical bacterial pneumonia included pneumonia in which C. pneumoniae, M. pneumoniae, or L. pneumophila was detected by NP/OP swab PCR or urinary antigen detection but without typical bacteria or viruses detected. Bacterial pneumonia was defined as pneumonia with detection of typical bacteria by blood, ET, BAL, PF culture, or PF PCR in any patient; bacterial detection by whole-blood PCR in children; or bacterial detection by urinary antigen testing or sputum culture in adults [23, 24]. Patients in the “bacterial-only” category had only typical bacteria detected without atypical bacteria or any virus detected.
Outcomes: Disease Severity and Length of Stay
For children and adults, severity was categorized according to the following mutually exclusive hierarchy: (1) severe pneumonia, defined by in-hospital death, need for extracorporeal membrane oxygenation, acute respiratory distress syndrome, shock, and/or invasive mechanical ventilatory support; (2) moderate pneumonia, defined by intensive care unit (ICU) admission without meeting criteria for severe pneumonia; and (3) mild pneumonia, which included all other hospitalized patients who did not require ICU admission [30]. We defined length of stay (LOS) as time from admission to hospital discharge (hours).
Statistical Analysis
Pediatric and adult analyses were performed separately. We compared clinical features and seasonality of detection in patients with PIV-1–, PIV-2–, and PIV-3–associated pneumonia, as distinguished by PCR (serology did not allow such distinction). Next, we compared features of PIV-only pneumonia, including all PIV cases identified by either PCR or serology and excluding co-detections with other pathogens, with those with other viral (sole or with co-detection of hMPV, RSV, AdV, CoV, influenza, or HRV), bacterial-only, atypical-only pneumonia, and pneumonia with no pathogens detected. These categories were mutually exclusive. Viral-bacterial, viral-atypical, atypical-bacterial, and viral-viral co-detections including PIV were excluded. Comparisons among these etiologic categories were limited to patients with at least 1 specimen available for both viral and bacterial pathogen detection.
We adjusted comparisons among PIV serotypes and between PIV and other etiologic categories by age, sex, and race using binary logistic or multinomial logistic regression for categorical variables or linear regression for continuous variables. Multivariable proportional odds regression was performed to evaluate the association between etiologic category and clinical disease severity using the 3-level ordinal outcome scale described above [31]. Multivariable proportional hazards regression was performed to evaluate the association between pneumonia etiologic category and time to hospital discharge (LOS) [32]. Covariates for the multivariable regression models included study site, year, age, race, sex, insurance status, influenza and pneumococcal vaccination history, antibiotic use prior to admission, duration of symptoms prior to admission, cigarette smoke exposure, and presence of an underlying comorbidity. For all subjects, comorbidities were assessed using medical record abstraction and included chronic kidney or liver disease, neurologic disorder, diabetes mellitus, immunosuppression, HIV infection with CD4 count greater than 200/mm3, and splenectomy. For children, comorbidities also included asthma/reactive airway disease, chromosomal disorders, and preterm birth (children <2 years of age). For adults, comorbidities also included chronic lung or chronic heart disease. We accounted for potential aggregation of observations at the hospital level using the Huber-White method for variance estimation in the regression models [33]. Analyses were conducted in Stata version 14.2 (StataCorp LLC, College Station, TX); P values less than or equal to .05 were considered statistically significant.
RESULTS
Parainfluenza Virus–Associated Pneumonia
Among 4651 patients with radiographic confirmation of pneumonia, PIV was detected by NP/OP PCR and/or serologic testing in 155 of 2354 children (6.6%) and 66 of 2297 adults (2.9%); this difference was significant (P < .001; data not shown in tables).
Among these 221 cases, 176 (80%) were detected by PCR. Among 1823 with acute and convalescent PIV serologies performed, 87 (5%) had a 4-fold or greater increase in PIV titers, and 42 of 221 (19%) PIV cases were identified by both PCR and serology.
In 111 of 221 (50%) PIV cases, PIV was the sole pathogen detected. Other viruses were commonly co-detected with PIV (82/221; 37%), including HRV (13%), RSV (12%), and AdV (11%). Bacterial pathogens were co-detected with PIV in 36 of 221 cases (16%). PIV was detected significantly less commonly in control children (14/726; 2%) and adults (0/262) than in children and adults with pneumonia (P < .001 and P = .005, respectively).
Serotype-specific Parainfluenza Virus–Associated Pneumonia
Among detections by PCR (n = 176), PIV-3 was the most commonly detected serotype in both children (81/121; 67%) and adults (28/55; 51%) (Tables 1 and 2). PIV-1 and PIV-3 were co-detected in 2 subjects; both were younger than 2 years of age. Among serotype-specific detections by PCR, other pathogens were co-detected in PIV-1, PIV-2, and PIV-3 cases in 15 of 27 (56%), 17 of 42 (40%), and 47 of 109 (43%) instances, respectively.
Table 1.
Select Characteristics of Hospitalized Children With PIV-1, PIV-2, and PIV-3 Pneumonia Including Co-detections With Other Pathogens
| PIV-1 (n = 19) | PIV-2 (n = 21) | PIV-3a (n = 79) | P b | |
|---|---|---|---|---|
| Female sex | 8 (42) | 7 (33) | 36 (46) | .578 |
| Median (IQR) age, m | 37 (21–95) | 53 (23–89) | 20 (10–39) | .013* |
| Age group | .051 | |||
| <2 y | 6 (32) | 6 (29) | 49 (62) | |
| 2–4 y | 6 (32) | 6 (29) | 19 (24) | |
| 5–9 y | 4 (21) | 6 (29) | 7 (9) | |
| 10–17 y | 3 (16) | 3 (14) | 4 (5) | |
| Race/ethnicity | .087 | |||
| Hispanic | 2 (11) | 8 (38) | 22 (28) | |
| Non-Hispanic black | 4 (21) | 4 (19) | 33 (42) | |
| Non-Hispanic white | 9 (47) | 7 (33) | 19 (24) | |
| Other | 4 (21) | 2 (10) | 5 (6) | |
| Any underlying comorbidityc | 9 (47) | 6 (29) | 31 (39) | .298 |
| Asthma | 6 (32) | 3 (14) | 24 (30) | .155 |
| Symptom duration prior to admission, d | 4 (2–8) | 3 (2–4) | 3 (2–6) | .754 |
| Clinical presentation | ||||
| Fever/feverish | 18 (95) | 21 (100) | 72 (91) | .505 |
| Cough | 19 (100) | 15 (71) | 75 (95) | .032* |
| Dyspnea | 12 (63) | 12 (57) | 48 (61) | .757 |
| Retractions | 9 (47) | 8 (38) | 28 (35) | .538 |
| Wheezing | 8 (42) | 3 (14) | 31 (39) | .116 |
| Rhinorrhea | 13 (68) | 12 (57) | 68 (86) | .040* |
| Oxygen requirement | 13 (68) | 11 (52) | 46 (58) | .578 |
| Radiographic features | .648 | |||
| Single lobar | 6 (32) | 7 (33) | 11 (14) | |
| Multilobar | 3 (16) | 3 (14) | 21 (27) | |
| Other consolidation | 0 (0) | 0 (0) | 3 (4) | |
| Other infiltrate | 9 (47) | 11 (52) | 34 (43) | |
| Mixed infiltrate | 1 (5) | 0 (0) | 10 (13) | |
| Empyema | 3 (16) | 4 (19) | 6 (8) | .613 |
| Length of stay, median (IQR), h | 61 (38–104) | 79 (39–101) | 68 (44–95) | .051 |
| Severity | .064 | |||
| Mild | 13 (68) | 16 (76) | 68 (86) | |
| Moderate | 2 (11) | 1 (5) | 9 (11) | |
| Severe | 4 (21) | 4 (19) | 2 (3) | |
| Co-detected with ≥1 other pathogen | 11 (58) | 11 (52) | 35 (44) | .470 |
Data are presented as n (%) unless otherwise indicated; N = 119.
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction; PIV, parainfluenza virus.
*Significant, P < .05.
aIndividual serotypes detected and distinguished by PCR; excludes cases of co-detection of >1 PIV serotype (n = 2).
bComparisons adjusted for age, sex, and race as appropriate.
cAny comorbidity including asthma, congenital heart disease, diabetes, chronic renal disease, chronic liver disease, immunosuppression, splenectomy, prematurity (assessed if <2 years), neurological disorder.
Table 2.
Select Characteristics of Hospitalized Adults With PIV-1, PIV-2, and PIV-3 Pneumonia Including Co-detections With Other Pathogens
| PIV-1 (n = 6) | PIV-2 (n = 21) | PIV-3a (n = 28) | P b | |
|---|---|---|---|---|
| Female sex | 1 (17) | 9 (43) | 11 (39) | .415 |
| Median (IQR) age, y | 52 (47–70) | 63 (51–77) | 59 (51.5–72) | .337 |
| Age group | .719 | |||
| 18–49 y | 3 (50) | 4 (19) | 3 (11) | |
| 50–64 y | 1 (17) | 7 (33) | 14 (50) | |
| 65–79 y | 2 (33) | 6 (29) | 5 (18) | |
| ≥80 y | 0 (0) | 4 (19) | 6 (21) | |
| Race/ethnicity | .732 | |||
| Hispanic | 1 (17) | 0 (0) | 4 (14) | |
| Non-Hispanic black | 1 (17) | 7 (33) | 12 (43) | |
| Non-Hispanic white | 4 (67) | 13 (62) | 10 (36) | |
| Other | 0 (0) | 1 (5) | 2 (7) | |
| Any underlying comorbidityc | 5 (83) | 19 (90) | 23 (82) | .776 |
| Symptom duration prior to admission, d | 4.5 (0–5) | 3 (1–5) | 3 (2–7.5) | .439 |
| Clinical presentation | ||||
| Fever/feverish | 4 (67) | 17 (81) | 23 (82) | .306 |
| Cough | 6 (100) | 20 (95) | 28 (100) | .438d |
| Dyspnea | 5 (83) | 17 (81) | 22 (79) | .712 |
| Wheezing | 1 (17) | 8 (38) | 12 (43) | .541 |
| Rhinorrhea | 3 (50) | 11 (52) | 16 (57) | .773 |
| Sore throat | 3 (50) | 8 (38) | 10 (36) | .904 |
| Oxygen requirement | 3 (50) | 9 (43) | 16 (57) | .488 |
| Radiographic features | .785 | |||
| Single lobar | 0 (0) | 7 (33) | 4 (14) | |
| Multilobar | 1 (17) | 5 (24) | 9 (32) | |
| Other consolidation | 1 (17) | 0 (0) | 2 (7) | |
| Other infiltrate | 4 (67) | 8 (38) | 13 (46) | |
| Mixed infiltrate | 0 (0) | 1 (5) | 0 (0) | |
| Empyema | 0 (0) | 5 (24) | 2 (7) | .268 |
| Length of stay, median (IQR), d | 2.5 (1–7) | 2 (2–5) | 2.5 (2–6) | .774 |
| Severity | .542 | |||
| Mild | 4 (67) | 16 (76) | 19 (68) | |
| Moderate | 1 (17) | 1 (5) | 6 (21) | |
| Severe | 1 (17) | 4 (19) | 3 (11) | |
| Co-detected with ≥1 other pathogen | 2 (33) | 6 (29) | 10 (36) | .957 |
Data are presented as n (%) unless otherwise indicated; N = 55.
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction; PIV, parainfluenza virus.
aIndividual serotypes detected and distinguished by PCR.
bComparisons adjusted for age, sex, and race as appropriate.
cAny comorbidity including chronic lung disease, chronic heart disease, diabetes, chronic renal disease, chronic liver disease, immunosuppression, splenectomy, neurological disorder.
d P value unadjusted, calculated using Fisher’s exact text due to small sample size.
An immunocompromising condition was present in 3 of 155 (2%) children and 21 of 66 (32%) adult cases of PIV-associated pneumonia (1/6 [17%] PIV-1, 9/21 [43%] PIV-2, 8/28 [29%] PIV-3 among 55 cases in which serotype determined).
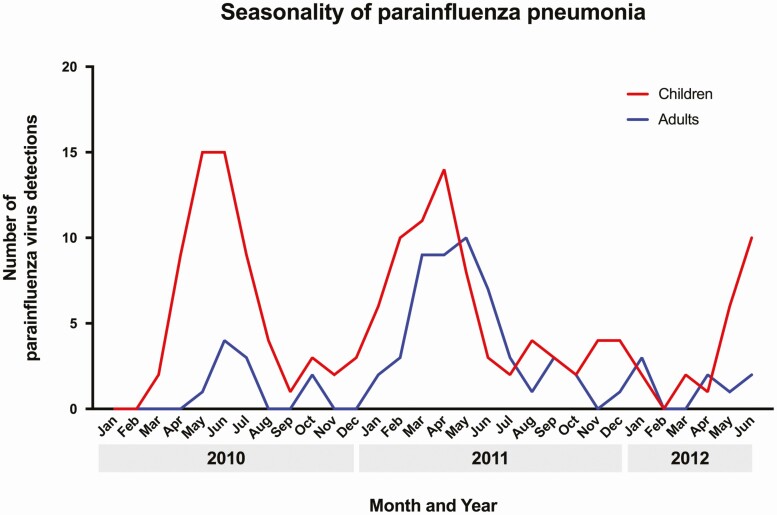
Parainfluenza Virus–Associated Pneumonia Seasonality
In 2010, peak PIV detections occurred in May–June (Figure 1), while in 2011, peak detections occurred in March–May. In both 2010 and 2011, increases in PIV activity in children tended to precede increases in activity in adults. PIV-2 and PIV-3 peaks occurred annually, with peak detections of PIV-3 typically occurring in the spring, while PIV-1 peaked only in late 2011 (Supplementary Figure 1).
Figure 1.
Seasonality of parainfluenza pneumonia.
Clinical Features of PIV-1, PIV-2, and PIV-3 Infections, Adjusted for Age, Sex, and Race
After exclusion of 2 instances of PIV-1 and PIV-3 co-detection, we compared patient characteristics and clinical features of PIV-1, -2, and -3 infections in children and adults. Children with PIV-3–associated pneumonia were significantly younger than children with PIV-1–associated and PIV-2–associated pneumonia; 62% of PIV-3 detections occurred in children younger than 2 years, compared with 29–32% of PIV-1 and PIV-2 detections (Table 1). Cough occurred significantly more commonly in children with PIV-1 and PIV-3 than in PIV-2 detections (P = .03), and rhinorrhea occurred most commonly in PIV-3 detections (P = .04). Other clinical features and radiographic features did not differ significantly between groups. Mild disease occurred in 68% of PIV-1, 76% of PIV-2, and 86% of PIV-3 pneumonia cases in children (P = .064).
In adults with PIV detected by PCR (n = 55), age was similar among PIV-1, PIV-2, and PIV-3 infections (Table 2). There were no differences in the proportion of patients with fever, cough, dyspnea, wheezing, oxygen requirement, or radiographic features between the PIV-1, PIV-2, and PIV-3 groups. Mild disease occurred in 67% of PIV-1, 76% of PIV-2, and 68% of PIV-3 pneumonia cases in adults (P = .54).
Features of Parainfluenza Virus–Associated Infections Compared With Other Viral, Bacterial, and Atypical Infections Adjusted for Age, Sex, and Race
In children, after exclusion of co-detections across pathogen groups, the presence of underlying comorbidities was significantly more common in children with PIV (48%) and other viral (51%) pneumonia than in those with bacterial pneumonia (27%; P < .001) (Table 3). Certain clinical features also varied by pathogen type. Wheezing occurred more commonly in PIV (46%) and other viral infections (49%) than in bacterial pneumonia (12%; P < .001). Abdominal pain occurred more commonly in bacterial (41%) and atypical (45%) pneumonia than in pneumonia associated with PIV (21%) or other viral (17%) pathogens (P = .002). Only 3% of PIV and 6% of other viral infections were classified as severe, compared with 29% of bacterial infections (P < .001).
Table 3.
Select Characteristics of Hospitalized Children With Only Parainfluenza Virus–Associated Pneumonia Compared With Other Viral, Bacterial, and Atypical Pneumonia
| PIV-only Pneumonia (n = 67) | Other Viral Pneumoniaa (n = 1342) | Bacterial-only Pneumonia (n = 41) | Atypical-only Pneumonia (n = 133) | No Pathogen Detected (n = 416) | P b | |
|---|---|---|---|---|---|---|
| Female sex | 26 (39) | 640 (48) | 14 (34) | 51 (38) | 178 (43) | .010* |
| Median (IQR) age, m | 33 (14–63) | 22 (10–49) | 55 (16–104) | 104 (67–140) | 57.5 (18–114.5) | <.001* |
| Age group | <.001* | |||||
| <2 y | 26 (39) | 711 (53) | 11 (27) | 10 (8) | 118 (28) | |
| 2–4 y | 23 (34) | 358 (27) | 12 (29) | 19 (14) | 92 (22) | |
| 5–9 y | 12 (18) | 184 (14) | 12 (29) | 52 (39) | 113 (27) | |
| 10–17 y | 6 (9) | 89 (7) | 6 (15) | 52 (39) | 93 (22) | |
| Race/ethnicity | <.001* | |||||
| Hispanic | 16 (24) | 273 (20) | 7 (17) | 19 (14) | 55 (13) | |
| Non-Hispanic black | 27 (40) | 497 (37) | 7 (17) | 21 (16) | 144 (35) | |
| Non-Hispanic white | 20 (30) | 457 (34) | 24 (59) | 87 (65) | 194 (47) | |
| Other | 4 (6) | 115 (9) | 3 (7) | 6 (5) | 23 (6) | |
| Received influenza vaccine | 24 (36) | 493 (37) | 9 (22) | 41 (31) | 151 (36) | .473 |
| Received PCV or PPSV | 58 (87) | 1182 (88) | 33 (80) | 91 (68) | 326 (78) | .953 |
| Daycare attendance (<6 y)c | 21 (40) | 377 (33) | 7 (30) | 10 (29) | 68 (28) | .571 |
| Any underlying comorbidityd | 32 (48) | 683 (51) | 11 (27) | 54 (41) | 204 (49) | <.001* |
| Asthma | 25 (37) | 497 (37) | 8 (20) | 37 (28) | 119 (29) | <.001* |
| Household smoke exposure | 19 (28) | 515 (38) | 16 (39) | 34 (26) | 126 (30) | .101 |
| Symptom duration prior to admission, d | 3 (1–6) | 3 (1–5) | 4 (2–8) | 7 (5–9) | 3 (1–7) | <.001* |
| Antibiotics prior to admission | 12 (18) | 291 (22) | 13 (32) | 73 (55) | 121 (29) | <.001* |
| Clinical presentation | ||||||
| Fever/feverish | 66 (99) | 1218 (91) | 39 (95) | 124 (93) | 375 (90) | .278 |
| Cough | 65 (97) | 1295 (96) | 37 (90) | 127 (96) | 371 (89) | <.001* |
| Dyspnea | 41 (61) | 978 (73) | 31 (76) | 82 (62) | 271 (65) | <.001* |
| Retractions | 26 (39) | 644 (48) | 18 (44) | 32 (24) | 163 (39) | .007* |
| Wheezing | 31 (46) | 655 (49) | 5 (12) | 33 (25) | 115 (28) | <.001* |
| Rhinorrhea | 51 (76) | 1017 (76) | 22 (54) | 54 (41) | 222 (53) | <.001* |
| Abdominal pain | 14 (21) | 231 (17) | 17 (41) | 60 (45) | 121 (29) | .002* |
| Ear pain | 10 (15) | 296 (22) | 7 (17) | 27 (20) | 66 (16) | .251 |
| Sore throat | 20 (30) | 356 (27) | 15 (37) | 63 (47) | 141 (34) | .973 |
| Oxygen requirement | 36 (54) | 894 (67) | 24 (59) | 85 (64) | 209 (50) | <.001* |
| WBC count >15 × 103 | 19 (28) | 329 (25) | 22 (54) | 24 (18) | 184 (44) | <.001* |
| Radiographic features | <.001* | |||||
| Single lobar | 18 (27) | 257 (19) | 14 (34) | 35 (26) | 139 (33) | |
| Multilobar | 14 (21) | 288 (21) | 15 (37) | 26 (20) | 104 (25) | |
| Other consolidation | 2 (3) | 87 (6) | 2 (5) | 4 (3) | 19 (5) | |
| Other infiltrate | 25 (37) | 614 (46) | 8 (20) | 58 (44) | 126 (30) | |
| Mixed infiltrate | 8 (12) | 96 (7) | 2 (5) | 10 (8) | 28 (7) | |
| Empyema | 4 (6) | 72 (5) | 27 (66) | 28 (21) | 77 (19) | <.001* |
| Length of stay, median (IQR), h | 61 (38–88) | 65 (43–102) | 160 (87–240) | 58 (41–88) | 62.5 (38–108) | .004* |
| Severity | <.001* | |||||
| Mild | 60 (90) | 1066 (79) | 23 (56) | 120 (90) | 327 (79) | |
| Moderate | 5 (7) | 194 (14) | 6 (15) | 12 (9) | 55 (13) | |
| Severe | 2 (3) | 82 (6) | 12 (29) | 1 (1) | 34 (8) |
Data are presented as n (%) unless otherwise indicated; N = 1999.
Abbreviations: AdV, adenovirus; CoV, coronavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; IQR, interquartile range; PCV, pneumococcal conjugate vaccine; PIV, parainfluenza virus; PPSV, pneumococcal polysaccharide vaccine;; RSV, respiratory syncytial virus; WBC, white blood cell.
*Significant for heterogeneity among the groups, P < .05.
a Other viral pneumonia was defined as pneumonia with ≥1 other virus detected (hMPV, RSV, AdV, CoV, influenza, or HRV, inclusive of co-detections of >1 of these viruses).
bcomparisons adjusted for age, sex, and race as appropriate.
cAny comorbidity including asthma, congenital heart disease, diabetes, chronic renal disease, chronic liver disease, immunosuppression, splenectomy, prematurity (assessed if <2 y), neurological disorder. Immunosuppression was present as a comorbidity in 2/67 (3%) PIV infections.
dDaycare attendance recorded for children less than 6 years of age (total n = 1478, PIV n = 53, other viral n = 1126, bacterial n = 23, atypical n = 35, no pathogen detected n = 241 children <6 y).
In adults, significant differences in certain clinical features were observed between PIV and other etiologic groups (Table 4). Wheezing was more commonly seen among patients with PIV (46%) and other viral (41%) pneumonia than among those with bacterial (27%) and atypical (19%) pneumonia (P < .001). The majority of PIV infections were associated with radiographic patterns characterized as “other infiltrate,” while in all other etiologic categories, the majority of infections were associated with a single or multilobar infiltrative pattern (P = .008). Ten percent of PIV and 8% of other viral infections were classified as severe, compared with 26% of bacterial infections, 10% of pneumonia with no pathogen detected, and 3% of atypical infections (P < .001).
Table 4.
Select Characteristics of Hospitalized Adults With Only Parainfluenza Virus–Associated Pneumonia Compared With Other Viral, Bacterial, and Atypical Pneumonia
| PIV-only Pneumonia (n = 39) | Other Viral Pneumoniaa (n = 478) | Bacterial-only Pneumonia (n = 169) | Atypical-only Pneumonia (n = 74) | No Pathogen Detected (n = 1389) | P b | |
|---|---|---|---|---|---|---|
| Female sex | 18 (46) | 274 (57) | 84 (50) | 32 (43) | 694 (50) | .031* |
| Median (IQR) age, y | 58 (50–72) | 55 (44–70) | 61 (50–71) | 51 (38–64) | 58 (47–71) | .002* |
| Age group | .021* | |||||
| 18–49 y | 9 (23) | 166 (35) | 38 (22) | 34 (46) | 402 (29) | |
| 50–64 y | 15 (38) | 146 (31) | 60 (36) | 22 (30) | 494 (36) | |
| 65–79 y | 8 (21) | 104 (22) | 50 (30) | 13 (18) | 304 (22) | |
| ≥80 y | 7 (18) | 62 (13) | 21 (12) | 5 (7) | 189 (14) | |
| Race/ethnicity | .147 | |||||
| Hispanic | 3 (8) | 58 (12) | 14 (8) | 13 (18) | 134 (10) | |
| Non-Hispanic black | 15 (38) | 185 (39) | 54 (32) | 23 (31) | 557 (40) | |
| Non-Hispanic white | 20 (51) | 215 (45) | 97 (57) | 38 (51) | 636 (46) | |
| Other | 1 (3) | 20 (4) | 4 (2) | 0 (0) | 62 (4) | |
| Received influenza vaccine | 13 (33) | 192 (40) | 59 (35) | 24 (32) | 529 (38) | .368 |
| Received PPSV | 17 (44) | 187 (39) | 65 (38) | 24 (32) | 524 (38) | .949 |
| Any underlying comorbidityc | 36 (92) | 365 (76) | 141 (83) | 43 (58) | 1099 (79) | .007* |
| Current smoker | 13 (33) | 136 (28) | 48 (28) | 18 (24) | 346 (25) | .583 |
| Symptom duration prior to admission, d | 3 (2–6) | 4 (2–7) | 3 (1–7) | 5 (3–7) | 4 (1–8) | .089 |
| Antibiotics prior to admission | 6 (15) | 91 (19) | 18 (11) | 16 (22) | 293 (21) | .026* |
| Clinical presentation | ||||||
| Fever/feverish | 31 (79) | 352 (74) | 112 (66) | 64 (86) | 909 (65) | <.001* |
| Cough | 38 (97) | 451 (94) | 134 (79) | 68 (92) | 1190 (86) | <.001* |
| Dyspnea | 31 (79) | 386 (81) | 126 (75) | 49 (66) | 1085 (78) | .049* |
| Wheezing | 18 (46) | 198 (41) | 45 (27) | 14 (19) | 342 (25) | <.001* |
| Rhinorrhea | 20 (51) | 241 (50) | 61 (36) | 16 (22) | 473 (34) | <.001* |
| Abdominal pain | 6 (15) | 104 (22) | 36 (21) | 10 (14) | 279 (20) | .440 |
| Ear pain | 6 (15) | 71 (15) | 17 (10) | 11 (15) | 152 (11) | .267 |
| Sore throat | 15 (39) | 187 (39) | 46 (27) | 21 (28) | 358 (26) | <.001* |
| Oxygen requirement | 17 (44) | 272 (57) | 112 (66) | 35 (47) | 717 (52) | .033* |
| WBC count >15 × 103 | 3 (8) | 98 (21) | 66 (39) | 14 (19) | 343 (25) | <.001* |
| Radiographic features | .008* | |||||
| Single lobar | 6 (15) | 131 (27) | 50 (30) | 36 (49) | 375 (27) | |
| Multilobar | 9 (23) | 126 (26) | 48 (28) | 20 (27) | 372 (27) | |
| Other consolidation | 2 (5) | 25 (5) | 13 (8) | 1 (1) | 56 (4) | |
| Other infiltrate | 20 (51) | 167 (35) | 47 (28) | 10 (14) | 492 (35) | |
| Mixed infiltrate | 2 (5) | 29 (6) | 11 (7) | 7 (9) | 94 (7) | |
| Empyema | 4 (10) | 28 (6) | 34 (20) | 6 (8) | 105 (8) | <.001* |
| Length of stay, median (IQR), d | 2 (2–5) | 3 (2–5) | 6 (3–11) | 3 (2–4) | 3 (2–6) | <.001* |
| Severity | <.001* | |||||
| Mild | 31 (79) | 382 (80) | 88 (52) | 63 (85) | 1093 (79) | |
| Moderate | 4 (10) | 58 (12) | 37 (22) | 9 (12) | 164 (12) | |
| Severe | 4 (10) | 38 (8) | 44 (26) | 2 (3) | 132 (10) |
Data are presented as n (%) unless otherwise indicated; N = 2149.
Abbreviations: AdV, adenovirus; CoV, coronavirus; hMPV, human metapneumovirus; HRV, human rhinovirus; IQR, interquartile range; PIV, parainfluenza virus; PPSV, pneumococcal polysaccharide vaccine; RSV, respiratory syncytial virus; WBC, white blood cell.
*Significant for heterogeneity among the groups, P < .05.
aOther viral pneumonia was defined as pneumonia with ≥1 other virus detected (hMPV, RSV, AdV, CoV, influenza, or HRV; inclusive of co-detections of >1 of these viruses).
bComparisons adjusted for age, sex, and race as appropriate.
cAny comorbidity including chronic lung disease, chronic heart disease, diabetes, chronic renal disease, chronic liver disease, immunosuppression, splenectomy, neurological disorder. Immunosuppression was present as a comorbidity in 15/39 (38%) PIV infections.
Clinical Severity and Length of Stay in Parainfluenza Virus–Associated Pneumonia Compared With Other Pathogens
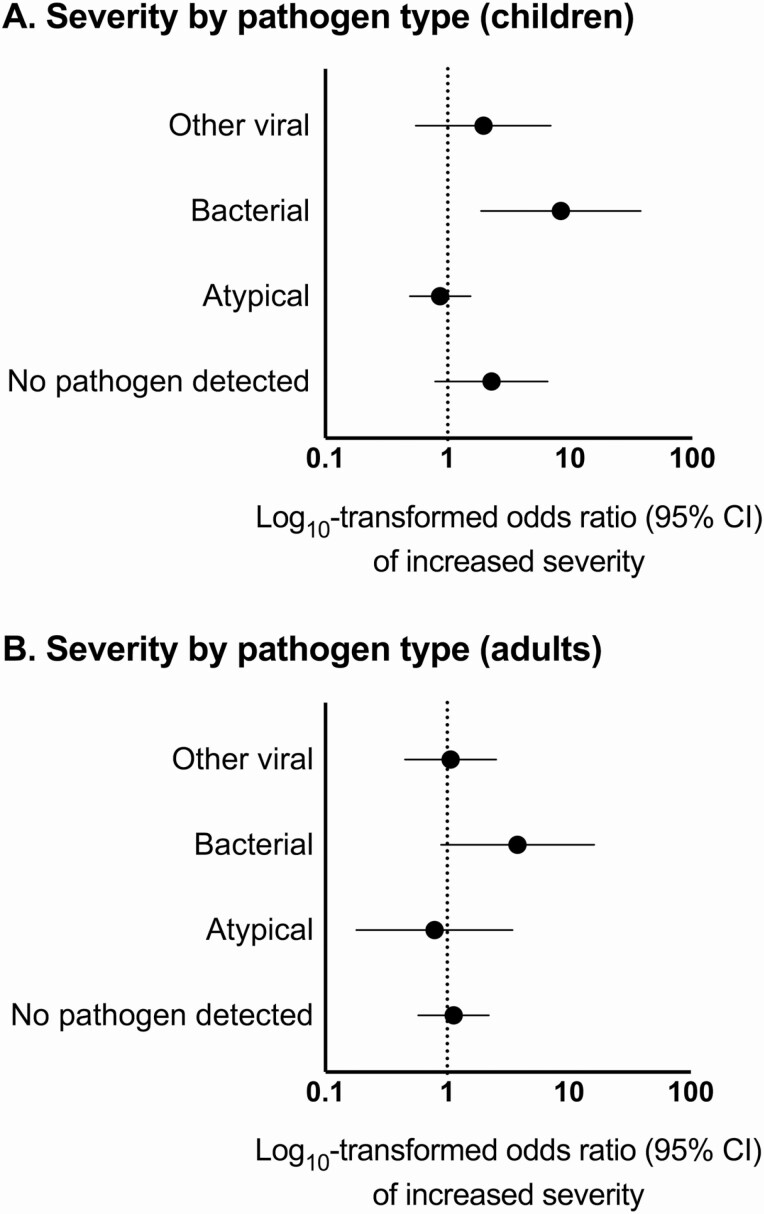
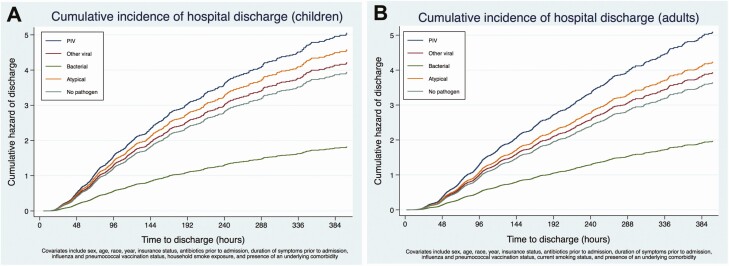
In multivariable regression analysis in children, compared with PIV-associated pneumonia, bacterial pneumonia was associated with increased odds of more severe disease (odds ratio [OR], 8.42; 95% confidence interval [CI], 1.88–37.80) (Figure 2A). However, odds of more severe disease did not significantly differ between PIV (reference) and other viruses (adjusted OR [aOR], 1.96; 95% CI, .55–6.97), atypical bacterial pathogens (aOR, .87; 95% CI, .49–1.54), or pneumonia with no pathogen detected (aOR, 2.28; 95% CI, .79–6.55). Compared with PIV-associated pneumonia, bacterial pneumonia (adjusted hazard ratio [aHR], .36; 95% CI, .24–.54) and pneumonia with no pathogen detected (aHR, .78; 95% CI, .67–.91) were associated with longer LOS (lower rate of discharge) (Figure 3A). The LOS for other viral (aHR, .83; 95% CI, .66–1.05) and atypical bacterial (aHR, .91; 95% CI, .57–1.45) pneumonia were similar to PIV pneumonia (reference).
Figure 2.
Proportional odds regression of severity (mild, moderate, severe) by pathogen type (reference: PIV) among (A) children and (B) adults. Abbreviations: CI, confidence interval; PIV, parainfluenza virus.
Figure 3.
A, Cumulative incidence of hospital discharge (children). B, Cumulative incidence of hospital discharge (adults). Abbreviation: PIV, parainfluenza virus.
In adults, compared with PIV (reference), odds of more severe disease did not significantly differ among those with pneumonia associated with other viruses (OR, 1.07; 95% CI, .45–2.52), atypical bacteria (OR, .79; 95% CI, .18–3.44), or pneumonia with no pathogen detected (OR, 1.13; 95% CI, .58–2.20) (Figure 2B). Bacterial pneumonia was associated with increased odds of severe disease (OR, 3.78; 95% CI, .89–16.11) compared with PIV but CIs overlapped 1.0. However, LOS differed significantly among groups, with other viral (aHR, .77; 95% CI, .63–.95), bacterial (aHR, .39; 95% CI, .26–.58), and pneumonia with no pathogen detected (aHR, .72; 95% CI, .57–.90) associated with longer LOS (Figure 3B) compared with PIV.
DISCUSSION
While PIV is recognized as an important etiology of respiratory illnesses in children, our findings underscore the importance of PIV as an etiology of pneumonia requiring hospitalization in both children and adults. In our study, PIV-associated pneumonia was associated with similar severity to other pathogens, except in children, in whom it was associated with reduced severity relative to bacterial pneumonia. We also found that clinical features varied little between PIV serotypes among cases of CAP. There was substantial overlap in presenting signs and symptoms of PIV infections and other etiologies, limiting the ability of clinical characteristics to reliably distinguish among pathogens at hospital admission.
In children younger than 5 years in the recent international Pneumonia Etiology for Child Health (PERCH) Study, PIV (including PIV-4) was among the 5 most common pathogens in 5 of 7 sites, detected in 12% of pneumonia cases and 5.9% of controls [4, 34]. Differences in populations and selection criteria between the PERCH study, which enrolled cases of World Health Organization–defined severe/very severe pneumonia, and our study should be considered when comparing detection estimates [35, 36].
Other studies among adults hospitalized with PIV-associated pneumonia have reported similar prevalence, ranging from 0.2% to 11% [7, 37, 38]. PIV is well known to cause severe infections in immunocompromised adults, and increasingly recognized to contribute to exacerbations of chronic obstructive pulmonary disease or congestive heart failure, while PIV infection in healthy adults is generally mild [7, 39, 40]. Our findings were consistent with these patterns, as the majority of adults hospitalized with PIV-associated pneumonia exhibited an underlying comorbidity.
We also observed that increases in PIV pneumonia in children preceded increases in PIV pneumonia in adults, suggesting that children may transmit PIV to adults [41]. The highest prevalence of PIV-3 infections occurred in the late spring–summer in 2010 and 2011. Small numbers of PIV-1 and PIV-2 cases and our relatively brief observation period, capturing only 2 complete calendar years, limited clear establishment of seasonal patterns for these serotypes with typical biennial circulation patterns [16, 21].
Although the clinical features of PIV-1, PIV-2, and PIV-3 pneumonia varied little, our study did not include the full spectrum of respiratory conditions associated with PIV, such as laryngotracheobronchitis or bronchiolitis, in which more serotype variation may be seen [16, 17]. Nevertheless, even in this multicenter study with relatively frequent PIV detections, the low number of sole detections of PIV limited our ability to compare serotypes, particularly in adults.
Our study has several strengths, including active surveillance, use of multiple modes of diagnostic testing, and a rigorous CAP case definition. The study also has several limitations. Community-acquired pneumonia is not the most commonly associated respiratory syndrome with PIV infection; thus, these findings may not represent the full spectrum of clinical manifestations of overall PIV infection. Lower respiratory tract specimens were infrequently obtained, and many patients were lacking convalescent sera. These diagnostic tests have limited sensitivity, particularly for bacterial detection. There was some discordance between PCR and serologic test results in our study, which may be explained by several factors, including limited sensitivity of the PCR or serological tests, timing of nasal or serum sample collection relative to illness onset, or the presence of heterotypic antibodies resulting in a false-positive antibody response [26, 42]. Antibiotic use preceded hospitalization in nearly one-quarter of subjects, which may have reduced the yield of bacterial cultures, likely underestimating the prevalence of bacterial pneumonia [43]. Finally, we did not test for PIV-4.
In summary, PIV infections were relatively common in children and less common in adults hospitalized with radiographically confirmed CAP. We found few differences among pneumonia cases by PIV serotype and no clinical characteristics were identified that could reliably distinguish PIV from other pathogen types at presentation. The severity of PIV-associated pneumonia was similar to severity of pneumonia cases associated with other viruses in both children and adults.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients who graciously consented to participate in this study. In addition, we thank the following: Associated Regional and University Pathologists (ARUP) Laboratories: Heather London, Torrance Meyer; BioFire Diagnostics: Mark A. Poritz; CDC: Suzette Bartley, Bernie Beall, Nicole Burcher, Robert Davidson, Michael Dillon, Barry Fields, Phalasy Juieng, Shelley Magill; Le Bonheur Children’s Hospital: Jody Cockroft, John Devincenzo, Tonya Galloway, Vivian Lebaroff, Moses Lockhart, Lakesha London, Tekita McKinney, Amanda Nesbit, Chirag Patel, Tina Pitt, Shante Richardson, Naeem Shaikh, Davida Singleton, Mildred Willis; Monroe Carell Jr Children’s Hospital: Thomas Abramo, Gretchen Edwards, Regina Ellis, Angela Harbeson, Deborah Hunter, Romina Libster, Angela Mendoza, Renee Miller, Deborah Myers, Natalee Rathert, Becca Smith, Bob Sparks, Kristy Spilman, Tanya Steinback, Scott Taylor, Sandy Yoder; Primary Children’s Hospital: Trenda Barney, Patrick Morris; St Jude Children’s Research Hospital: Edwina Anderson, Nancy Foster, Donna Nance, Ryan Heine, Amanda-Anderson Green, Amy Iverson, Shane Gansebom, Pat Flynn, Randall Hayden, Kim Allison; University of Utah: Fumiko Alger, Alexandra Burringo, Christopher Carlson, Lacey Collom, Gabriel Cortez, Kristina Grim, Keith Gunnerson, David Halladay, Caroline Heyrend, Jarrett Killpack, Kevin Martin, Brittany McDowell, Francesca Nichols, Parker Plant, Margaret Reid, Joshua Shimizu, Luke Schunk, Melanie Sperry, John Sweeley, and Lucy Williams.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. L. M. H. is supported by the National Institutes of Health under award number 1K23AI141621. This work was performed by L. M. H. as a Young Investigator Award recipient of the IDSA Education and Research Foundation (ERF) sponsored by Pfizer. C. G. G. was supported in part by the National Institutes of Health (grant number NIAID K24AI148459).
Potential conflicts of interest . L. M. H. reports that the Vanderbilt University Medical Center has received grant support from Pfizer and CDC; W. H. S. reports receiving research funding and fees for serving on advisory boards for Merck and Pfizer; R. G. W. reports receiving consulting fees from Roche, the Medicines Company, Vical, Cubist Pharmaceuticals, Bayer, Cerexa, and Visterra; D. J. W. has received research support form Biomerieux, CDC, National Institutes of Health, and the Agency for Healthcare Research and Quality; C. G. G. has received consulting fees from Pfizer, Sanofi, and Merck and received research support from Sanofi-Pasteur, Campbell Alliance, the CDC, National Institutes of Health, the Food and Drug Administration, and the Agency for Healthcare Research and Quality; E. J. A. reports receiving grant support through his institution from MedImmune, GlaxoSmithKline, Pfizer, Merck, Sanofi-Pasteur, PaxVax, Novavax, and Micron Biomedical, and consulting fees from AbbVie and Pfizer; S. R. A. reports receiving grant support from GlaxoSmithKline; K. A. reports receiving fees through his institution from GlaxoSmithKline, National Institute of Allergy and Infectious Disease, and Cubist Pharmaceuticals for the enrollment of patients in other studies, and collaborating with BioFire Diagnostics on grants funded by the National Institutes of Health (grant number 1U181P000303), Epidemiology and Etiology of Pneumonia in Children from CDC, and consulting fees to the institution from Merck; A. T. P. reports receiving fees for serving on an advisory board for BioFire Diagnostics, grants from the National Institutes of Health, fees for creation of content from WebMD, consulting fees from Genentech and Merck, fees for the preparation of educational material from Medscape, and royalties from Antimicrobial Therapy; and K. M. E. reports serving on a data and safety monitoring board for Novartis, Sanofi, Pfizer, Sequiras, X4 Pharmaceuticals, and Moderna for which her institution receives fees; advisor fees from Bionet and Merck; and payments from Bionet, the National Institutes of Health, and Roche, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD; New Vaccine Surveillance Network Study Group . Epidemiology of respiratory infections in young children: insights from the New Vaccine Surveillance Network. Pediatr Infect Dis J 2004; 23:S188–92. [DOI] [PubMed] [Google Scholar]
- 2. Iwane MK, Edwards KM, Szilagyi PG, et al. ; New Vaccine Surveillance Network . Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004; 113:1758–64. [DOI] [PubMed] [Google Scholar]
- 3. Weinberg GA, Hall CB, Iwane MK, et al. ; New Vaccine Surveillance Network . Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr 2009; 154:694–9. [DOI] [PubMed] [Google Scholar]
- 4. Feikin DR, Fu W, Park DE, et al. ; PERCH Study Group . Is higher viral load in the upper respiratory tract associated with severe pneumonia? Findings from the PERCH Study. Clin Infect Dis 2017; 64:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Budge PJ, Griffin MR, Edwards KM, et al. ; RESPIRA-PERU Group . A household-based study of acute viral respiratory illnesses in Andean children. Pediatr Infect Dis J 2014; 33:443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 2008; 134:1141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russell E, Ison MG. Parainfluenza virus in the hospitalized adult. Clin Infect Dis 2017; 65:1570–6. [DOI] [PubMed] [Google Scholar]
- 8. Álvarez-Argüelles ME, Rojo-Alba S, Pérez Martínez Z, et al. . New clinical and seasonal evidence of infections by human parainfluenzavirus. Eur J Clin Microbiol Infect Dis 2018; 37:2211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar D, Husain S, Chen MH, et al. . A prospective molecular surveillance study evaluating the clinical impact of community-acquired respiratory viruses in lung transplant recipients. Transplantation 2010; 89:1028–33. [DOI] [PubMed] [Google Scholar]
- 10. Maeng SH, Yoo HS, Choi SH, et al. . Impact of parainfluenza virus infection in pediatric cancer patients. Pediatr Blood Cancer 2012; 59:708–10. [DOI] [PubMed] [Google Scholar]
- 11. Srinivasan A, Wang C, Yang J, Shenep JL, Leung WH, Hayden RT. Symptomatic parainfluenza virus infections in children undergoing hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17:1520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danziger-Isakov L, Englund J, Green M, Posfay-Barbe KM, Zerr DM. Cytomegalovirus in pediatric hematopoietic stem cell transplantation: a case-based panel discussion of current challenges. J Pediatric Infect Dis Soc 2018; 7:72–4. [DOI] [PubMed] [Google Scholar]
- 13. Paulsen GC, Danziger-Isakov L. Respiratory viral infections in solid organ and hematopoietic stem cell transplantation. Clin Chest Med 2017; 38:707–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parainfluenza infections in the elderly 1976–82. Br Med J (Clin Res Ed) 1983; 287:1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood 2001; 98:573–8. [DOI] [PubMed] [Google Scholar]
- 16. Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clin Infect Dis 2006; 43:1016–22. [DOI] [PubMed] [Google Scholar]
- 17. Walker TA, Khurana S, Tilden SJ. Viral respiratory infections. Pediatr Clin North Am 1994; 41:1365–81. [DOI] [PubMed] [Google Scholar]
- 18. Frost HM, Robinson CC, Dominguez SR. Epidemiology and clinical presentation of parainfluenza type 4 in children: a 3-year comparative study to parainfluenza types 1-3. J Infect Dis 2014; 209:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lau SK, To WK, Tse PW, et al. . Human parainfluenza virus 4 outbreak and the role of diagnostic tests. J Clin Microbiol 2005; 43:4515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao NG, Duan ZJ, Xie ZP, et al. . Human parainfluenza virus types 1-4 in hospitalized children with acute lower respiratory infections in China. J Med Virol 2016; 88:2085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeGroote NP, Haynes AK, Taylor C, et al. . Human parainfluenza virus circulation, United States, 2011–2019. J Clin Virol 2020; 124:104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maykowski P, Smithgall M, Zachariah P, et al. . Seasonality and clinical impact of human parainfluenza viruses. Influenza Other Respir Viruses 2018; 12:706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jain S, Williams DJ, Arnold SR, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Self WH, Williams DJ, Zhu Y, et al. . Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 2016; 213:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Sakthivel SK, Bramley A, et al. . Serology enhances molecular diagnosis of respiratory virus infections other than influenza in children and adults hospitalized with community-acquired pneumonia. J Clin Microbiol 2017; 55:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinberg GA, Schnabel KC, Erdman DD, et al. . Field evaluation of TaqMan Array Card (TAC) for the simultaneous detection of multiple respiratory viruses in children with acute respiratory infection. J Clin Virol 2013; 57:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blaschke AJ, Heyrend C, Byington CL, et al. . Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J 2011; 30:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blaschke AJ, Heyrend C, Byington CL, et al. . Rapid identification of pathogens from positive blood cultures by multiplex polymerase chain reaction using the FilmArray system. Diagn Microbiol Infect Dis 2012; 74:349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams DJ, Zhu Y, Grijalva CG, et al. . Predicting severe pneumonia outcomes in children. Pediatrics 2016; 138:e20161019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong BG, Sloan M. Ordinal regression models for epidemiologic data. Am J Epidemiol 1989; 129:191–204. [DOI] [PubMed] [Google Scholar]
- 32. Cox DR. Regression models and life-tables. J R Stat Soc 1972; 34:187–220. [Google Scholar]
- 33. Tanner-Smith EE, Tipton E. Robust variance estimation with dependent effect sizes: practical considerations including a software tutorial in Stata and SPSS. Res Synth Methods 2014; 5:13–30. [DOI] [PubMed] [Google Scholar]
- 34. The Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet 2019; 394:757–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fancourt N, Deloria Knoll M, Barger-Kamate B, et al. . Standardized interpretation of chest radiographs in cases of pediatric pneumonia from the PERCH study. Clin Infect Dis 2017; 64:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levine OS, O’Brien KL, Deloria-Knoll M, et al. . The pneumonia etiology research for child health project: a 21st century childhood pneumonia etiology study. Clin Infect Dis 2012; 54(Suppl 2):S93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alimi Y, Lim WS, Lansbury L, Leonardi-Bee J, Nguyen-Van-Tam JS. Systematic review of respiratory viral pathogens identified in adults with community-acquired pneumonia in Europe. J Clin Virol 2017; 95:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Angeles Marcos M, Camps M, Pumarola T, et al. . The role of viruses in the aetiology of community-acquired pneumonia in adults. Antivir Ther 2006; 11:351–9. [PubMed] [Google Scholar]
- 39. Branche AR, Falsey AR. Parainfluenza virus infection. Semin Respir Crit Care Med 2016; 37:538–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marx A, Gary HE Jr, Marston BJ, et al. . Parainfluenza virus infection among adults hospitalized for lower respiratory tract infection. Clin Infect Dis 1999; 29:134–40. [DOI] [PubMed] [Google Scholar]
- 41. MacIntyre CR, Ridda I, Seale H, et al. . Respiratory viruses transmission from children to adults within a household. Vaccine 2012; 30:3009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lennette EH, Jensen FW, Guenther RW, Magoffin RL. Serologic responses to para-influenza viruses in patients with mumps virus injection. J Lab Clin Med 1963; 61:780–8. [PubMed] [Google Scholar]
- 43. Harris AM, Bramley AM, Jain S, et al. . Influence of antibiotics on the detection of bacteria by culture-based and culture-independent diagnostic tests in patients hospitalized with community-acquired pneumonia. Open Forum Infect Dis 2017; 4:ofx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.